Application of Multi-Isotopes and Geochemical Modeling for Delineating Recharge and Salinization Sources in Dahab Basin Aquifers (South Sinai, Egypt)
Abstract
:1. Introduction
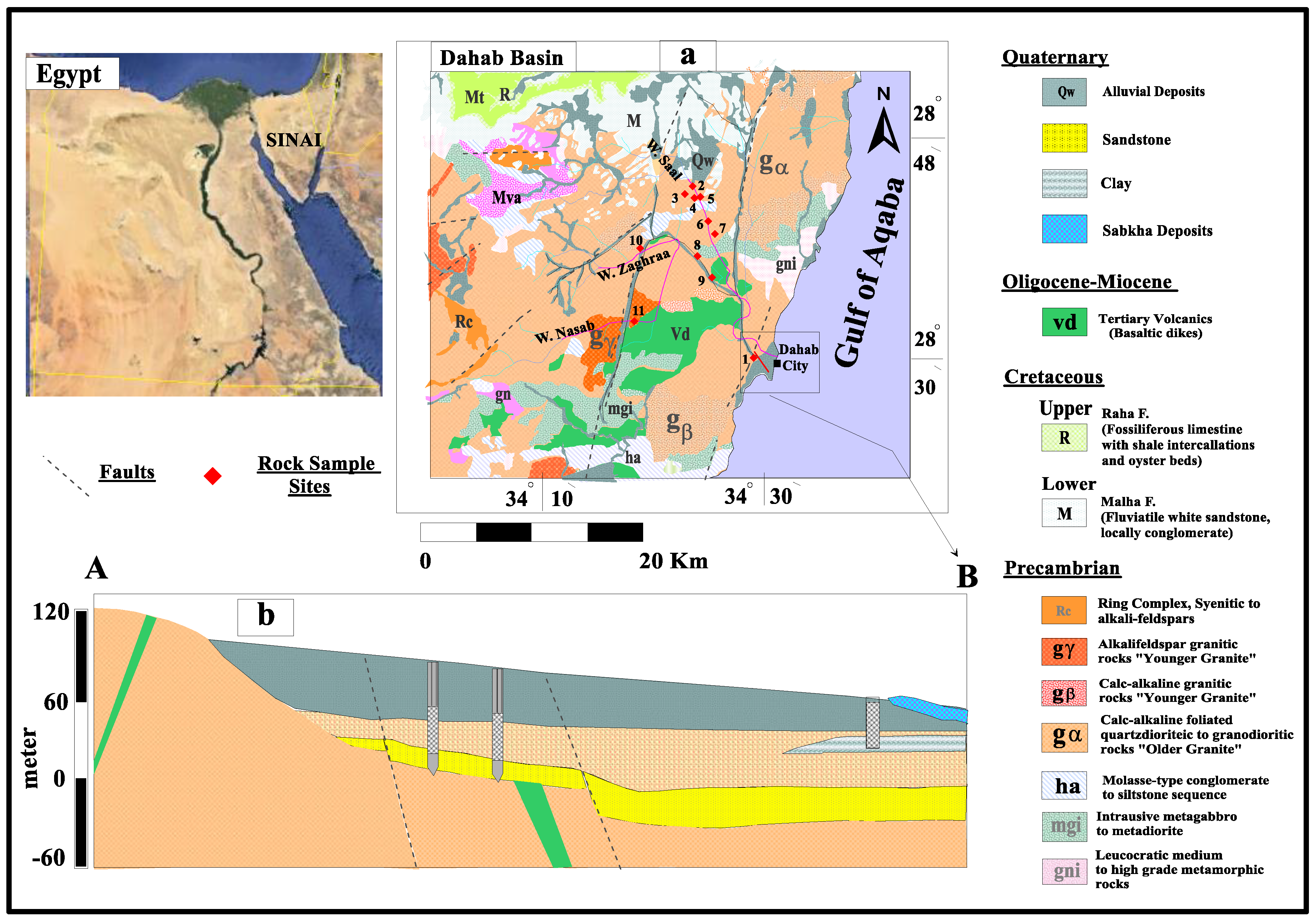
2. The Study Area, Geology, and Hydrogeology
3. Methodology
3.1. Field and Laboratory Work
3.2. Water–Rock Reaction Modeling
4. Results and Discussion
4.1. Groundwater Chemistry
4.2. Sources of Groundwater Recharge
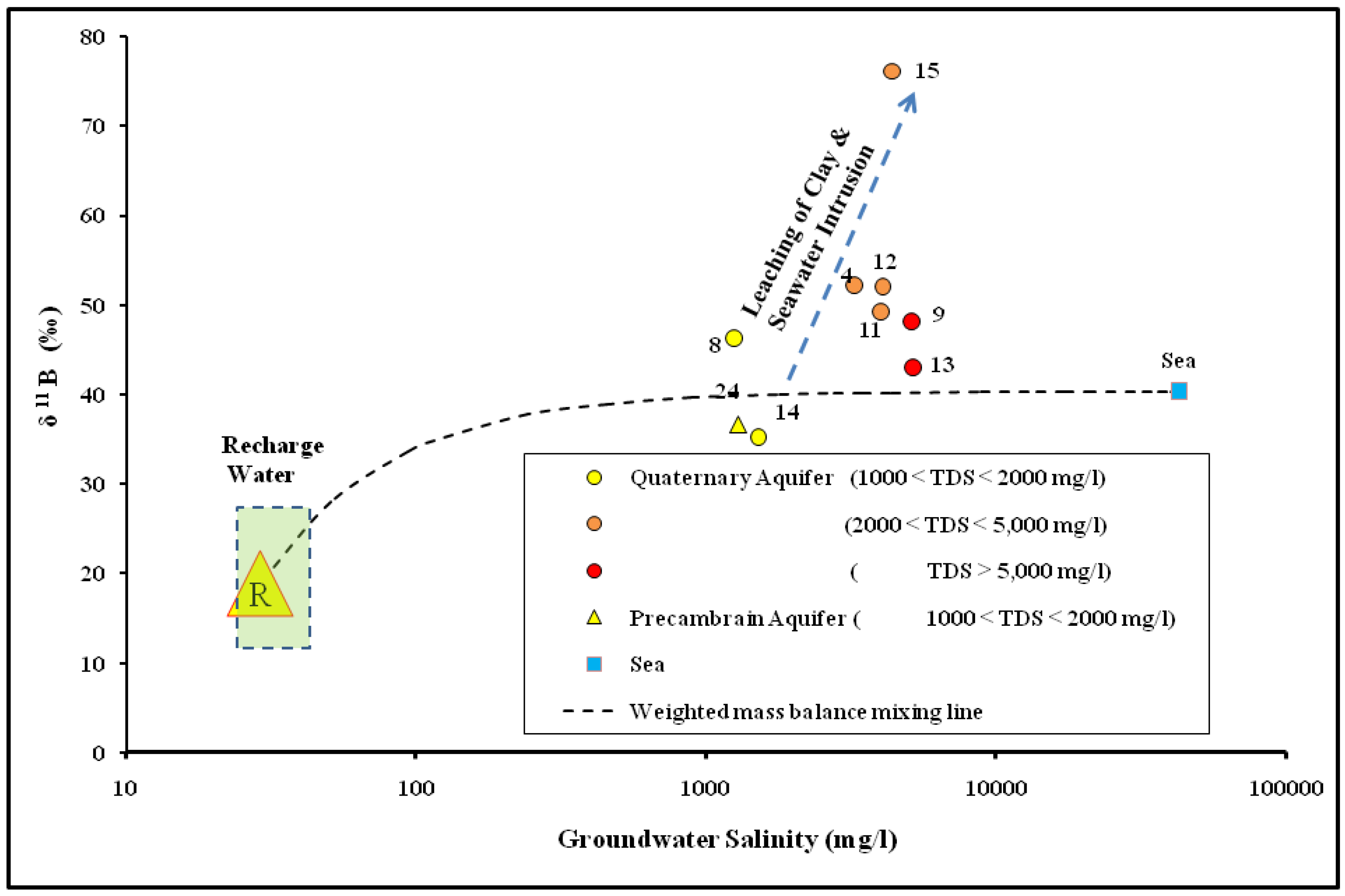
4.3. The Genesis of Groundwater Salinization
4.4. 87Sr/86Sr as an Indicator for Water–Rock Interaction
4.5. Inverse Geochemical and Mixing Model
4.6. Corrected Age Dating 14C Model
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Sherif, M.M.; Singh, V.P. Saltwater Intrusion. In Hydrology of Disasters; Water Science and Technology Library Series; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1996; pp. 269–319. [Google Scholar]
- Bear, J.; Cheng, A.H.-D.; Sorek, S.; Ouazar, D.; Herrera, I. Seawater Intrusion in Coastal Aquifers—Concepts, Methods and Practices; Springer: Berlin, Germany, 1999; ISSN 0924-6118. [Google Scholar]
- Gaaloul, N. GIS-Based Numerical Modeling of Aquifer Recharge and Salt Water Intrusion in Arid South Eastern Tunisia. J. Hydrol. Eng. 2012, 19, 777–789. [Google Scholar] [CrossRef]
- Gnanachandrasamy, G.; Ramkumar, T.; Venkatramanan, S.; Chung, S.Y.; Vasudevan, S. Identification of saline water intrusion in part of Cauvery deltaic region, Tamil Nadu, Southern India: Using GIS and VES methods. Mar. Geophys. Res. 2016, 37, 113–126. [Google Scholar] [CrossRef]
- Eissa, M. Integrative management of saltwater intrusion in poorly-constrained semiarid coastal aquifer at Ras El-Hekma, Northwestern Coast, Egypt. Groundw. Sustain. Dev. 2018, 6, 57–70. [Google Scholar] [CrossRef]
- Zhao, Q.; Su, X.; Kang, B.; Zhang, Y.; Wu, X.; Liu, M.A. Hydrogeochemistry and multi-isotope (Sr, O, H, and C) study of groundwater salinity origin and hydrogeochemcial processes in the shallow confined aquifer of northern Yangtze River downstream coastal plain, China. Appl. Geochem. 2017, 86, 49–58. [Google Scholar] [CrossRef]
- Paniconi, C.; Khalifi, I.; Lecca, G.; Giacomeli, A.; Tarhouni, J. Modeling and Analysis of Seawater Intrusion in the Coastal Aquifer of Eastern Cap-Bon. Tunisia. Transp. Porous Media 2001, 43, 3–28. [Google Scholar] [CrossRef]
- El-Kiki, M.F.; Eweida, E.A.; El Refeai, A.A. Hydrogeology of the Aqaba rift border province. In Proceedings of the 3rd Conference of the Geology and Sinai Development, Ismailia, Egypt, 1992; pp. 91–100. [Google Scholar]
- El-Refeai, A.A. Water Resources of Southern Sinai Egypt Geomorphological and Hydrogeological Studies. Ph.D. Thesis, Faculty of Science Cairo University, Cairo, Egypt, 1992; 357p. [Google Scholar]
- Awwad, R.A.; Olsthoorn, T.N.; Zhou, Y.; Uhlenbrook, S.; Smidt, E. Optimum Pumping-Injection System for Saline Groundwater Desalination in Sharm El Sheikh; Water Mill Working Paper No. 11; Water Mill: Lexington, MA, USA, 2008; 21p. [Google Scholar]
- Isawi, H.; El-Sayed, M.H.; Eissa, M.; Shouakar-Stash, O.; Shawky, H.; Abdel Mottaleb, M.S. Integrated geochemistry, isotopes, and geostatistical techniques to investigate groundwater sources and salinization origin in the Sharm EL-Shiekh Area, South Sinia, Egypt. Water Air Soil Pollut. 2016, 227, 151. [Google Scholar] [CrossRef]
- Eissa, M.A.; Thomas, J.M.; Pohll, G.; Shouakar-Stash, O.; Hershey, R.L.; Dawoud, M. Groundwater recharge and salinization in the arid coastal plain aquifer of the Wadi Watir delta, Sinai, Egypt. Appl. Geochem. 2016, 71, 48–62. [Google Scholar] [CrossRef]
- Missimer, T.M.; Ghaffour, N.; Abdullah, H.A.D.; Rachman, R.; Maliva, R.G.; Amy, G. Subsurface intakes for seawater reverse osmosis facilities: Capacity limitation, water quality improvement, and economics. Desalination 2013, 322, 37–51. [Google Scholar] [CrossRef]
- Edmunds, W.M. Renewable and non-renewable groundwater in semi-arid and arid regions. Dev. Water Sci. 2003, 50, 265–280. [Google Scholar]
- Vengosh, A.; Kloppmann, W.; Marie, A.; Livshitz, Y.; Gutierrez, A.; Bana, M.; Guerrot, C.; Pankratov, I.; Ranan, H. Sources of salinity and boron in Gaza Strip: Natural contaminant flow in southern Mediterranean Coastal aquifer. Water Res. 2005, 41, W01013. [Google Scholar] [CrossRef]
- Bennetts, D.A.; Webb, J.A.; Stone, D.J.M.; Hill, D.M. Understanding the salinization process for groundwater in an area of south-eastern Australia, using hydrochemical and isotopic evidence. J. Hydrol. 2006, 323, 178–192. [Google Scholar] [CrossRef]
- Issar, A.; Gilad, D. Groundwater flow system in the arid crystalline province of southern Sinai. Hydrol. Sci. J. 1982, 27, 309–325. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Walton, N.R.G. A geochemical and isotopic approach torecharge evaluation in semi-arid zones, past and present. In Arid-ZoneHydrology, Investigations with Isotope Techniques; International Atomic Energy Agency: Vienna, Austrian, 1980; pp. 47–68. [Google Scholar]
- Simmers, I. Natural groundwater recharge estimation in (semi-)arid zones; some state of-the-art observations. In Sahel Forum on the State-of the-Art of Hydrology and Hydrogeology in the Arid and Semi-Arid Areas of Aji-Ica; Stout, G.E., Demissie, M., Eds.; UNESCO: New York, NY, USA, 1990; pp. 373–386. [Google Scholar]
- Edmunds, W.M.; Gaye, C.B. Estimating the spatial variability of groundwater recharge in the Sahel using chloride. J. Hydrol. 1994, 156, 47–59. [Google Scholar] [CrossRef]
- Edmunds, W.M. Characterization of groundwaters in semi-arid and arid zones using minor elements. In Groundwater Quality; Nash, H.G., McCall, J., Eds.; Chapman & Hall: London, UK, 1995; pp. 19–30. [Google Scholar]
- Gat, J.R.; Mazor, E.; Tzur, Y. The stable isotope composition of mineral waters in the Jordan Rift Valley, Israel. J. Hydrol. 1969, 76, 334–352. [Google Scholar] [CrossRef]
- Gammonsa, C.H.; Poulsonb, S.R.; Pellicoria, D.A.; Reedc, P.J.; Roeslera, A.J. The hydrogen and oxygen isotopic composition of precipitation, evaporated mine water, and river water in Montana, USA. J. Hydrol. 2006, 328, 319–330. [Google Scholar] [CrossRef]
- Guay, B.E.; Eastoe, C.J.; Bassett, R.; Long, A. Identifying sources of groundwater in the lower Colorado River Valley, USA, with δ18O, δD, and 3H: Implications for river water accounting. J. Hydrogeol. 2006, 14, 146–158. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- Mook, G.W. Environmental Isotopes in the Hydrological Cycle; IHP-V Technical Document in Hydrology No. 39 VI; UNESCO: Paris, France, 2001. [Google Scholar]
- Albarede, F.; Michard, A. Evidence for slowly changing 87Sr/86Sr in runoff from freshwater limestones of southern France. Chem. Geol. 1987, 64, 55–65. [Google Scholar] [CrossRef]
- Casanova, J.; Negrel, P.H.; Kloppmann, W.; Aranyossy, J.F. Origin of deep saline groundwaters in the Vienne granitoids (France). Geofluids 2001, 1, 91–101. [Google Scholar] [CrossRef]
- Eissa, M. Groundwater Resource Sustainability in Wadi Watir Watershed, Sinai, Egypt. Ph.D. Thesis, Hydrogeology University of Nevada, Reno, NV, USA, August 2012. UMI Number: 3539186. [Google Scholar]
- Faure, G. Principles of Isotope Geology, 2nd ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Davidson, G.R.; Bassett, R.L. Application of boron isotopes identifying contaminants such as fly ash leachate in groundwater. Environ. Sci. Technol. 1993, 27, 172–176. [Google Scholar] [CrossRef]
- Quast, K.W.; Lansey, K.; Arnold, R.; Bassett, R.L.; Rincon, M. Boron Isotopes as an Artificial Tracer. Groundwater 2006, 44, 453–466. [Google Scholar] [CrossRef]
- Du, Y.; Ma, Y.; Chen, L.; Shan, H.; Xiao, C.; Lu, Y.; Liu, C.; Cai, H. Genesis of salinized groundwater in Quaternary aquifer system of the coastal plain, Laizhou Bay, China: Geochemical evidence, especially from bromine stable isotope. Appl. Geochem. 2015, 59, 155–165. [Google Scholar] [CrossRef]
- Vengosh, A.; Chivas, A.R.; McCulloch, M.T.; Starisnky, A.; Kolodny, Y. Boron isotope geochemistry of Australian salt lakes. Geochim. Cosmochim. Acta 1991, 55, 2591–2606. [Google Scholar] [CrossRef]
- Stotler, R.L.; Frape, S.K.; Shouakar-Stash, O. An isotopic survey of d81Br and d37Cl of dissolved halides in the Canadian and Fennos Canadian Shields. Chem. Geol. 2010, 274, 38–55. [Google Scholar] [CrossRef]
- Sherif, M.M. The Nile Delta Aquifer in Egypt. In Theory and Application of Transport in Porous Media; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 559–590. [Google Scholar]
- Surour, A.A.; EL-Kammar, A.A.; Arafa, E.H.; Korany, H.M. Dahab stream sediments, southeastern Sinai, Egypt: A potential source of Gold, magnetite, and zircon. J. Geochem. Explor. 2003, 77, 25–43. [Google Scholar] [CrossRef]
- Soliman, F.A. Geology of WadiSa’al Area with Special Emphasis of Metamorphism and Tectonics, Central Sinai of Egypt. Ph.D. Thesis, Faculty of Science, Suez Canal University, Ismailia, Egypt, 1 January 1986; 240p. [Google Scholar]
- Hassen, I.S.; Ibrahim, S.K.; El Emer, P.M. Evolution and origin of the metavolcanics at WadiSaâl area, south Sinai, Egypt. Ann. Geol. Surv. Egypt 2004, 27, 61–78. [Google Scholar]
- Hassan, O.A.; Ahmed, M.H.; Arafat, S.M. Environmental land use/land cover use change detection in coastal zones of the Gulf of Aqaba, Egypt, using multi-temporal Landsat imagery. Egypt. J. Remote Sens. Space Sci. 2005, 8, 21–38. [Google Scholar]
- El-Sheshtawy, Y.A.; Aly, M.M.; Ahmed, A.M. Geochemistry and tectonic environments of the granite-pegmatite dikes around Wadi El-Markh area, Sinai, Egypt. Mansoura Sci. Bull. 1988, 15, 205–226. [Google Scholar]
- Wassif, N.A. Palaeomagnetism and opaque mineral oxides of some basalt from west central Sinai, Egypt. Geophys. J. Int. 1991, 104, 319–330. [Google Scholar] [CrossRef]
- El-Metwally, A.A.; El-Assay, I.E.; Essawy, M.A.; El-Mowafy, A.A. Petrological, structural and geochemical studies on the basement rocks of Gabal Um Zariq-Wadi Kid area, southeastern Sinai, Egypt. Egypt. J. Geol. 1999, 43, 147–180. [Google Scholar]
- Egyptian Geological Survey and Mining Authority (EGSMA). Geological Map of South Sinai Scale 1:250,000; Egyptian Geological Survey Annal; Egyptian Geological Survey and Mining Authority: Cairo, Egypt, 1994.
- CONOCO. Geological Map of Egypt, (Scale 1:500,000); Conoco Continental Oil Company: Houston, TX, USA, 1987. [Google Scholar]
- Khalifa, I.H.; Soliman, F.A.; Ibrahim, S.K.; El-Bialy, M.Z. Petrological and petrochemical characteristics of some old granites in South Sinai. In Proceedings of the 7th Conference of Sinai for Development, Ismailia, Egypt, June 2004; pp. 81–92. [Google Scholar]
- Omran, A.; Hahn, M.; Hochschild, V.; El-Rayes, A.; Geriesh, M. Lithological Mapping of Dahab Basin, South Sinai, Egypt, Using ASTER Data; PFG Photogrammetrie, Fernerkundung, Geoinformation; Springer: Heidelberg, Germany, 2012; pp. 711–726. [Google Scholar]
- Hassan, A.A. A new Carboniferous occurrence in the Abu Durba, Sinai, Egypt. In Proceedings of the 6th Arabian Petroleum Conference, Baghdad, Iraq, 6–13 March 1967; Volume 2. [Google Scholar]
- Said, R. Explanatory Notes to Accompany the Geological Map of Egypt; Ministry of Industry, Petroleum, and Mineral Wealth, Geological Survey of Egypt and Mining Authority: Cairo, Egypt, 1971; 123p.
- Shabana, A.R. Geology of Water Resources in Some Catchments Areas Draining in the Gulf of Aqaba, Sinai-Egypt. Ph.D. Thesis, Faculty of Science Ain Shams University, Cairo, Egypt, September 1998; 246p. [Google Scholar]
- Issawi, B.; Jux, U. Contribution on the stratigraphy of the Paleozoic rocks in Egypt. Geol. Surv. Egypt 1982, 64, 28. [Google Scholar]
- Abdel-Rahman, I.F. Physical and chemical characteristics of silica sand deposits of Wadi Watir region, Sinai. Acta Miner. Petrogr. Szeged 2002, 43, 79–83. [Google Scholar]
- El-Shazly, E.M.; Abddel-Hady, M.A.; El-Ghawaby, M.A.; El-Kassas, I.A.; ElShazly, M.M. Geology of Sinai Peninsula from ERTS-1 Satellite Images; Remote Sensing Research Project, Academy of Scientific Research and Technology: Cairo, Egypt, 1974; p. 20. [Google Scholar]
- Eyal, M.; Bartov, Y.; Shimron, A.E.; Bentor, Y.K. Sinai Geologic Map, Scale 1: 500,000; Contour interval 10 gammas, based on four separate surveys; Institute for Petroleum Research and Geophysics, and Ministry of Energy and Infrastructure, Administration for Research in Earth Sciences: Holon, Israel, 1980. [Google Scholar]
- El Rayes, A. Hydrogeological Studies of Saint Katherine Area, South Sinai, Egypt. Master’s Thesis, Suez Canal University, Ismailia, Egypt, January 1992; p. 95. [Google Scholar]
- Japan International Cooperation Agency (JICA); Water Resources Research Institute (WRRI). South Sinai Groundwater Resources Study in the Arab The Republic of Egypt; Main Report; Pacific Consultants International: Tokyo, Japan, 1999.
- Greenwood, N. A Physical Geography of the Sinai Peninsula; University of Texas Press: Austin, TX, USA, 1997; ISBN 78-0-292-72799-1. [Google Scholar]
- Abd El Samei, S.G.; Sadek, M.A. Groundwater recharge and flow in the lower Cretaceous Nubian sandstone aquifer in the Sinai Peninsula, using isotopic techniques and hydrochemistry. Hydrogeol. J. 2001, 9, 378–389. [Google Scholar]
- Rainwater, F.H.; Thatcher, L.L. Methods for Collection and Analysis of Water Samples; U.S. Geological Survey Water Supply; Paper 1454; USGS: Washington, DC, USA, 1960.
- Fishman, M.J.; Friedman, L.C. Methods for determination of inorganic substances in water and fluvial sediments, U.S. Geological Survey Book 5, Chapter A1. Open-File Report 84:85–495 Denver Colorado U.S.A. for hydrogen isotope analysis. Anal. Chem. 1985, 63, 910–912. [Google Scholar]
- Morrison, J.; Brockwell, T.; Merren, T.; Fourel, F.; Phillips, A.M. On-line high-precision stable hydrogen isotopic analyses on nanoliter water samples. Anal. Chem. 2001, 73, 3570–3575. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.; Mayeda, T.K. Variations of 18O content of waters from natural sources. Geochim. Cosmochim. Acta 1953, 4, 213–224. [Google Scholar] [CrossRef]
- McNutt, R.H.; Frape, S.K.; Fritz, P.; Jones, M.G.; MacDonald, I.M. The 87Sr/86Sr values of Canadian shield brines and fracture minerals with applications to groundwater mixing, fracture history, and geochronology. Geochim. Cosmochim. Acta 1990, 54, 202–215. [Google Scholar] [CrossRef]
- Shouakar-Stash, O.; Frape, S.K.; Drimmie, R.J. Determination of bromine stable isotopes using continuous-flow isotope ratio mass spectrometry. Anal. Chem. 2005, 77, 4027e4033. [Google Scholar] [CrossRef] [PubMed]
- Plummer, L.N.; Prestemon, E.C.; Parkhurst, D.L. NETPATH: An interactive code for interpreting NET geochemical reactions from chemical and isotopic data along a flow PATH. In Proceedings of the International Symposium on Water-Rock Interaction 7th Park City Utah, Rotterdam, Balkema, 9–23 July 1992; Kharaka, Y., Maest, A.S., Eds.; pp. 239–242. [Google Scholar]
- Hershey, R.L.; Heilweil, V.M.; Gardner, P.; Lyles, B.; Earman, S.; Thomas, J.; Lundmark, K.W. Ground-Water Chemistry Interpretations Supporting the Basin and Range Regional Carbonate-Rock Aquifer System (BARCAS) Study, Eastern Nevada and Western Utah; DHS Publication No. 41230; Desert Research Institute, Nevada System of Higher Education: Reno, NV, USA; US Geological Survey: Reston, VA, USA, 2007.
- Chung, Y.S. Hydrogeochemical Processes of Groundwater Using Multivariate Statistical Analyses and Inverse Geochemical Modeling in Samrak Park of Nakdong River Basin, Korea. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 12–17 April 2015. [Google Scholar]
- Said, M.M. Geochemistry of Groundwater in Coastal Areas, South Sinai, Egypt. Ph.D. Thesis, Faculty of Science, Ain Shams University, Cairo, Egypt, September 2004; 820p. [Google Scholar]
- Eissa, M.A.; Thomas, J.M.; Hershey, R.L.; Dawoud, M.I.; Pohll, G.; Gomaa, M.A.; Kamal, A.D. Geochemical and isotopic evolution of groundwater in the Wadi Watir Watershed, Sinai Peninsula, Egypt. Environ. Earth Sci. J. 2013. [Google Scholar] [CrossRef]
- Carpenter, A.B. Origin and chemical evolution of brines in sedimentary basins. In Proceedings of the SPE Annual Fall Technical Conference and Exhibition, Houston, TX, USA, 1–3 October 1978. [Google Scholar]
- Alcala, F.J.; Custodio, E. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J. Hydrol. 2008, 359, 189–207. [Google Scholar] [CrossRef]
- Katz, B.G.; Eberts, S.M.; Kauffman, L.J. Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: A review and examples from principal aquifers in the United States. J. Hydrol. 2010, 397, 151–166. [Google Scholar] [CrossRef]
- Vengosh, A. Salinization and saline environments. Environmental geochemistry. In Treatise in Geochemistry, 2nd ed.; Sherwood Lollar, B., Ed.; Elsevier: New York, NY, USA, 2014; Volume 9, pp. 325–378. [Google Scholar]
- Barbecot, F.; Marlin, C.; Gibert, E.; Dever, L. Hydrochemical and isotopic characterization of the Bathonian and Bajocian coastal aquifer of the Caen area (northern France). Appl. Geochem. 2000, 15, 791–805. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water analysis. Am. Geophys. Union Trans. 1953, 25, 914–923. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable Isotopes in Precipitation; Wiley: Hoboken, NJ, USA, 1964; Volume 16, pp. 436–468. [Google Scholar]
- Gat, J.R.; Gonfiantini, R. Stable Isotope Hydrology Deuterium and Oxygen-18 in the Water Cycle; Technical Reports Series No 210; IAEA: Vienna, Austria, 1981. [Google Scholar]
- Herczeg, A.L.; Dogramaci, S.S.; Leaney, F.W.J. Origin of dissolved salts in a large, semi-arid groundwater system: Murray Basin, Australia. Marine Freshw. Res. 2001, 52, 41–52. [Google Scholar] [CrossRef]
- Rosenthal, E.; Zilberbrand, M.; Livshitz, Y. The hydrochemical evolution of brackish groundwater in central and northern Sinai (Egypt) and in the western Negev (Israel). J. Hydrol. 2007, 337, 294–314. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Wright, E.P. Groundwater recharge and palaeoclimate in the Sirte and Kufra basins, Libya. J. Hydrol. 1979, 40, 215–241. [Google Scholar] [CrossRef]
- Jasechko, S.; Perrone, D.; Cardenas, B.; Ferguson, G.; Gleeson, T.; Luijendijk, E.; McDonnell, J.; Taylor, R.; Wada, Y.; Kirchner, J. Global aquifers dominated by fossil groundwaters but wells vulnerable to modern contamination. Nat. Geosci. 2017, 10, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Eissa, M.; Thomas, J.M.; Pohll, G.M.; Hershey, R.L.; Dahab, K.; Dawoud, M.; Gomaa, M.; El Shiekh, A. Groundwater resource sustainability in the WadiWatir delta, Gulf of Aqaba. Hydrogeol. J. 2013, 21, 1833–1851. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Samei, S.G. Isotopic and Hydrochemical Studies on the Groundwater of Sinai Peninsula. Dissertation, Ain Shams University, Cairo, Egypt, 1995. [Google Scholar]
- Salem, W.M.; Sadek, M.A. Groundwater recharge and salinity evolution at Wadi El-ain and El-saghier basin, Gulf of Aqaba province, Sinai, Egypt. Isot. Radiat. Res. 2003, 35, 431–454. [Google Scholar]
- Anker, Y. Evolution of the Carbonate System in the Hyper-Arid Environment—Central Arava Area, Israel, and Its Implications for 14C-dating. Master’s Thesis, Hebrew University, Jerusalem, Israel, 19 March 2003. (In Hebrew, English Abstract). [Google Scholar]
- IAEA & WISER. Water Isotope System for Data Analysis, Visualization, and Electronic Retrieval. WISER Version 0.7. 2008. Available online: https://websso.iaea.org (accessed on 1 May 2013).
- Shouakar-Stash, O.; Alexeev, S.V.; Frape, S.K.; Alexeeva, L.P.; Drimmie, R.J. Geochemistry and stable isotope signatures, including chlorine and bromine isotopes, of the deep groundwaters of the Siberian Platform, Russia. Appl. Geochem. 2007, 22, 589–605. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Barth, S. 11B/10B variations of dissolved boron in a freshwater–seawater mixing plume (Elbe Estuary, North Sea). Mar. Chem. 1998, 62, 1–14. [Google Scholar] [CrossRef]
- Vengosh, A.; Kolodny, Y.; Starisnky, A.; Chivas, A.R.; McCulloch, M.T. Coprecipitation and isotopic fractionation of boron in modem biogenic carbonates. Geochim. Cosmochim. Acta 1991, 55, 2901–2910. [Google Scholar] [CrossRef]
- Vengosh, A.; Starisnky, A.; Kolodny, Y.; Chivas, A.R. Boron isotope geochemistry as a tracer for the evolution of brines and associated hot springs from the Dead Sea. Geochim. Cosmochim. Acta 1991, 55, 1689–1695. [Google Scholar] [CrossRef]
- Negrel, P.H.; Casanova, J.; Kolppman, W.; Aranyossy, J.F. A combined isotopic tool box for the investigation of water-rock interaction: An overview of Sr, B, O, H isotopes and U-series in deep groundwaters from the Vienne granitoid (France). Water Rock Interact. 2002, 40, 1–38. [Google Scholar]
- Miyata, Y.; Takayuki, T.; Amakawa, H.; Uemastu, M.; Yoshiyuki, N. Boron isotope variations in the atmosphere. Tellus 2000, 52, 1057–1065. [Google Scholar] [CrossRef]
- Palmer, M.R.; Spivack, A.J.; Edmond, J.M. Temperature and pH controls over isotopic fractionation during adsorption of boron on marine clay. Geochim. Cosmochim. Acta 1987, 51, 2319–2323. [Google Scholar] [CrossRef]
- Kloppmann, W.; Petelet-Giraud, E.; Guerrot, C.; Cary, L.; Pauwels, H. Extreme boron isotope ratios in groundwater. Procedia Earth Planet. Sci. 2015, 13, 296–300. [Google Scholar] [CrossRef]
- McNutt, R.H.; Frape, S.K.; Fritz, P. Strontium isotopic composition of some brines from the Precambrian shield of Canada. Chem. Geol. Isot. Geosci. 1984, 2, 205–215. [Google Scholar] [CrossRef]
- Lyons, W.B.; Tyler, S.W.; Gaudette, H.E.; Long, D.T. The use of strontium isotopes in determining groundwater mixing and brine fingering in a playa spring zone, Lake Tyrrell, Australia. J. Hydrol. 1995, 167, 225–239. [Google Scholar] [CrossRef]
- Issar, A.; Nativ, R.; Karnieli, A.; Gat, J.R. Isotopic evidence of the origin of groundwater in arid zones. In Isotope Hydrology; International Atomic Energy Agency: Vienna, Austria, 1984; pp. 85–104. [Google Scholar]









| Constraints | Phases | Processes |
|---|---|---|
| Calcium, Carbon, Magnesium, Potassium, Sodium Sulfur, Chloride | (±) Calcite, (±) Dolomite, (±) Gypsum, (+) Halite (NaCl), (−) Silica, (+) Plagioclase, (+) Microcline, (+) Chlorite (−) Na-Montmorillonite, (−) illite, (−) Mafic Montmorillonite, (±) Exchange | (Evaporation and/or Mixing) |
| No. | Calcite | Dolomite | Gypsum | Silica | Calcedony | PlgAn | Mcln | Mica | Chlrt | Mont | Illite |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 0.07 | −0.13 | −1.64 | −0.66 | 0.21 | −3.38 | 0.21 | 6.77 | 1.96 | 0.27 | 1.39 |
| 4 | −0.37 | −0.38 | −0.98 | −0.71 | 0.15 | −3.93 | 0.02 | 6.59 | −2.23 | 1.69 | 1.24 |
| 8 | 0.06 | −1.02 | −1.59 | −0.71 | 0.15 | −3.76 | 0.00 | 5.86 | −6.06 | 1.01 | 0.46 |
| 9 | 0.48 | 0.23 | −0.94 | −0.59 | 0.27 | −3.16 | 0.44 | 6.67 | −3.36 | 1.88 | 1.34 |
| 11 | −0.44 | −0.23 | −1.39 | −0.76 | 0.10 | −4.14 | −0.08 | 6.537 | −1.71 | 1.53 | 1.16 |
| 12 | 0.06 | 0.48 | −1.11 | −0.65 | 0.21 | −3.73 | 0.19 | 6.64 | −1.23 | 1.79 | 1.38 |
| 13 | 0.41 | 0.412 | −0.56 | −0.97 | −0.13 | −3.96 | −0.54 | 6.01 | −4.32 | 0.81 | 0.38 |
| 14 | 0.04 | −0.46 | −1.36 | −0.63 | 0.24 | −3.72 | 0.56 | 7.02 | −5.87 | 1.89 | 1.41 |
| 15 | −0.14 | 0.52 | −0.98 | −0.83 | 0.03 | −4.39 | −0.21 | 6.02 | −0.19 | 0.86 | 0.73 |
| 21 | 0.97 | 1.79 | −1.41 | −0.74 | 0.12 | −4.15 | −0.67 | 4.63 | −0.55 | 2.30 | −0.16 |
| 22 | 0.15 | 0.10 | −1.35 | −0.610 | 0.25 | −0.84 | 0.97 | 6.5 | −1.58 | 0.98 | 1.16 |
| 24 | 0.48 | 0.76 | −1.14 | −0.89 | −0.03 | −4.39 | 0.38 | 6.00 | −2.34 | 0.06 | 0.40 |
| 25 | 0.31 | 0.41 | −1.40 | −0.64 | 0.23 | −3.98 | 1.15 | 6.65 | −1.59 | 0.87 | 1.21 |
| 27 | 0.16 | −0.02 | −1.36 | −0.70 | 0.17 | −4.01 | 0.56 | 7.06 | −5.42 | 1.43 | 1.26 |
| 28 | −0.03 | −0.46 | −1.26 | −0.75 | 0.11 | −4.13 | −0.95 | 5.11 | −4.73 | 0.99 | 0.04 |
| 29 | 0.25 | 0.20 | −1.21 | −0.61 | 0.26 | −3.75 | 1.09 | 7.51 | −4.93 | 1.93 | 1.79 |
| 30 | 0.38 | 0.50 | −1.41 | −0.70 | 0.16 | −4.03 | 0.97 | 6.54 | −1.89 | 0.71 | 1.04 |
| 31 | 0.15 | −0.58 | −0.40 | −0.66 | 0.20 | −2.91 | 0.59 | 7.20 | −3.81 | 2.05 | 1.60 |
| Rain | −3.72 | −8.27 | −3.47 | −1.39 | −0.52 | −9.62 | -- | -- | −32.02 | −1.47 | -- |
| Sea | −0.25 | 0.42 | −0.55 | −1.27 | −0.41 | −4.80 | −0.28 | 6.55 | −0.95 | −0.95 | 0.46 |
| Basin | Aquifer | Initial (Site1) | Final Water | Carbon Isotopic Exchange (mmol/L) | 13C | 14C | Age (year) | ||
|---|---|---|---|---|---|---|---|---|---|
| Computed (‰) | Observed (‰) | Computed (pmc) | Observed (pmc) | ||||||
| UpstreamDahab Watershed | Quaternary | 21 | 4 | 4.3 | −5.93 | 6.0 | 26.6 | 37 | Moden Water |
| DownstreamDahabDelta | Early Cambrian | 21 | 19 | 5.4 | −5.35 | −5.40 | 30.6 | 42 | Moden Water |
| 21 | 16 | 1.8 | −8.36 | −8.40 | 51.43 | 37.0 | 2723 | ||
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, M.A. Application of Multi-Isotopes and Geochemical Modeling for Delineating Recharge and Salinization Sources in Dahab Basin Aquifers (South Sinai, Egypt). Hydrology 2018, 5, 41. https://doi.org/10.3390/hydrology5030041
Eissa MA. Application of Multi-Isotopes and Geochemical Modeling for Delineating Recharge and Salinization Sources in Dahab Basin Aquifers (South Sinai, Egypt). Hydrology. 2018; 5(3):41. https://doi.org/10.3390/hydrology5030041
Chicago/Turabian StyleEissa, Mustafa A. 2018. "Application of Multi-Isotopes and Geochemical Modeling for Delineating Recharge and Salinization Sources in Dahab Basin Aquifers (South Sinai, Egypt)" Hydrology 5, no. 3: 41. https://doi.org/10.3390/hydrology5030041
APA StyleEissa, M. A. (2018). Application of Multi-Isotopes and Geochemical Modeling for Delineating Recharge and Salinization Sources in Dahab Basin Aquifers (South Sinai, Egypt). Hydrology, 5(3), 41. https://doi.org/10.3390/hydrology5030041




