Hydro-Geochemistry and Water Quality Index Assessment in the Dakhla Oasis, Egypt
Abstract
1. Introduction
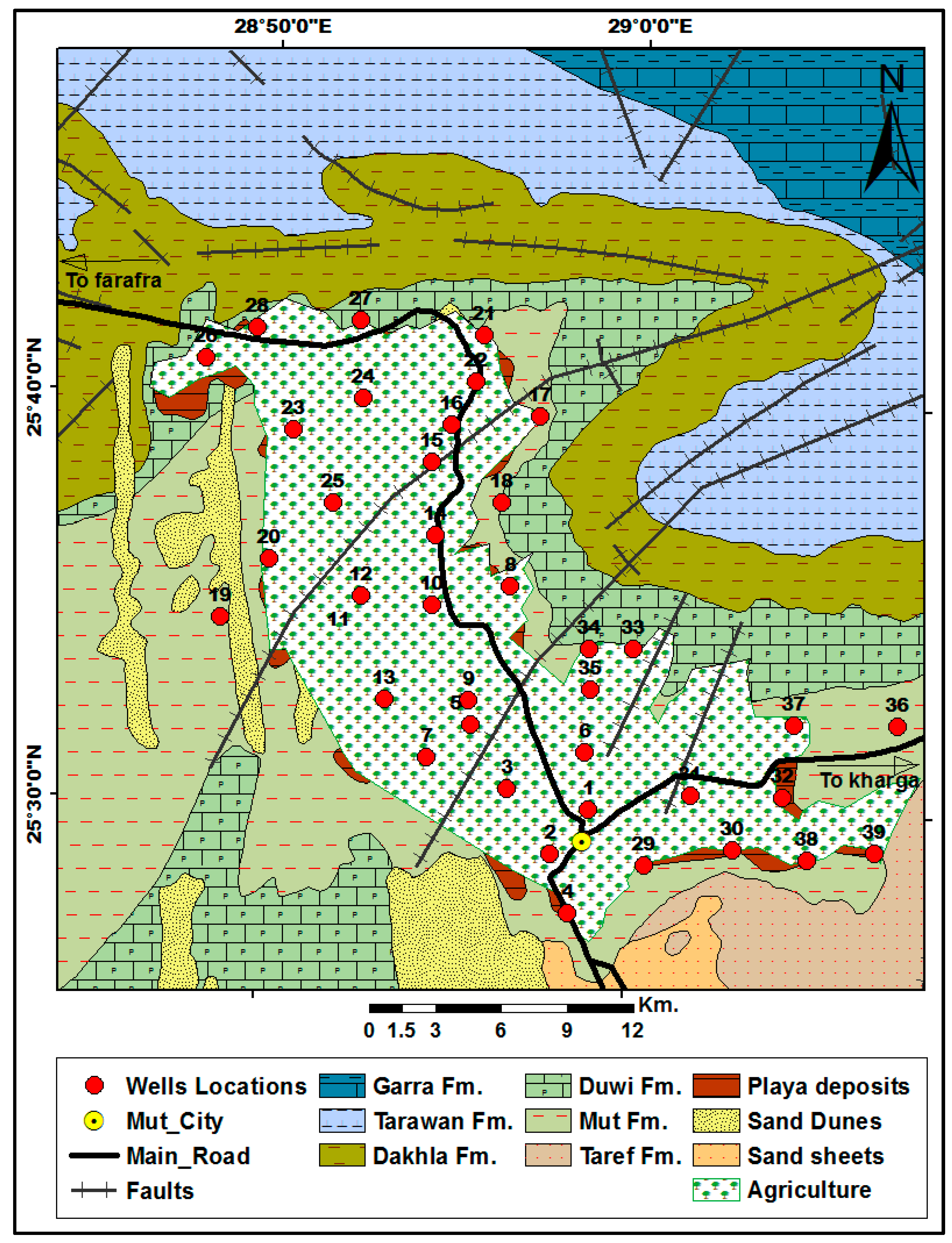
2. Study Area Description
Geology and Hydro-Geology
3. Materials and Methods
4. Results and Discussions
4.1. Physical Characteristics of Water
4.1.1. Temperature
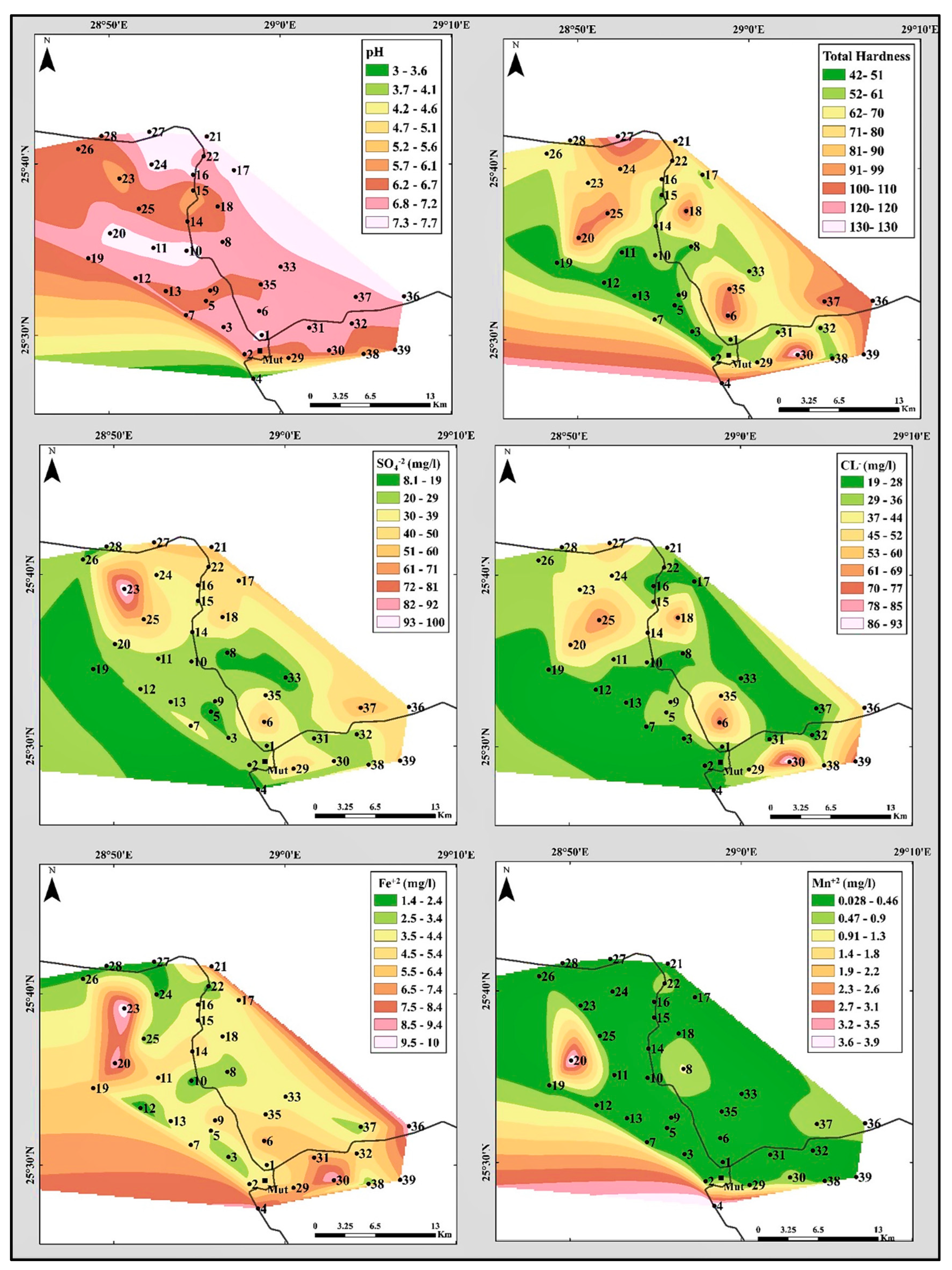
4.1.2. Hydrogen Ion Concentration (pH)
4.2. Chemical Characteristics of Water
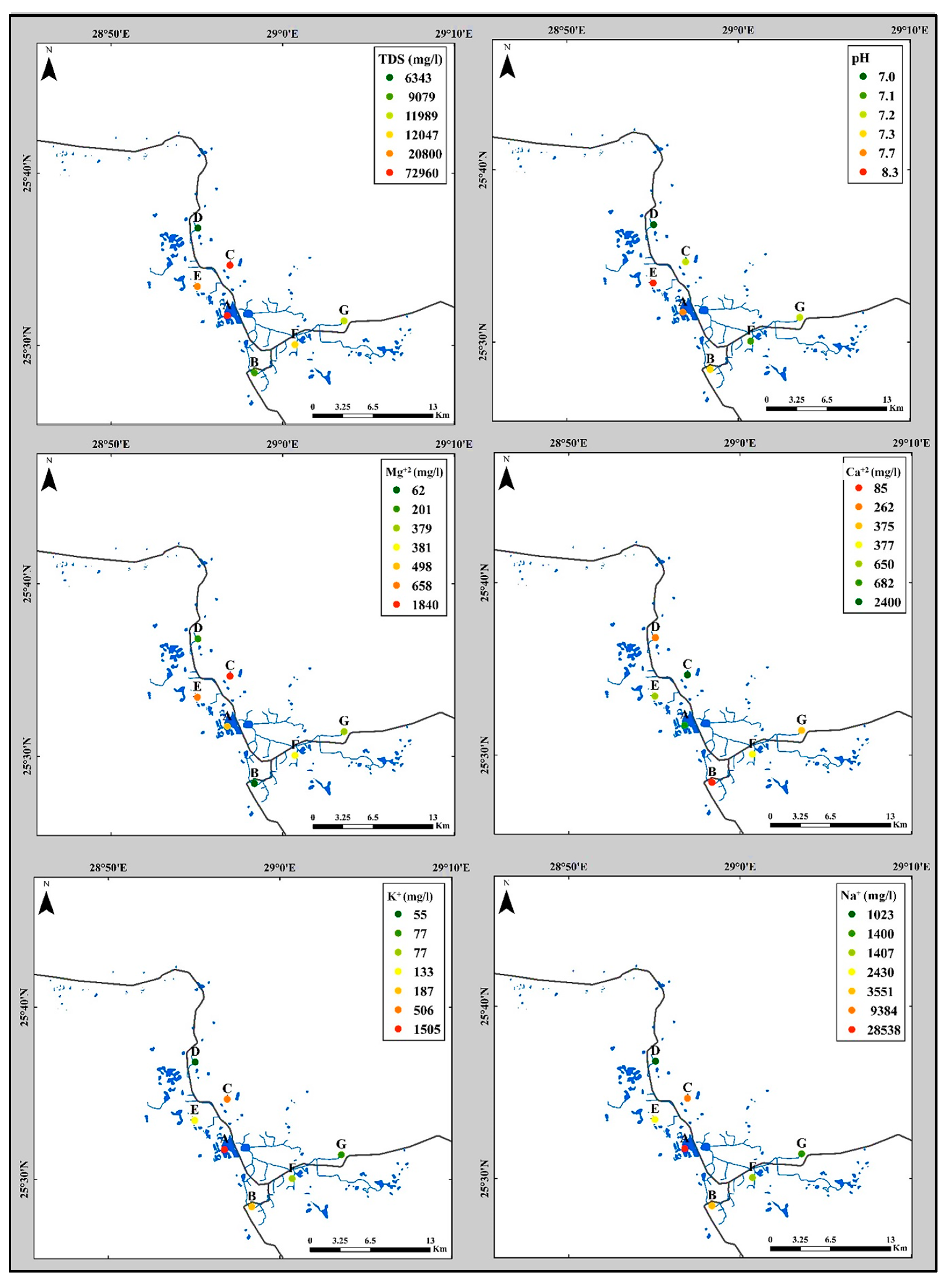
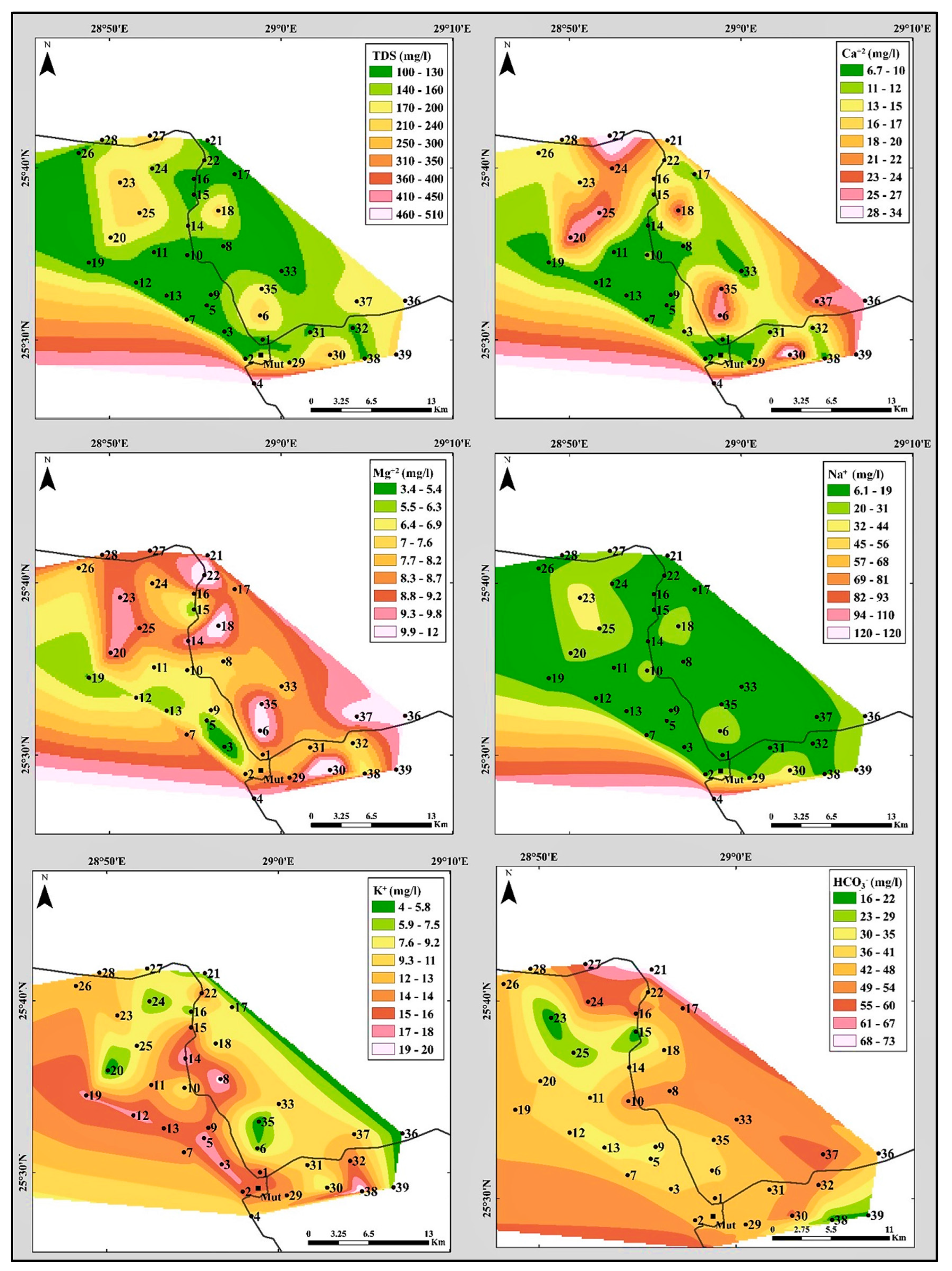
4.2.1. Total Dissolved Solids (TDS)
4.2.2. Total Hardness (TH)
4.2.3. Electrical Conductivity (EC)
4.2.4. Ion Concentrations and Distribution
- A. Major Ions:
- Calcium and magnesium ion concentrations and distribution:
- Sodium and potassium ions concentrations and distribution:
- Bicarbonate ions concentration and distribution:
- Sulphate ions concentrations and distribution:
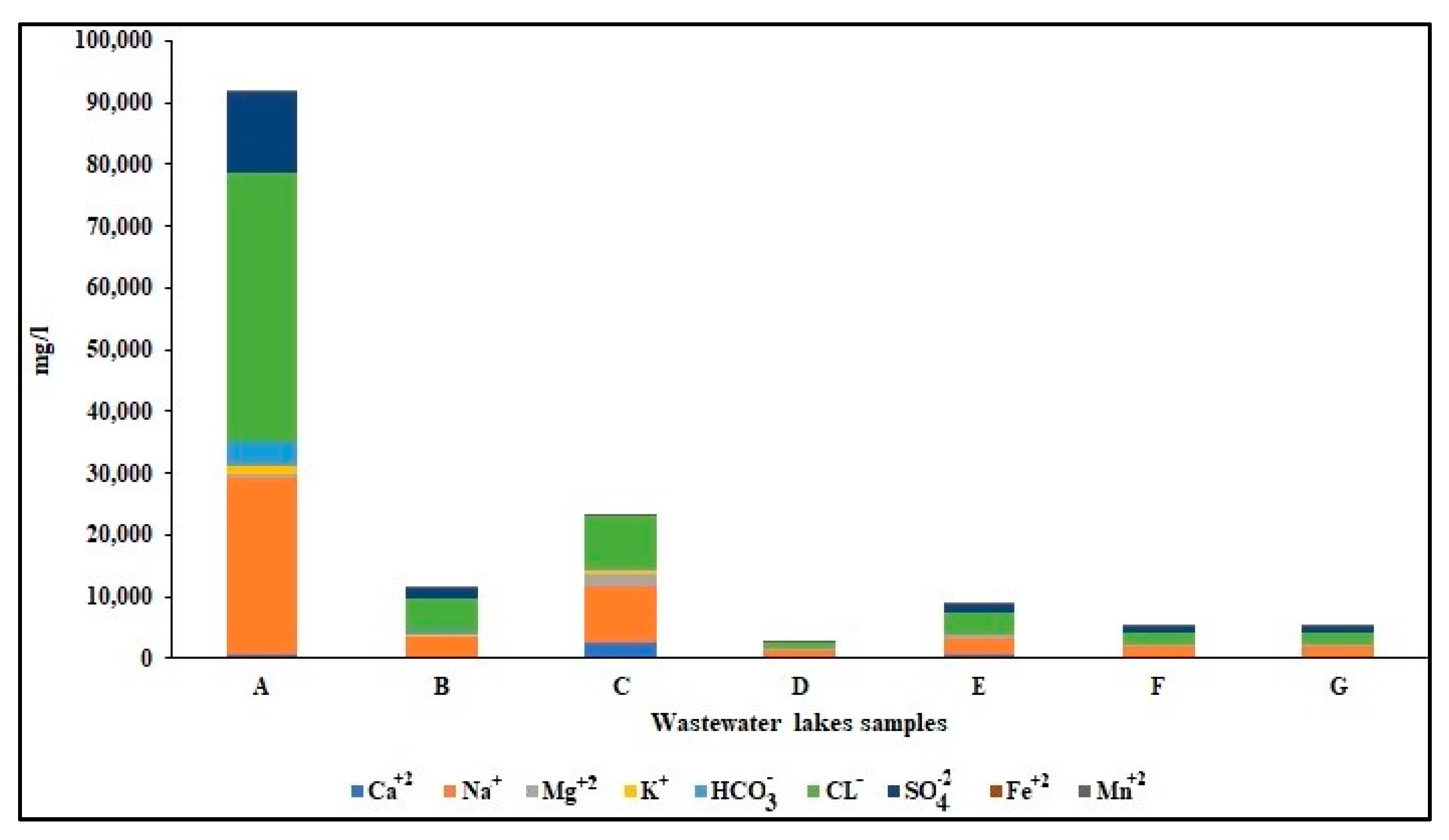
- Chloride ions concentrations and distribution:
- B. Trace elements:
- Iron (Fe+2) and Manganese (Mn+2)
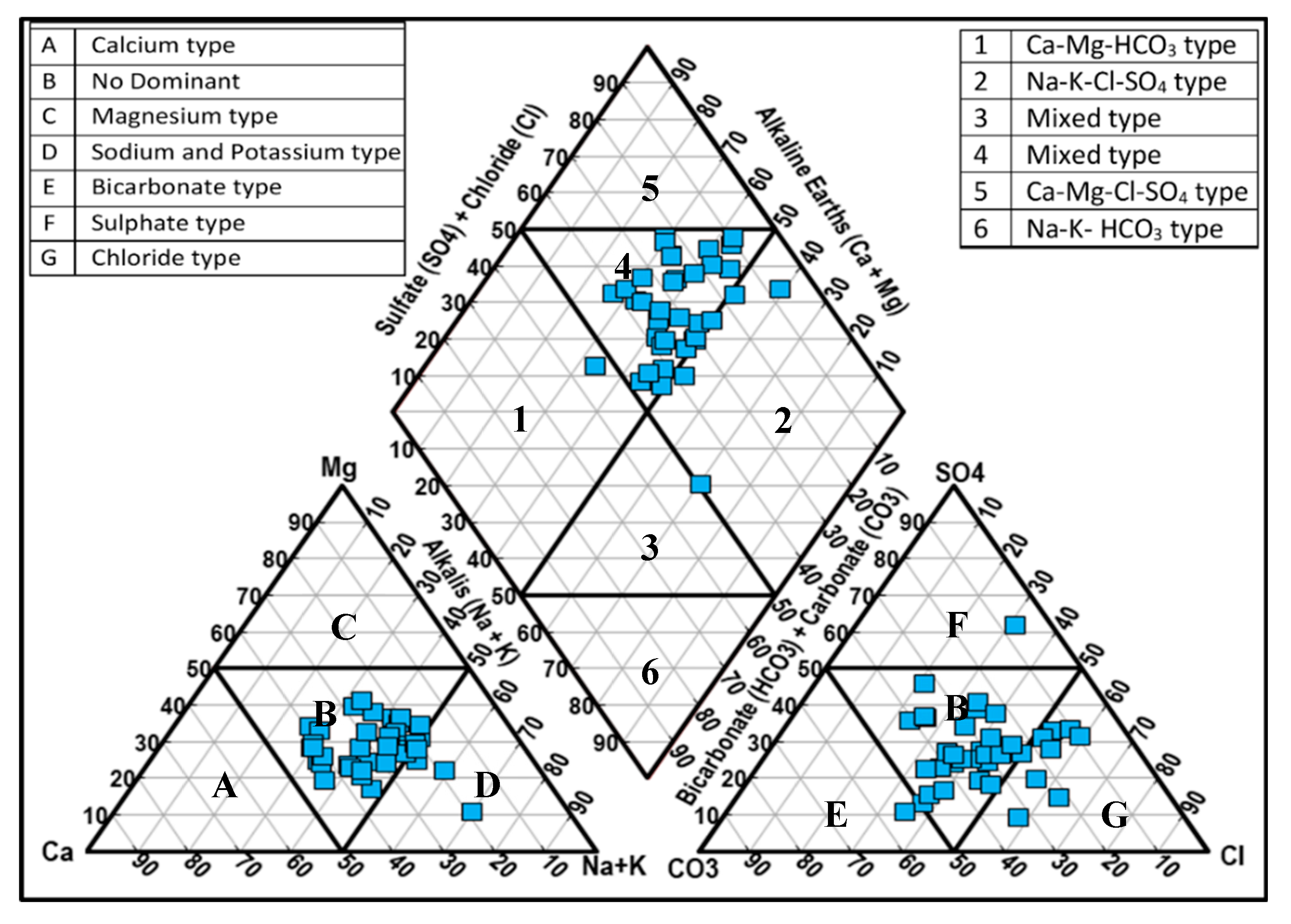
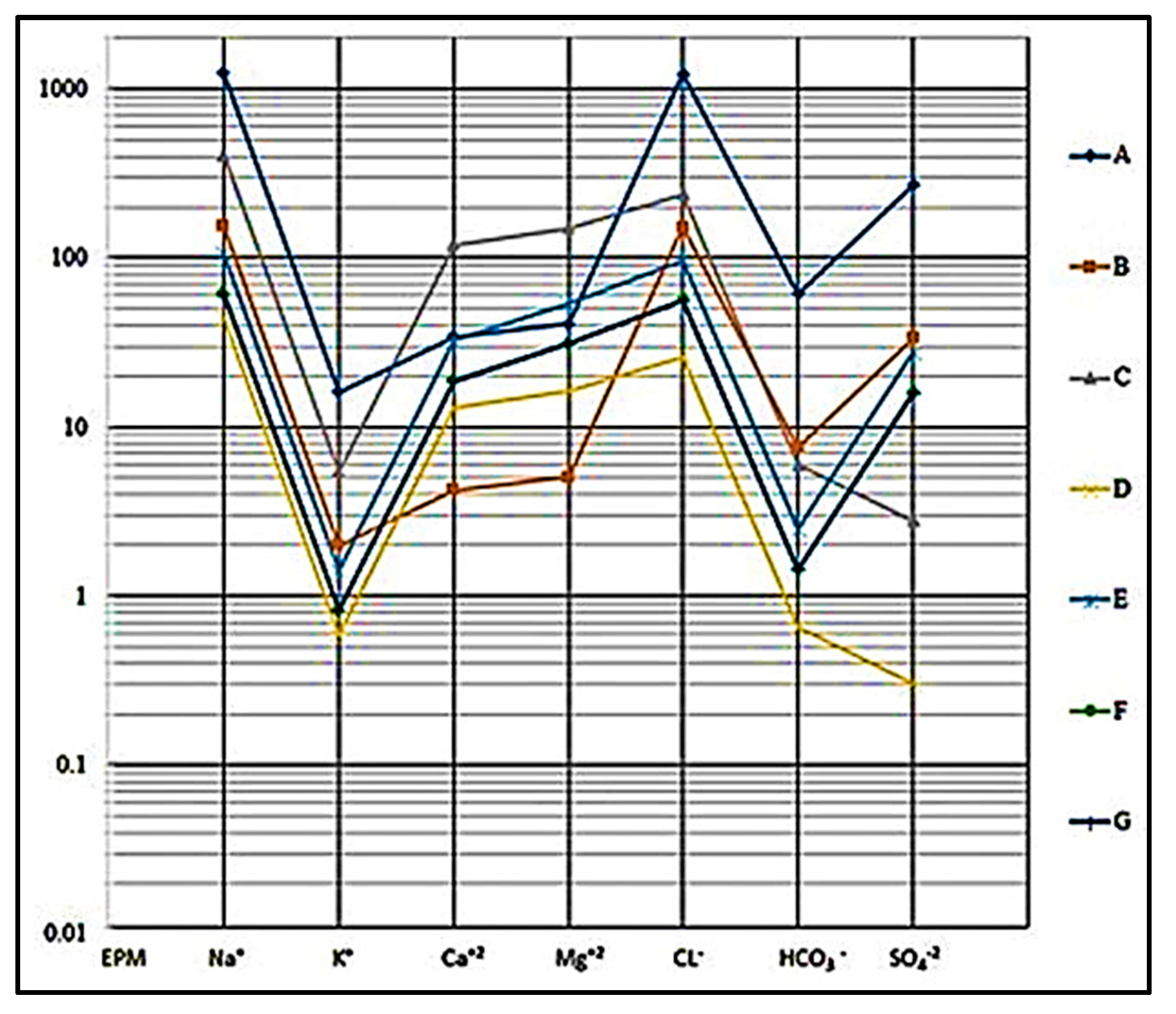
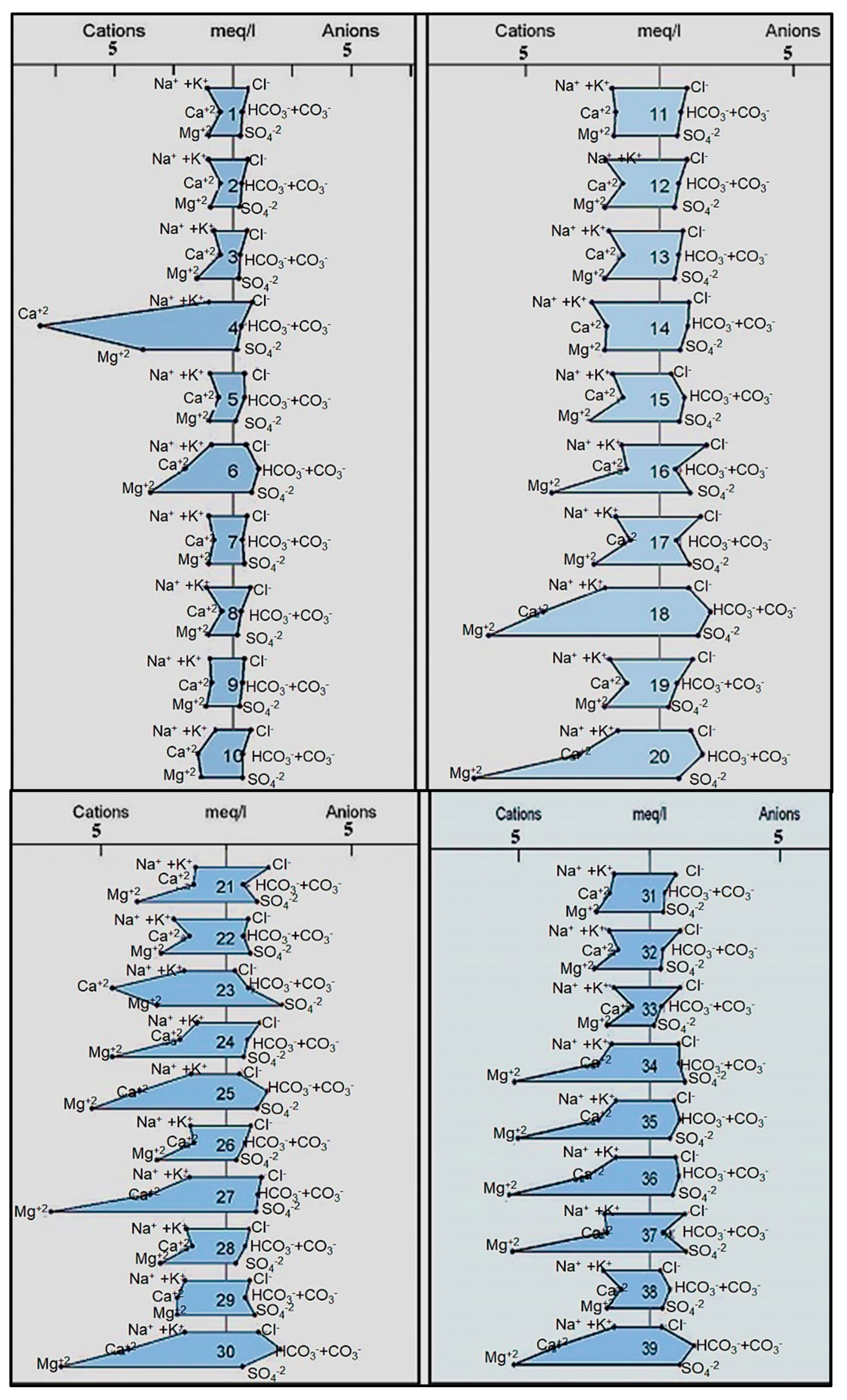
4.2.5. Hydro-Chemical Classifications
- Trilinear diagram:
- Semilogarithmic diagram:
- Stiff diagram
4.2.6. Water Type
- Na+ > Mg+2(Ca+2) > Ca+2 (Mg+2) and Cl− > SO4−2(HCO3−) > HCO3−(SO4−2), the groundwater type is sodium–chloride.
- Na+ > Mg+2(Ca+2) > Ca+2(Mg+2) and HCO3− > SO4−2(Cl−) > Cl−(SO4−2), the groundwater type is sodium–bicarbonate.
- Na+ > Mg+2(Ca+2) > Ca+2(Mg+2) and SO4−2 > HCO3−(Cl−) > Cl−(HCO3−), the groundwater type is sodium–sulphate.
- Ca+2 > Na+(Mg+2) > Mg+2(Na+) and Cl− > SO4−2(HCO3−) > HCO3−(SO4−2), the groundwater type is calcium–chloride.
- Ca+2 > Na+(Mg+2) > Mg+2(Na+) and SO4−2 > HCO3− > Cl−, the groundwater is calcium–sulphate.
- Ca+2 > Na+(Mg+2) > Mg+2(Na+) and HCO3− > SO4−2 > Cl−, the groundwater is calcium–bicarbonate.
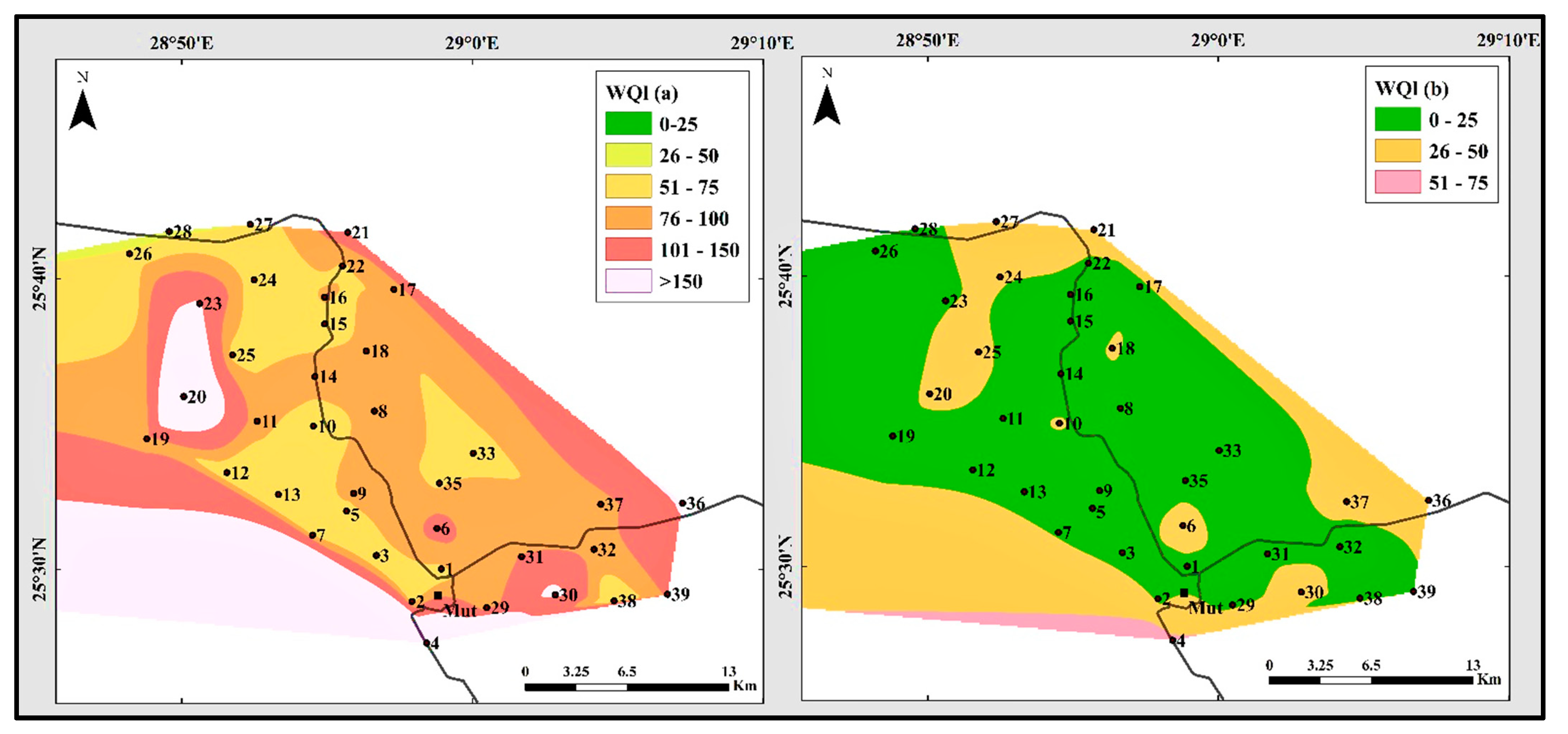
5. Water Quality Index (WQI)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, H.H.; Khan, A.; Ahmed, S.; Perrin, J. GIS-based impact assessment of land-use changes on groundwater quality: Study from a rapidly urbanizing region of South India. Environ. Earth Sci. 2011, 63, 1289–1302. [Google Scholar] [CrossRef]
- Siraj, G.; Khan, H.H.; Khan, A. Dynamics of surface water and groundwater quality using water quality indices and GIS in river Tamsa (Tons), Jalalpur, India. HydroResearch 2023, 6, 89–107. [Google Scholar] [CrossRef]
- Adimalla, N.; Wu, J. Groundwater quality and associated health risks in a semi-arid region of south India: Implication to sustainable groundwater management. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 191–216. [Google Scholar] [CrossRef]
- Kammoun, S.; Trabelsi, R.; Re, V.; Zouari, K.; Henchiri, J. Groundwater quality assessment in semi-arid regions using integrated approaches: The case of Grombalia aquifer (NE Tunisia). Environ. Monit. Assess. 2018, 190, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ostad-Ali-Askari, K.; Shayannejad, M.; Eslamian, S.; Zamani, F.; Shojaei, N.; Navabpour, B.; Majidifar, Z.; Sadri, A.; Ghasemi-Siani, Z.; Nourozi, H. Deficit irrigation: Optimization models. In Handbook of Drought and Water Scarcity; CRC Press: Boca Raton, FL, USA, 2017; pp. 375–391. [Google Scholar]
- Talebmorad, H.; Ostad-Ali-Askari, K. Hydro geo-sphere integrated hydrologic model in modeling of wide basins. Sustain. Water Resour. Manag. 2022, 8, 118. [Google Scholar] [CrossRef]
- Bodrud-Doza, M.; Bhuiyan, M.A.H.; Islam, S.D.-U.; Rahman, M.S.; Haque, M.M.; Fatema, K.J.; Ahmed, N.; Rakib, M.; Rahman, M.A. Hydrogeochemical investigation of groundwater in Dhaka City of Bangladesh using GIS and multivariate statistical techniques. Groundw. Sustain. Dev. 2019, 8, 226–244. [Google Scholar] [CrossRef]
- Mallick, J.; Singh, C.K.; AlMesfer, M.K.; Kumar, A.; Khan, R.A.; Islam, S.; Rahman, A. Hydro-geochemical assessment of groundwater quality in Aseer Region, Saudi Arabia. Water 2018, 10, 1847. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Unigwe, C.O.; Omeka, M.E.; Ayejoto, D.A. Urban groundwater quality assessment using pollution indicators and multivariate statistical tools: A case study in southeast Nigeria. Int. J. Environ. Anal. Chem. 2023, 103, 3324–3350. [Google Scholar] [CrossRef]
- Abrahão, R.; Causapé, J.; García-Garizábal, I.; Merchán, D. Implementing irrigation: Water balances and irrigation quality in the Lerma basin (Spain). Agric. Water Manag. 2011, 102, 97–104. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.-J.; Shen, J.-M.; Nie, Z.-L.; Meng, L.-Q.; Cao, L.; Wei, S.-B.; Zeng, X.-F. Groundwater characteristics and climate and ecological evolution in the Badain Jaran Desert in the southwest Mongolian Plateau. China Geol. 2021, 4, 421–432. [Google Scholar] [CrossRef]
- Gabr, M.E.; Soussa, H.; Fattouh, E. Groundwater quality evaluation for drinking and irrigation uses in Dayrout city Upper Egypt. Ain Shams Eng. J. 2021, 12, 327–340. [Google Scholar] [CrossRef]
- Darwish, M.H.; Sayed, A.G.; Hassan, S.H. Assessment of Water for Different Uses at Some Localities of the Dakhla Oasis, Western Desert, Egypt. Water Air Soil Pollut. 2023, 234, 516. [Google Scholar] [CrossRef]
- Massoud, M.A. Assessment of water quality along a recreational section of the Damour River in Lebanon using the water quality index. Environ. Monit. Assess. 2012, 184, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Rosemond, S.D.; Duro, D.C.; Dubé, M. Comparative analysis of regional water quality in Canada using the Water Quality Index. Environ. Monit. Assess. 2009, 156, 223–240. [Google Scholar] [CrossRef]
- Kannel, P.R.; Lee, S.; Lee, Y.-S.; Kanel, S.R.; Khan, S.P. Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environ. Monit. Assess. 2007, 132, 93–110. [Google Scholar] [CrossRef]
- Boyacioglu, H.; Boyacioglu, H. Surface water quality assessment by environmetric methods. Environ. Monit. Assess. 2007, 131, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Debels, P.; Figueroa, R.; Urrutia, R.; Barra, R.; Niell, X. Evaluation of water quality in the Chillán River (Central Chile) using physicochemical parameters and a modified water quality index. Environ. Monit. Assess. 2005, 110, 301–322. [Google Scholar] [CrossRef]
- Cox, C.R.; World Health Organization. Operation and Control of Water Treatment Processes; World Health Organization: Geneva, Switzerland, 1964.
- Horton, R.K. An index number system for rating water quality. J. Water Pollut. Control Fed. 1965, 37, 300–306. [Google Scholar]
- Drever, J.I. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Prentice Hall: Hoboken, NJ, USA, 1997. [Google Scholar]
- Akter, T.; Jhohura, F.T.; Akter, F.; Chowdhury, T.R.; Mistry, S.K.; Dey, D.; Barua, M.K.; Islam, M.A.; Rahman, M. Water Quality Index for measuring drinking water quality in rural Bangladesh: A cross-sectional study. J. Health Popul. Nutr. 2016, 35, 4. [Google Scholar] [CrossRef] [PubMed]
- Asadi, E.; Isazadeh, M.; Samadianfard, S.; Ramli, M.F.; Mosavi, A.; Nabipour, N.; Shamshirband, S.; Hajnal, E.; Chau, K.-W. Groundwater quality assessment for sustainable drinking and irrigation. Sustainability 2019, 12, 177. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Lin, Y.; Fang, Y.; Qian, H.; Liu, R.; Ma, H. Hydrogeochemical characteristics and quality assessment of groundwater in an irrigated region, Northwest China. Water 2019, 11, 96. [Google Scholar] [CrossRef]
- Cash, K.; Wright, R. Canadian Water Quality Guidelines for the Protection of Aquatic Life; CCME: Ottawa, ON, Canada, 2001. [Google Scholar]
- Ranganathan, M.; Karuppannan, S.; Murugasen, B.; Brhane, G.K.; Panneerselvam, B. Assessment of groundwater prospective zone in Adigrat Town and its surrounding area using geospatial technology. In Climate Change Impact on Groundwater Resources: Human Health Risk Assessment in Arid and Semi-Arid Regions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 387–405. [Google Scholar]
- Darwish, M.H.; Galal, W.F. Spatiotemporal effects of wastewater ponds from a geoenvironmental perspective in the Kharga region, Egypt. Prog. Phys. Geogr. Earth Environ. 2020, 44, 376–397. [Google Scholar] [CrossRef]
- Darwish, M.H.; Megahed, H.A.; Farrag, A.E.-H.A.; Sayed, A.G. Geo-Environmental Changes and Their Impact on the Development of the Limestone Plateau, West of Assiut, Egypt. J. Indian Soc. Remote Sens. 2020, 48, 1705–1727. [Google Scholar] [CrossRef]
- Galal, W.F.; Darwish, M.H. Geoenvironmental assessment of the mut wastewater ponds in the Dakhla Oasis, Egypt. Geocarto Int. 2022, 37, 3293–3311. [Google Scholar] [CrossRef]
- Brookes, I.A. Geomorphology and Quaternary geology of the Dakhla Oasis region, Egypt. Quat. Sci. Rev. 1993, 12, 529–552. [Google Scholar] [CrossRef]
- Egyptian Meteorological Authority. Climatic Atlas of Egypt; Arab Republic of Egypt, Ministry of Transport: Cairo, Egypt, 1996.
- Ghoubachi, S. Hydrogeological Characteristics of the Nubia Sandstone Aquifer System in Dakhla Depression, Western Desert Egypt. Master’s Thesis, Faculty of Science, Ain Shams University, Cairo, Egypt, 2001. [Google Scholar]
- Hermina, M. The surroundings of Kharga, Dakhla and Farafra oases. In The Geology of Egypt; Routledge: Oxfordshire, UK, 2017; pp. 259–292. [Google Scholar]
- Awad, G.H. Zonal stratigraphy of Kharga Oasis. Ann. Geol. Surv. Egypt 1966, 34, 1–77. [Google Scholar]
- Issawi, B. Review of Upper Cretaceous-Lower Tertiary stratigraphy in central and southern Egypt. AAPG Bull. 1972, 56, 1448–1463. [Google Scholar]
- Pachur, H.-J. Paläo-Environment und Drainagesysteme der Ostsahara im Spätpleistozän und Holozän. In Nordost-Afrika Strukturen und Ressourcen. Deutsche Forschungemeinschaft; Wiley-VCH Verlag: Weinheim, Germany, 1999. [Google Scholar]
- Said, R. The Geology of Egypt; Routledge: Oxfordshire, UK, 2017. [Google Scholar]
- Thorweihe, U.; Heinl, M. Grundwasserressourcen im Nubischen Aquifersystem. In Nordost-Afrika Strukturen und Ressourcen. Deutsche Forschungemeinschaft; Wiley-VCH Verlag: Weinheim, Germany, 1999. [Google Scholar]
- Van Ginkel, S.W.; Hassan, S.H.A.; Oh, S.E. Detecting endocrine disrupting compounds in water using sulfur-oxidizing bacteria. Chemosphere 2010, 81, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Bridgewater, L.; American Public Health Association (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Raghunath, H.M. Ground Water: Hydrogeology, Ground Water Survey and Pumping Tests, Rural Water Supply and Irrigation Systems; New Age International: New Delhi, India, 1987. [Google Scholar]
- Appelo, C.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; Balkema: Leiden, The Netherlands, 2005. [Google Scholar]
- Dahab, K. Review on the hydrostratigraphic section in Egypt with special emphasis on their recharging sources. Bull. Geol. Surv. Cairo Egypt 1998, 45, 61–89. [Google Scholar]
- Idris, H.; Nour, S. Present groundwater status in Egypt and the environmental impacts. Environ. Geol. Water Sci. 1990, 16, 171–177. [Google Scholar] [CrossRef]
- Davis, S.N.; DeWiest, R.J. Hydrogeology; John Wiley and Sons: New York, NY, USA, 1966. [Google Scholar]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 2nd ed.; US Government Printing Office: Washington, DC, USA, 1970.
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; Department of the Interior, US Geological Survey: Reston, VA, USA, 1985.
- Saber, M.; Mokhtar, M.; Bakheit, A.; Elfeky, A.M.; Gameh, M.; Mostafa, A.; Sefelnasr, A.; Kantoush, S.A.; Sumi, T.; Hori, T.; et al. An integrated assessment approach for fossil groundwater quality and crop water requirements in the El-Kharga Oasis, Western Desert, Egypt. J. Hydrol. Reg. Stud. 2022, 40, 101016. [Google Scholar] [CrossRef]
- Porter, D.O.; Marek, T. Irrigation management with saline water. In Proceedings of the 2006 Central Plains Irrigation Conference, Colby, KS, USA, 21–22 February 2006; Colorado State University Libraries: Fort Collins, CO, USA, 2006. [Google Scholar]
- Rabeiy, R.E. Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ. Sci. Pollut. Res. 2018, 25, 30808–30817. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304.
- Correns, C.W. The geochemistry of the halogens. Phys. Chem. Earth 1956, 1, 181–233. [Google Scholar] [CrossRef]
- Fipps, G. Irrigation Water Quality Standards and Salinity Management Strategies; Texas FARMER Collection: Waco, TX, USA, 2003.
- Am, P. A graphic procedure in the geochemical interpretation of water analysis. Trans.-Am. Geophys. Union 1944, 25, 914–923. [Google Scholar]
- Scholler, H. Geochemie des eaux souterraines. Rev. De I’institut Fr. Du Pet. 1962, 10, 230–244. [Google Scholar]
- Kumar, P. Interpretation of groundwater chemistry using piper and Chadha’s diagrams: A comparative study from Perambalur Taluk. Elixir Geosci. 2013, 54, 12208–12211. [Google Scholar]
- Stiff, H.A., Jr. The interpretation of chemical water analysis by means of patterns. J. Pet. Technol. 1951, 3, 15. [Google Scholar] [CrossRef]
- Megahed, H.A.; GabAllah, H.M.; AbdelRahman, M.A.; D’Antonio, P.; Scopa, A.; Darwish, M.H. Geomatics-Based Modeling and Hydrochemical Analysis for Groundwater Quality Mapping in the Egyptian Western Desert: A Case Study of El-Dakhla Oasis. Water 2022, 14, 4018. [Google Scholar] [CrossRef]
- Megahed, H.A.; GabAllah, H.M.; Ramadan, R.H.; AbdelRahman, M.A.; D’Antonio, P.; Scopa, A.; Darwish, M.H. Groundwater Quality Assessment Using Multi-Criteria GIS Modeling in Drylands: A Case Study at El-Farafra Oasis, Egyptian Western Desert. Water 2023, 15, 1376. [Google Scholar] [CrossRef]
- Igwe, O.; Omeka, M. Hydrogeochemical and pollution assessment of water resources within a mining area, SE Nigeria, using an integrated approach. Int. J. Energy Water Resour. 2022, 6, 161–182. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Yan, H.; Zhou, B.; Huang, X.; Hao, Q.; Zhang, Y.; Zhang, Y.; Liao, X.; Yin, S. Hydrogeochemical constraints on groundwater resource sustainable development in the arid Golmud alluvial fan plain on Tibetan plateau. Environ. Earth Sci. 2021, 80, 1–17. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Ren, W.; Wang, H.; Liu, F.; Yang, F. Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 2020, 260, 121006. [Google Scholar] [CrossRef]
- Tirkey, P.; Bhattacharya, T.; Chakraborty, S.; Baraik, S. Assessment of groundwater quality and associated health risks: A case study of Ranchi city, Jharkhand, India. Groundw. Sustain. Dev. 2017, 5, 85–100. [Google Scholar] [CrossRef]
- Batabyal, A.K.; Chakraborty, S. Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking uses. Water Environ. Res. 2015, 87, 607–617. [Google Scholar] [CrossRef]
- Ramakrishnaiah, C.; Sadashivaiah, C.; Ranganna, G. Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. J. Chem. 2009, 6, 523–530. [Google Scholar]
- Sahoo, S.; Khaoash, S. Impact assessment of coal mining on groundwater chemistry and its quality from Brajrajnagar coal mining area using indexing models. J. Geochem. Explor. 2020, 215, 106559. [Google Scholar] [CrossRef]
- Samal, P.; Mohanty, A.K.; Khaoash, S.; Mishra, P. Hydrogeochemical evaluation, groundwater quality appraisal, and potential health risk assessment in a coal mining region of Eastern India. Water Air Soil Pollut. 2022, 233, 324. [Google Scholar] [CrossRef]
- Silva, M.; Gonçalves, A.; Lopes, W.; Lima, M.; Costa, C.; Paris, M.; Firmino, P.; De Paula Filho, F. Assessment of groundwater quality in a Brazilian semiarid basin using an integration of GIS, water quality index and multivariate statistical techniques. J. Hydrol. 2021, 598, 126346. [Google Scholar] [CrossRef]
- Şener, Ş.; Şener, E.; Davraz, A. Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci. Total Environ. 2017, 584, 131–144. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Singh, A.K.; Mahato, M.K. Assessment of groundwater quality of Pratapgarh district in India for suitability of drinking purpose using water quality index (WQI) and GIS technique. Sustain. Water Resour. Manag. 2018, 4, 601–616. [Google Scholar] [CrossRef]






| Groundwater Samples | Wastewater Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Minimum | Maximum | Mean | STDEV | Minimum | Maximum | Mean | STDEV | |
| pH | 5.7 | 7.7 | 6.8 | 0.53 | 7 | 8.3 | 7.5 | 0.45 | |
| EC (µS/cm) | 168 | 866.7 | 276 | 126.4 | 6766 | 114,000 | 40,526 | 39,481 | |
| TH | (mg/L) | 41.5 | 129.6 | 73.6 | 27.77 | 467.4 | 13,482 | 4676 | 4358 |
| TDS | 101 | 520 | 165 | 75.8 | 6343 | 72,960 | 30,080 | 26,833 | |
| Ca+2 | 6.6 | 34.1 | 15.8 | 8.56 | 84.9 | 2400 | 813 | 782 | |
| Mg+2 | 3.4 | 12 | 8.2 | 2.02 | 62 | 1840 | 658 | 590.5 | |
| Na+ | 6 | 120 | 21.2 | 18.92 | 1023 | 28,538 | 8588 | 10,004 | |
| K+ | 4 | 20 | 11.4 | 4.81 | 55 | 1505 | 456 | 527.1 | |
| HCO3− | 15 | 74 | 42.5 | 13.1 | 40 | 3752 | 972 | 1351.8 | |
| Cl− | 19.2 | 94.7 | 38.1 | 18.19 | 929 | 43,693 | 12,293 | 15,326 | |
| SO4−2 | 7.5 | 103.5 | 35.4 | 19.8 | 15 | 12,935 | 3388 | 4634.7 | |
| Fe+2 | 1.4 | 10.6 | 4.7 | 2.43 | 0.01 | 0.15 | 0.06 | 0.06 | |
| Mn+2 | 0.02 | 4 | 0.5 | 0.87 | 0.07 | 0.9 | 0.35 | 0.323 | |
| Hem [47] | Davis and Dewiest [46] | Present Study | |||
|---|---|---|---|---|---|
| Water Type | TDS (mg/L) | Water Type | TDS (mg/L) | Groundwater | Wastewater |
| Freshwater | <1000 | Freshwater | <1000 | 100% of the samples | --------- |
| Moderately saline water | 3000–10,000 | Brackish | 1000–10,000 | ----------- | (B and D samples) |
| Very saline water | 10,000–35,000 | Salty | 10,000–100,000 | ----------- | (E–F–G samples) |
| Briny water | >35,000 | brine | >100,000 | ----------- | (A and C samples) |
| Class | Total Hardness (mg/L) | Present Study | |
|---|---|---|---|
| Groundwater | Wastewater | ||
| Soft | 0–60 | 21 samples (53.85%) | ------------ |
| Moderately hard | 61–120 | 16 samples (41.02%) | ------------ |
| Hard | 121–180 | 2 samples (5.13%) | ------------ |
| Very hard | >180 | ------------ | All samples (100%) |
| Sample | (%) | (%) | Water Type | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Na+ & K+ | Ca+2 | Mg+2 | CL− | HCO3− | SO4−2 | ||
| 1 | 41 | 23 | 36 | 38 | 38 | 24 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 2 | 44 | 22 | 34 | 38 | 38 | 24 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 3 | 47 | 36 | 17 | 36 | 41 | 23 | Na+ > Ca+2 > Mg+2 | Sodium -Bicarbonate |
| HCO3− > CL− > SO4−2 | ||||||||
| 4 | 70 | 19 | 11 | 37 | 50 | 13 | Na+ > Ca+2 > Mg+2 | Sodium -Bicarbonate |
| HCO3− > CL− > SO4−2 | ||||||||
| 5 | 30 | 24 | 26 | 58 | 33 | 9 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 6 | 39 | 37 | 24 | 54 | 16 | 30 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 7 | 44 | 20 | 37 | 35 | 31 | 34 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 8 | 45 | 23 | 32 | 38 | 47 | 15 | Na+ > Mg+2 > Ca+2 | Sodium -Bicarbonate |
| HCO3− > CL− > SO4−2 | ||||||||
| 9 | 50 | 22 | 28 | 45 | 31 | 24 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 10 | 52 | 23 | 25 | 35 | 38 | 27 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 11 | 50 | 19 | 31 | 42 | 31 | 27 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 12 | 49 | 22 | 29 | 41 | 34 | 25 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 13 | 49 | 23 | 28 | 43 | 30 | 27 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 14 | 48 | 17 | 35 | 46 | 28 | 26 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 15 | 47 | 29 | 24 | 53 | 14 | 33 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 16 | 26 | 40 | 34 | 24 | 40 | 36 | Na+ > Ca+2 > Mg+2 | Sodium -Bicarbonate |
| HCO3− > SO4−2 > CL− | ||||||||
| 17 | 21 | 28 | 39 | 26 | 37 | 37 | Na+ > Mg+2 > Ca+2 | Sodium -Bicarbonate |
| HCO3− > SO4−2 > CL− | ||||||||
| 18 | 42 | 33 | 25 | 52 | 17 | 31 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 19 | 49 | 24 | 27 | 40 | 44 | 16 | Na+ > Mg+2 > Ca+2 | Sodium -Bicarbonate |
| HCO3− > CL− > SO4−2 | ||||||||
| 20 | 33 | 42 | 25 | 56 | 24 | 20 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 21 | 29 | 38 | 33 | 26 | 37 | 37 | Ca+2 > Mg+2 > Na+ | Calcium -Bicarbonate |
| HCO3− > SO4−2 > CL− | ||||||||
| 22 | 38 | 24 | 38 | 35 | 26 | 39 | Na+ > Mg+2 > Ca+2 | Sodium -Sulphate |
| SO4−2 > CL− > HCO3− | ||||||||
| 23 | 59 | 19 | 22 | 31 | 7 | 62 | Na+ > Mg+2 > Ca+2 | Sodium -Sulphate |
| SO4−2 > CL− > HCO3− | ||||||||
| 24 | 34 | 42 | 24 | 39 | 36 | 25 | Ca+2 > Na+ > Mg+2 | Calcium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 25 | 44 | 36 | 20 | 56 | 11 | 33 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 26 | 40 | 32 | 28 | 46 | 35 | 19 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 27 | 37 | 44 | 19 | 42 | 27 | 31 | Ca+2 > Na+ > Mg+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 28 | 39 | 29 | 32 | 48 | 34 | 18 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 29 | 50 | 21 | 29 | 34 | 25 | 41 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > SO4−2 >HCO3− | ||||||||
| 30 | 40 | 37 | 23 | 63 | 22 | 15 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 31 | 44 | 25 | 31 | 37 | 37 | 26 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > HCO3− > SO4−2 | ||||||||
| 32 | 45 | 26 | 29 | 33 | 45 | 22 | Na+ > Mg+2 > Ca+2 | Sodium -Bicarbonate |
| HCO3− > CL− > SO4−2 | ||||||||
| 33 | 34 | 25 | 41 | 35 | 54 | 11 | Na+ > Mg+2 > Ca+2 | Sodium -Bicarbonate |
| HCO3− > CL− > SO4−2 | ||||||||
| 34 | 29 | 41 | 30 | 40 | 23 | 37 | Ca+2 > Mg+2 > Na+ | Calcium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 35 | 30 | 42 | 28 | 50 | 23 | 27 | Ca+2 > Na+ > Mg+2 | Calcium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 36 | 33 | 41 | 26 | 47 | 24 | 29 | Na+ > Ca+2 > Mg+2 | Sodium -Bicarbonate |
| HCO3− > SO4−2 > CL− | ||||||||
| 37 | 30 | 42 | 28 | 21 | 33 | 46 | Ca+2 > Na+ > Mg+2 | Calcium -Sulphate |
| SO4−2 > HCO3− > CL− | ||||||||
| 38 | 51 | 21 | 28 | 55 | 16 | 29 | Na+ > Mg+2 > Ca+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| 39 | 43 | 35 | 22 | 59 | 9 | 32 | Na+ > Ca+2 > Mg+2 | Sodium -Chloride |
| CL− > SO4−2 > HCO3− | ||||||||
| Sample No. | (%) | (%) | Water Type | |||||
|---|---|---|---|---|---|---|---|---|
| Na+ & K+ | Ca+2 | Mg+2 | CL− | HCO3− | SO4−2 | |||
| A | 94.4 | 2.6 | 3.0 | 78.8 | 3.9 | 17.77 | Na+ > Mg+2 > Ca+2 CL− > SO4−2 > HCO3− | Sodium -Chloride |
| B | 94.4 | 2.6 | 3.0 | 78.8 | 3.9 | 17.25 | Na+ > Mg+2 > Ca+2 CL− > SO4−2 > HCO3− | Sodium -Chloride |
| C | 61 | 17.2 | 21.8 | 96.47 | 2.4 | 1.1 | Na+ > Mg+2 > Ca+2 CL− > HCO3− > SO4−2 | Sodium -Chloride |
| D | 61 | 17.3 | 21.7 | 96.46 | 2.4 | 1.2 | Na+ > Mg+2 > Ca+2 CL− > HCO3− > SO4−2 | Sodium -Chloride |
| E | 55.7 | 16.6 | 27.7 | 76.45 | 2.0 | 21.6 | Na+ > Mg+2 > Ca+2 CL− > SO4−2 > HCO3− | Sodium -Chloride |
| F | 55.8 | 16.6 | 27.6 | 76.45 | 2 | 21.58 | Na+ > Mg+2 > Ca+2 CL− > SO4−2 > HCO3− | Sodium -Chloride |
| G | 55.8 | 16.6 | 27.6 | 76.4 | 1.8 | 21.8 | Na+ > Mg+2 > Ca+2 CL− > SO4−2 > HCO3− | Sodium -Chloride |
| Parameter | Unit | WHO [52] | Weight (Wi) | (Wi) |
|---|---|---|---|---|
| pH | --- | 8.5 | 3 | 0.107142857 |
| TDS | mg/L | 500 | 5 | 0.178571429 |
| Ca+2 | 75 | 2 | 0.071428571 | |
| Mg+2 | 30 | 2 | 0.071428571 | |
| Na+ | 50 | 2 | 0.071428571 | |
| Cl− | 250 | 3 | 0.107142857 | |
| SO4−2 | 200 | 4 | 0.142857143 | |
| HCO3− | 200 | 3 | 0.107142857 | |
| K+ | 20 | 2 | 0.071428571 | |
| Fe2+ | 0.3 | 1 | 0.035714286 | |
| Mn2+ | 0.1 | 1 | 0.035714286 |
| Well No. | WQI | Well No. | WQI | ||
|---|---|---|---|---|---|
| Parameters with (Fe2+ and Mn2+) | Parameters without (Fe2+ and Mn2+) | Parameters with (Fe2+ and Mn2+) | Parameters without (Fe2+ and Mn2+) | ||
| 1 | 70.5 | 22.8 | 21 | 123 | 27.5 |
| 2 | 62.8 | 19.6 | 22 | 69.8 | 23.8 |
| 3 | 60.8 | 19.4 | 23 | 156 | 24.3 |
| 4 | 297 | 55.5 | 24 | 57.1 | 25.6 |
| 5 | 61.3 | 14.7 | 25 | 65.3 | 26.8 |
| 6 | 115 | 29.4 | 26 | 53.1 | 16.2 |
| 7 | 73.5 | 18.9 | 27 | 52.2 | 37.5 |
| 8 | 86 | 18.7 | 28 | 43 | 17.5 |
| 9 | 87 | 16.9 | 29 | 85.9 | 16.8 |
| 10 | 51.4 | 25.8 | 30 | 168 | 35.3 |
| 11 | 85.9 | 22 | 31 | 100.8 | 20.1 |
| 12 | 55.4 | 17.2 | 32 | 88.9 | 18.8 |
| 13 | 62.5 | 14.7 | 33 | 66.7 | 16.7 |
| 14 | 91.3 | 17.6 | 34 | 90.2 | 29.2 |
| 15 | 57.4 | 9.2 | 35 | 69.8 | 20.6 |
| 16 | 79.7 | 21.7 | 36 | 157 | 27.8 |
| 17 | 76.8 | 22.4 | 37 | 82.4 | 28.5 |
| 18 | 84.2 | 26.5 | 38 | 43.3 | 12 |
| 19 | 90.6 | 16.2 | 39 | 105 | 23.3 |
| 20 | 278 | 28 | |||
| WQI Values with (Fe2+ and Mn2+) | Water Quality | Possible Usages | No. of Samples and Percentage |
|---|---|---|---|
| 0–25 | Excellent | Drinking, irrigation, and industrial | -------- |
| 26–50 | Good | Domestic, irrigation, and industrial | 2 (5%) |
| 51–75 | Poor | Irrigation and industrial | 15 (38.5%) |
| 76–100 | Very poor | Irrigation | 12 (30.8%) |
| 101–150 | Unsuitable | Restricted use for irrigation | 4 (10.3%) |
| > 150 | Unfit for drinking | Proper treatment is required before any use | 6 (15.4%) |
| WQI values Without (Fe2+ and Mn2+) | Water Quality | Possible usages | No. of samples |
| 0–25 | Excellent | Drinking, irrigation, and industrial | 26 (66.6%) |
| 26–50 | Good | Domestic, irrigation, and industrial | 12 (30.8%) |
| 51–75 | Poor | Irrigation and industrial | 1 (2.6%) |
| 76–100 | Very poor | Irrigation | ------- |
| 101–150 | Unsuitable | Restricted use for Irrigation | -------- |
| >150 | Unfit for drinking | Proper treatment is required before any use | --------- |
| Wastewater | WQI Values | WQI Values | Possible Usages | No. of Samples |
|---|---|---|---|---|
| Mut Lake (A) | 10,440 | >150 | Treatment is required before any use | 7 (100%) |
| Mut canal (B) | 1300 | |||
| El-Rashda Lake (C) | 4686 | |||
| El-Rashda canal (D) | 511 | |||
| El-Qalamun Lake (E) | 1624 | |||
| Al-Masarah canal (F) | 936 | |||
| Ismant wastewater canal (G) | 932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, M.H.; Megahed, H.A.; Sayed, A.G.; Abdalla, O.; Scopa, A.; Hassan, S.H.A. Hydro-Geochemistry and Water Quality Index Assessment in the Dakhla Oasis, Egypt. Hydrology 2024, 11, 160. https://doi.org/10.3390/hydrology11100160
Darwish MH, Megahed HA, Sayed AG, Abdalla O, Scopa A, Hassan SHA. Hydro-Geochemistry and Water Quality Index Assessment in the Dakhla Oasis, Egypt. Hydrology. 2024; 11(10):160. https://doi.org/10.3390/hydrology11100160
Chicago/Turabian StyleDarwish, Mahmoud H., Hanaa A. Megahed, Asmaa G. Sayed, Osman Abdalla, Antonio Scopa, and Sedky H. A. Hassan. 2024. "Hydro-Geochemistry and Water Quality Index Assessment in the Dakhla Oasis, Egypt" Hydrology 11, no. 10: 160. https://doi.org/10.3390/hydrology11100160
APA StyleDarwish, M. H., Megahed, H. A., Sayed, A. G., Abdalla, O., Scopa, A., & Hassan, S. H. A. (2024). Hydro-Geochemistry and Water Quality Index Assessment in the Dakhla Oasis, Egypt. Hydrology, 11(10), 160. https://doi.org/10.3390/hydrology11100160








