Hydrochemical Analysis of Groundwater in Remah and Al Khatim Regions, United Arab Emirates
Abstract
:1. Introduction
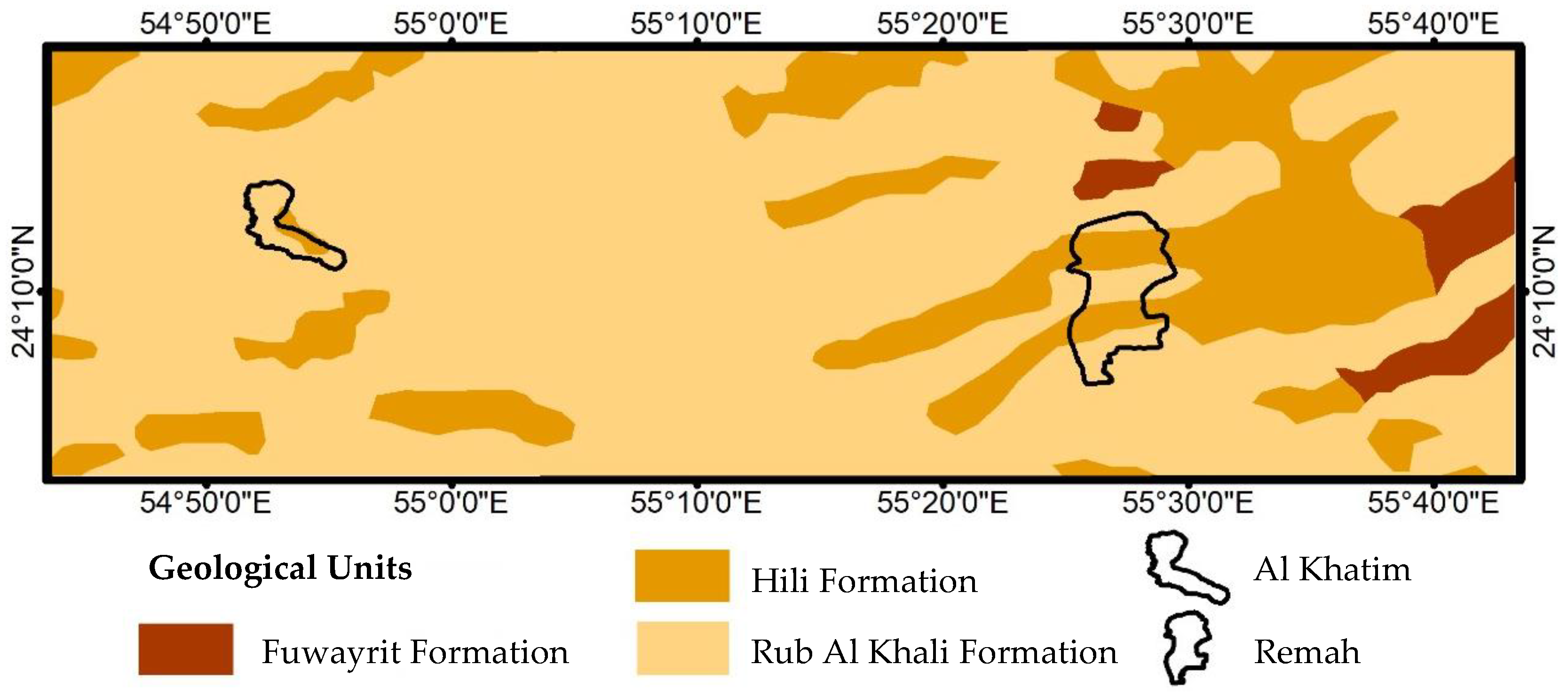
2. Study Area
2.1. Geology and Hydrogeology
2.2. Hydrogeological Units
3. Materials and Methods
3.1. Sample Collection and Analysis
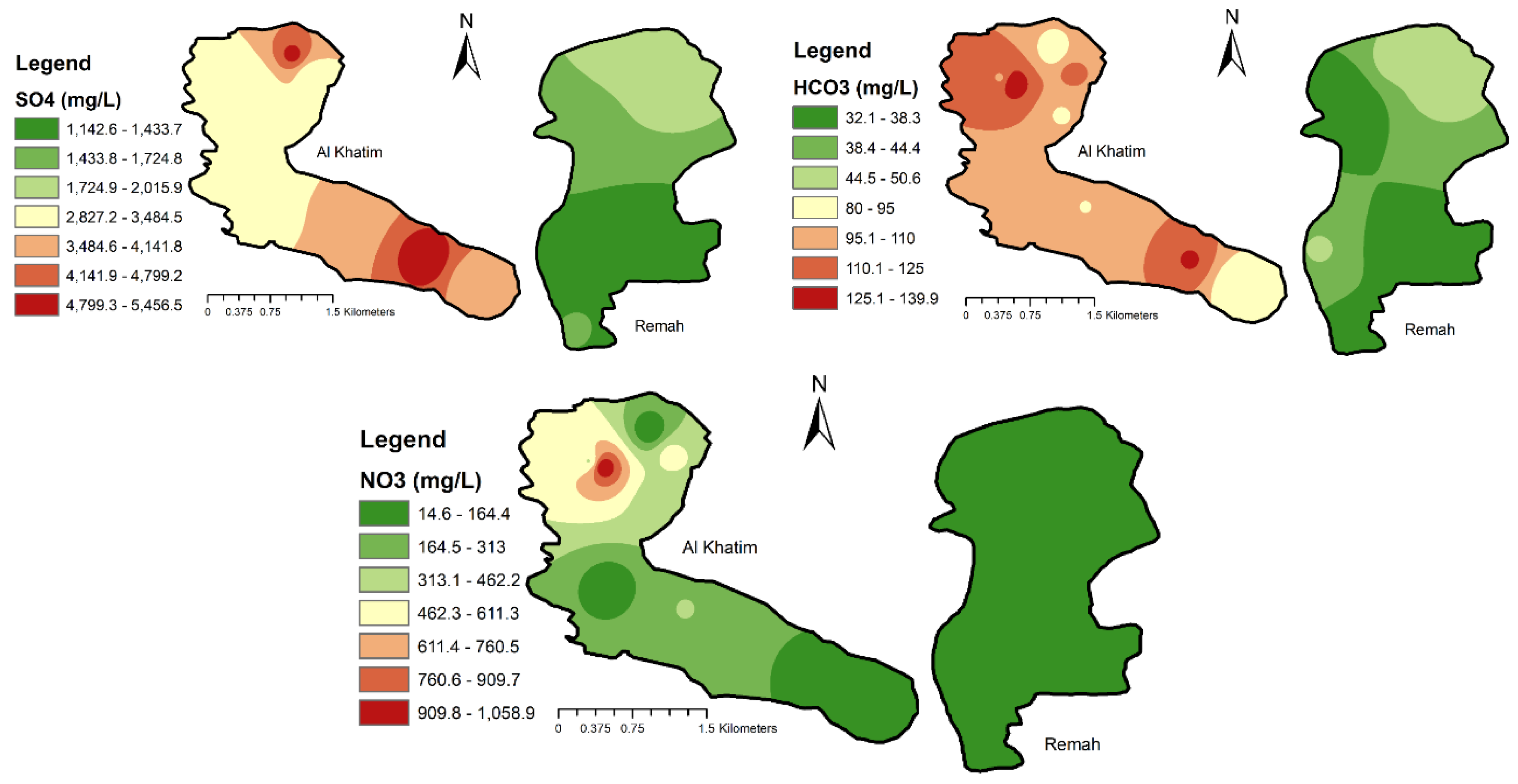
3.2. Development of Water Quality Maps
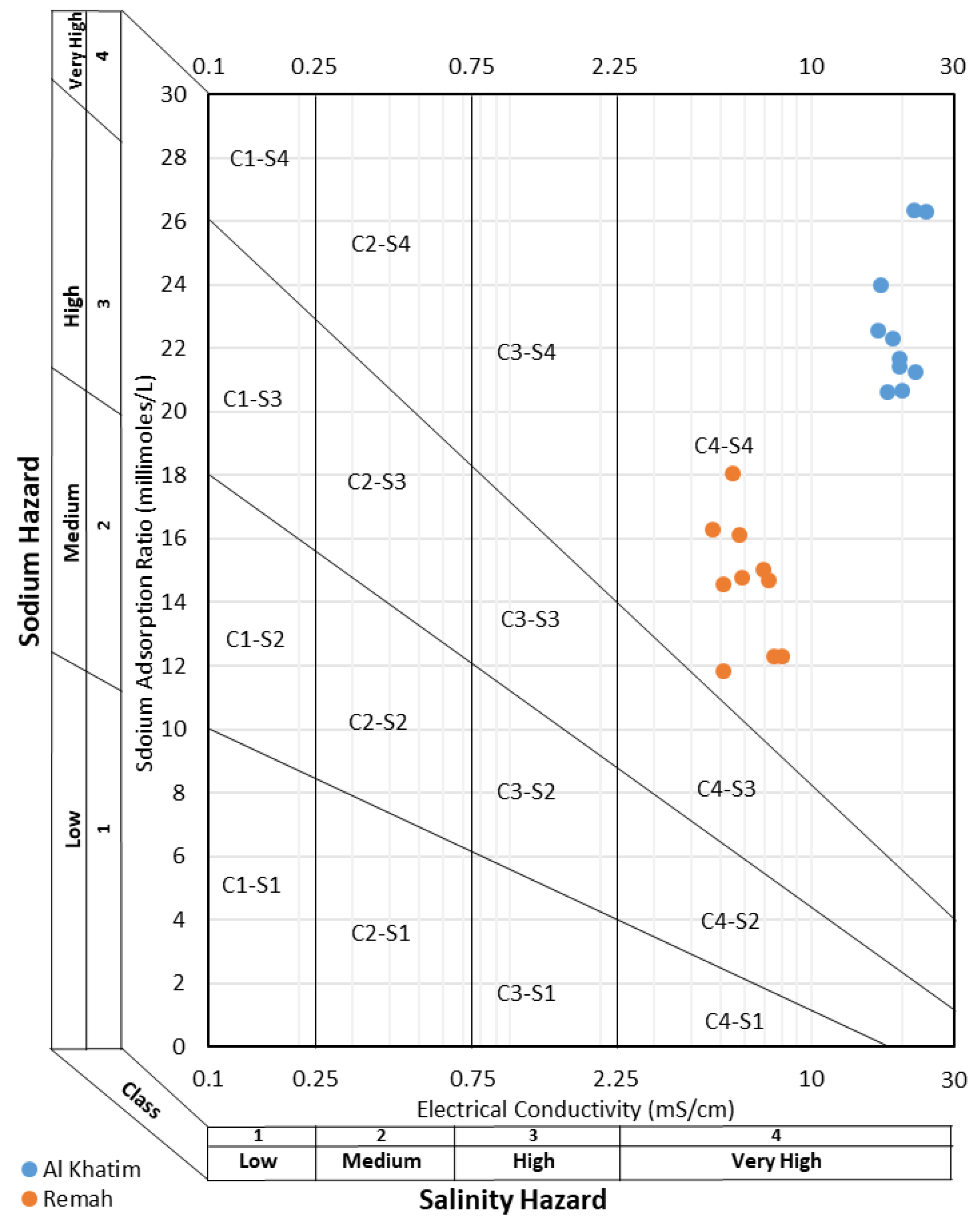
3.3. Suitability of Groundwater for Irrigation Use
4. Results
4.1. Hydrochemical Analysis of Results
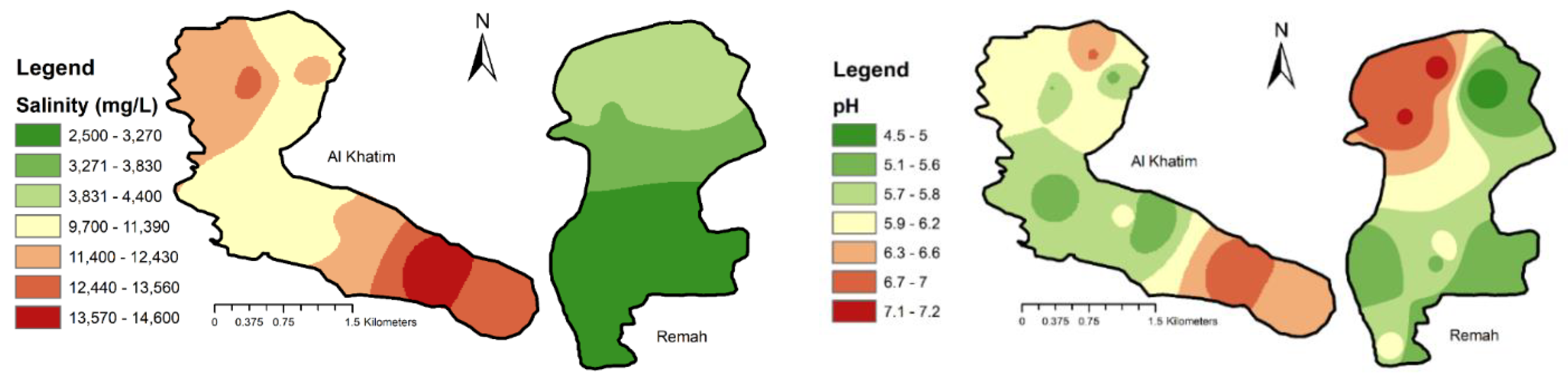
4.1.1. Electrical Conductivity and Salinity
4.1.2. Hydrogen-Ion Concentration (pH) and Temperature
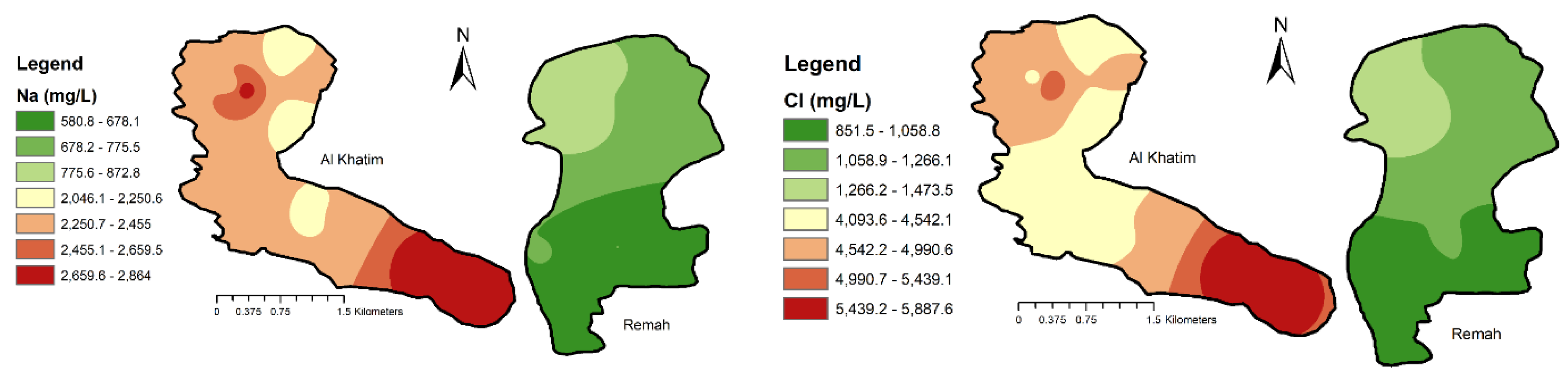
4.1.3. Major Ions
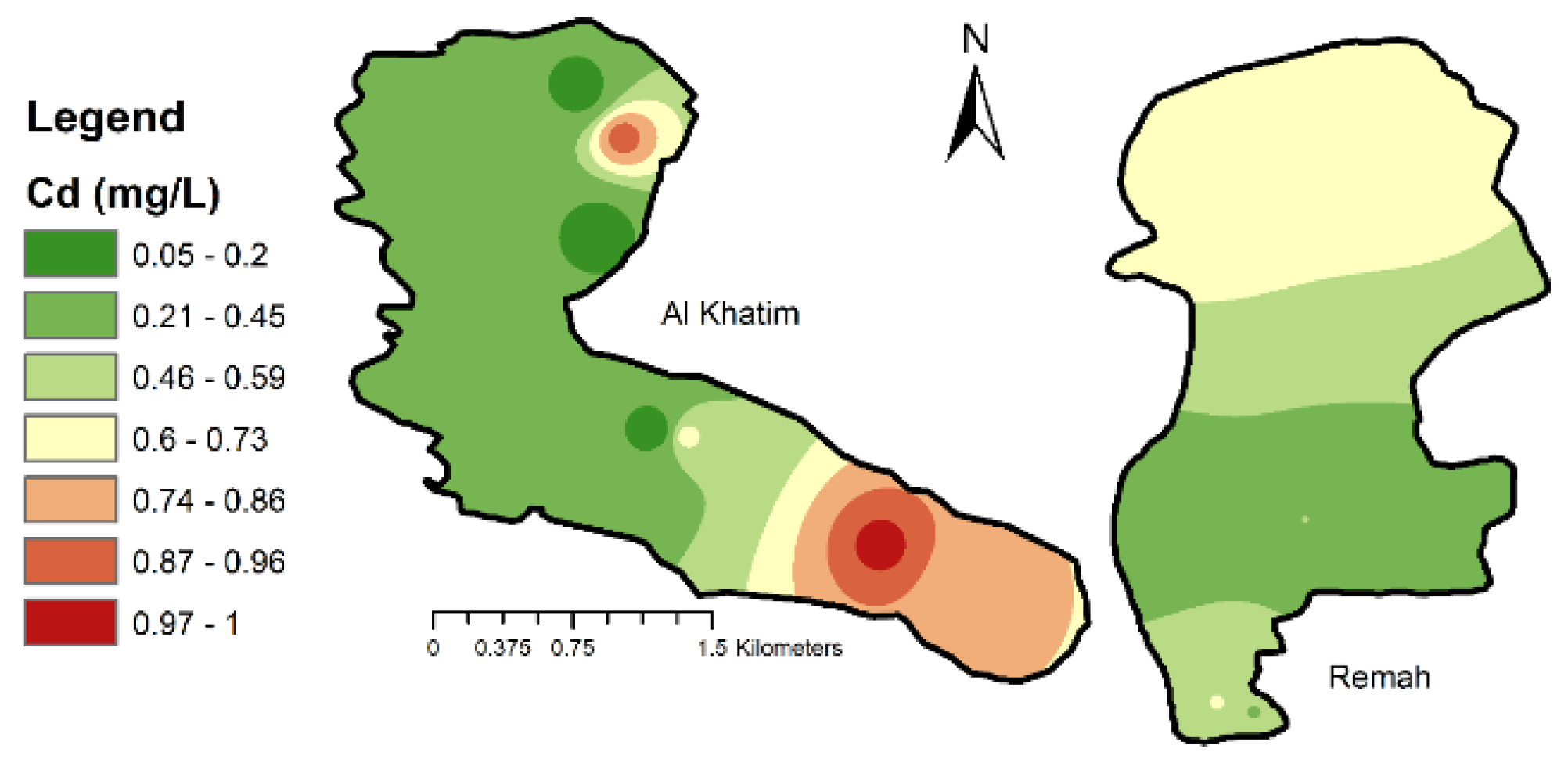
4.1.4. Trace Metals
4.2. Groundwater Levels and Salinity
4.3. Suitability for Irrigation Use
5. Discussion
- ➢
- Shallow groundwater level
- ➢
- Higher EC and salinity
- ➢
- Higher concentrations of all ions
- ➢
- Higher concentrations of cadmium, with all other metals being approximately equal to Remah
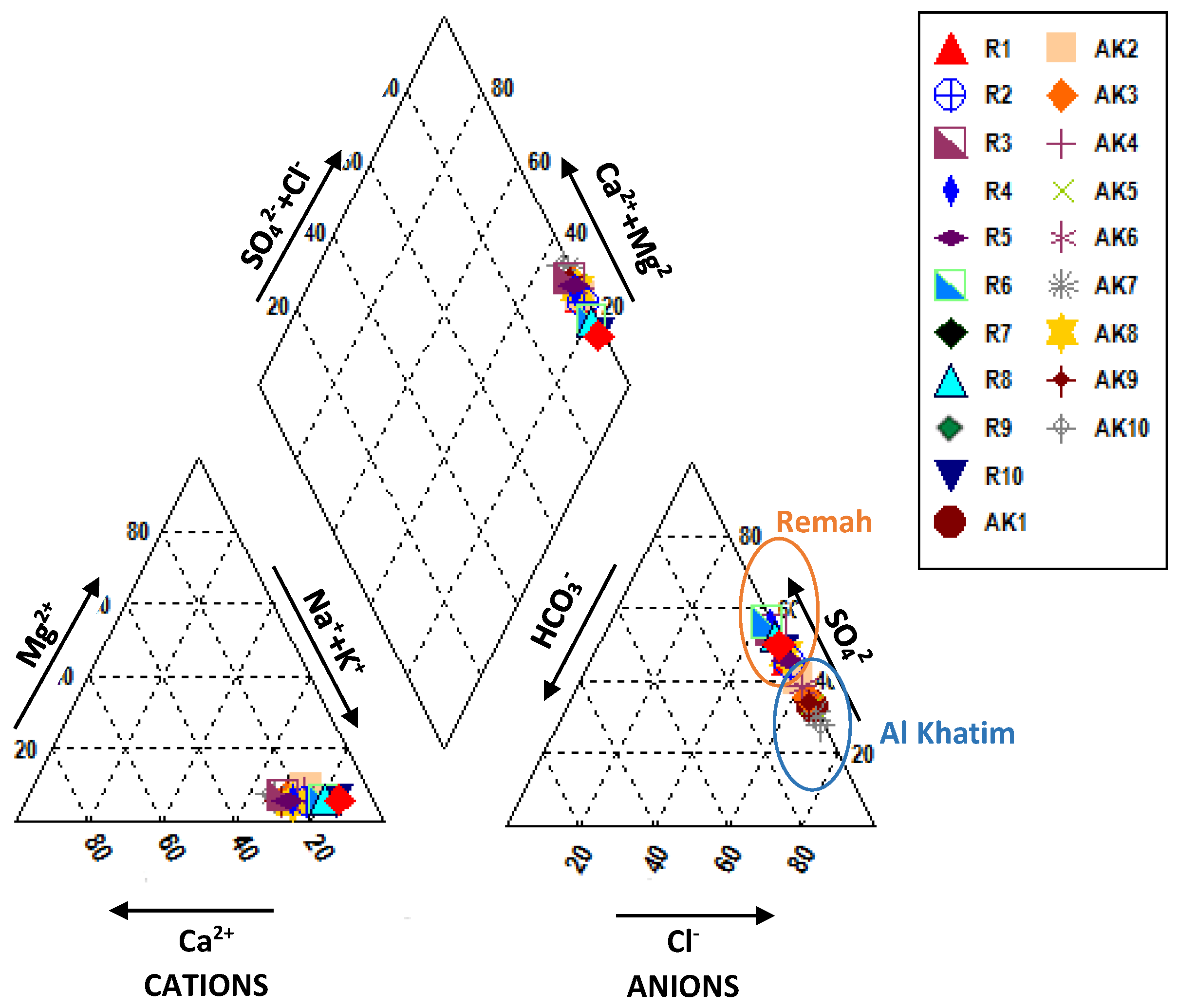
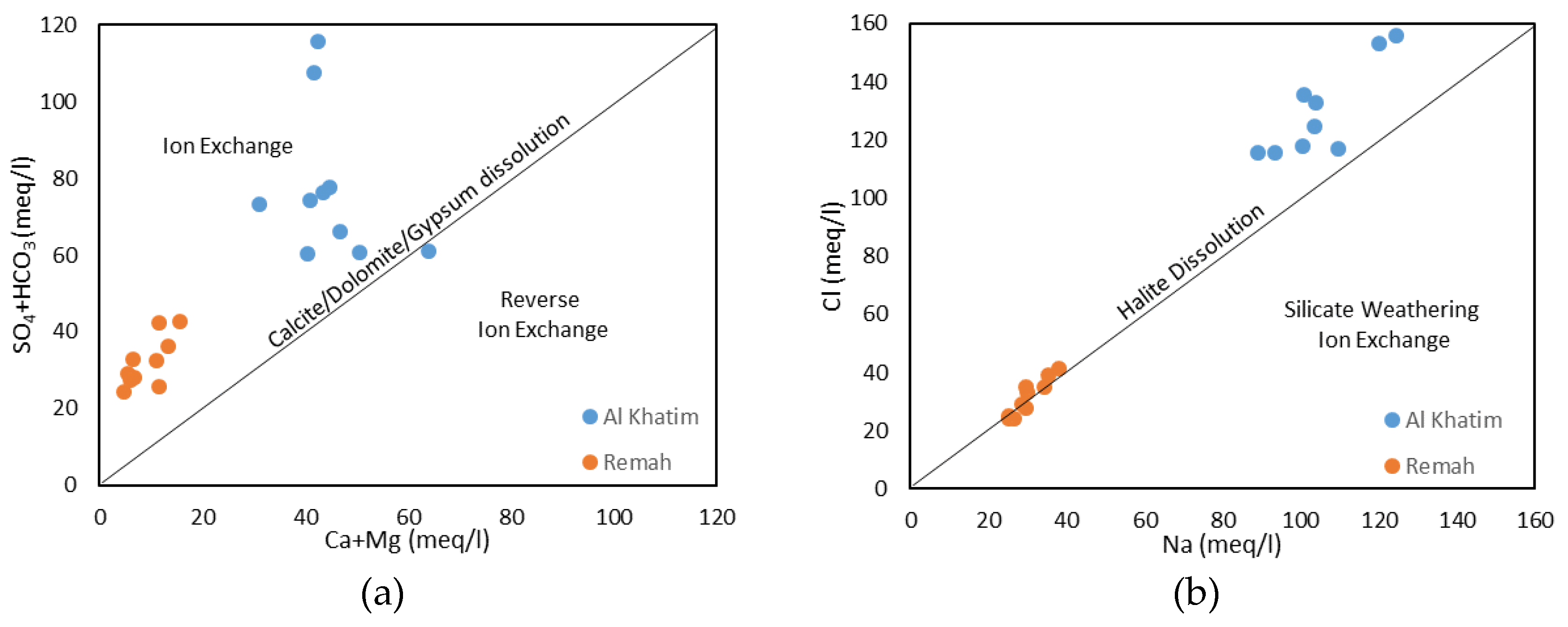
5.1. General Hydrochemistry
- Na–Ca–SO4–Cl (Samples: R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, AK8)
- Na–Ca–Cl–SO4 (Samples: AK1, AK2, AK3, AK4, AK5, AK6, AK7, AK9, AK10)
5.2. Trace Metal Contamination
5.3. Suitability for Irrigation
5.4. Effect of Agriculture on Groundwater Quality
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keesari, T.; Kulkarni, U.P.; Deodhar, A.; Ramanjaneyulu, P.S.; Sanjukta, A.K.; Kumar, U.S. Geochemical characterization of groundwater from an arid region in India. Environ. Earth Sci. 2014, 71, 4869–4888. [Google Scholar] [CrossRef]
- Tsujimura, M.; Abe, Y.; Tanaka, T.; Shimada, J.; Higuchi, S.; Yamanaka, T.; Davaa, G.; Oyunbaatar, D. Stable isotopic and geochemical characteristics of groundwater in Kherlen River basin, a semi-arid region in eastern Mongolia. J. Hydrol. 2007, 333, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Rizk, Z.S.; Alsharhan, A.S. Water resources in the United Arab Emirates. Dev. Water Sci. 2003, 50, 245–264. [Google Scholar]
- Lapworth, D.J.; MacDonald, A.M.; Tijani, M.N.; Darling, W.G.; Gooddy, D.C.; Bonsor, H.C.; Araguás-Araguás, L.J. Residence times of shallow groundwater in West Africa: Implications for hydrogeology and resilience to future changes in climate. Hydrogeol. J. 2013, 21, 673–686. [Google Scholar] [CrossRef]
- Brkić, Ž.; Briški, M.; Marković, T. Use of hydrochemistry and isotopes for improving the knowledge of groundwater flow in a semiconfined aquifer system of the Eastern Slavonia (Croatia). Catena 2016, 142, 153–165. [Google Scholar] [CrossRef]
- Eissa, M.A.; Thomas, J.M.; Hershey, R.L.; Dawoud, M.I.; Pohll, G.; Dahab, K.A.; Gomaa, M.A.; Shabana, A.R. Geochemical and isotopic evolution of groundwater in the Wadi Watir watershed, Sinai Peninsula, Egypt. Environ. Earth Sci. 2014, 71, 1855–1869. [Google Scholar]
- Sophocleous, M. Groundwater management practices, challenges, and innovations in the High Plains aquifer, USA—lessons and recommended actions. Hydrogeol. J. 2010, 18, 559–575. [Google Scholar] [CrossRef]
- Jeevanandam, M.; Kannan, R.; Srinivasalu, S.; Rammohan, V. Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district, South India. Environ. Monit. Assess. 2007, 132, 263. [Google Scholar] [CrossRef]
- Jianhua, S.; Qi, F.; Xiaohu, W.; Yonghong, S.; Haiyang, X.; Zongqiang, C. Major ion chemistry of groundwater in the extreme arid region northwest China. Environ. Geol. 2009, 57, 1079–1087. [Google Scholar] [CrossRef]
- Zhu, G.F.; Li, Z.Z.; Su, Y.H.; Ma, J.Z.; Zhang, Y.Y. Hydrogeochemical and isotope evidence of groundwater evolution and recharge in Minqin Basin, Northwest China. J. Hydrol. 2007, 333, 239–251. [Google Scholar] [CrossRef]
- Cartwright, I.; Weaver, T.R. Hydrogeochemistry of the Goulburn Valley region of the Murray Basin, Australia: Implications for flow paths and resource vulnerability. Hydrogeol. J. 2005, 13, 752–770. [Google Scholar] [CrossRef]
- Wells, C.M.; Price, J.S. The hydrogeologic connectivity of a low-flow saline-spring fen peatland within the Athabasca oil sands region, Canada. Hydrogeol. J. 2015, 23, 1799. [Google Scholar] [CrossRef]
- Adams, S.; Titus, R.; Pietersen, K.; Tredoux, G.; Harris, C. Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J. Hydrol. 2001, 241, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Tóth, J. Groundwater as a geologic agent: An overview of the causes, processes, and manifestations. Hydrogeol. J. 1999, 7, 1–14. [Google Scholar]
- Ahmed, I.; Tariq, N.; Al Muhery, A. Hydrochemical characterization of groundwater to align with sustainable development goals in the Emirate of Dubai, UAE. Environ. Earth Sci. 2019, 78, 44. [Google Scholar] [CrossRef]
- Al-Muaini, A.; Green, S.; Dakheel, A.; Abdullah, A.-H.; Dahr, W.A.A.; Dixon, S.; Kemp, P.; Clothier, B. Irrigation management with saline groundwater of a date palm cultivar in the hyper-arid United Arab Emirates. Agric. Water Manag. 2019, 211, 123–131. [Google Scholar] [CrossRef]
- Al-Katheeri, E.S.; Howari, F.M.; Murad, A.A. Hydrogeochemistry and pollution assessment of quaternary–tertiary aquifer in the Liwa area, United Arab Emirates. Environ. Earth Sci. 2009, 59, 581. [Google Scholar] [CrossRef]
- Iqbal, J.; Nazzal, Y.; Howari, F.; Xavier, C.; Yousef, A. Hydrochemical processes determining the groundwater quality for irrigation use in an arid environment: The case of Liwa Aquifer, Abu Dhabi, United Arab Emirates. Groundw. Sustain. Dev. 2018, 7, 212–219. [Google Scholar] [CrossRef]
- Mohamed, A.M.O. Groundwater Management. Available online: https://www.taylorfrancis.com/ (accessed on 12 July 2018).
- Sanford, W.E.; Wood, W.W. Hydrology of the coastal sabkhas of Abu Dhabi, United Arab Emirates. Hydrogeol. J. 2001, 9, 358–366. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Hassane, A.B. Hydrochemistry assessment of groundwater quality in Al-Ain city, UAE. Environ. Earth Sci. 2016, 75, 353. [Google Scholar] [CrossRef]
- Murad, A.; Mahgoub, F.; Hussein, S. Hydrogeochemical variations of groundwater of the northern Jabal Hafit in eastern part of Abu Dhabi Emirate, United Arab Emirates (UAE). Int. J. Geosci. 2012, 3, 410. [Google Scholar] [CrossRef]
- Imes, J.L.; Wood, W.W. Solute and isotope constraint of groundwater recharge simulation in an arid environment, Abu Dhabi Emirate, United Arab Emirates. Hydrogeol. J. 2007, 15, 1307–1315. [Google Scholar] [CrossRef]
- Elmahdy, S.I.; Mohamed, M.M. Groundwater of Abu Dhabi Emirate: A regional assessment by means of remote sensing and geographic information system. Arab. J. Geosci. 2015, 8, 11279–11292. [Google Scholar] [CrossRef]
- Othman, A.K. Quantitative and Qualitative Assessment of Groundwater Resources in Al-Khatim Area, UAE. Master’s Thesis, United Arab Emirates University, Al-Ain, UAE, 2005. [Google Scholar]
- Embabi, N.S.; Yahia, M.A.; Al Sharhan, A.S. The National Atlas of UAE; United Arab Emirates University: Al Ain, UAE, 1993. [Google Scholar]
- El-Ghawaby, M.A.; El-Sayed, M.I. The structural and sedimentological setting of the Quaternary deposits in Al-Ain area, UAE. J. Arid Environ. 1997, 35, 627–640. [Google Scholar] [CrossRef]
- El-Shami, F. The hydrogeochemistry of the spring of Ain Bu Sukhanah, UAE. Arab Gulf J. Sci. Res. 1990, 8, 34–36. [Google Scholar]
- Abou El-Enin, H.S. Structurally controlled features in Jabal Hafit. Bull. Geog. Soc. Kuwait 1993, 151, 1–63. [Google Scholar]
- Baghdady, A.R. Petrography, Mineralogy and Environmental Impacts of the Sand Dune Fields of the Greater Al Ain Area, United Arab Emirates. Ph.D. Thesis, Faculty of Science, Ain Shams University, Ain Shams, Egypt, 1998. [Google Scholar]
- Garamoon, H.K. Hydrogeological and Geomorphological Studies on the Abu Dhabi-Dubai-Al Ain Triangle, UAE. Ph.D. Thesis, Ain Shames University, Cairo, Egypt, 1996. Unpublished. [Google Scholar]
- Hamdan, A.A.; Anan, H.S. Cretaceous/Tertiary boundary in the United Arab Emirates; Earth Science Series 7; MERC, Ain Shams University: Cairo, Egypt, 1993; Volume 7, pp. 223–231. [Google Scholar]
- Hamdan, A.R.A.; Bahr, S. Lithostratigraphy of the Paleogene succession of northern Jabal Hafit, Al Ain area, United Arab Emirates; Earth Science Series; Middle East Research Center, Ain Shams University: Cairo, Egypt, 1992; Volume 6, pp. 201–224. [Google Scholar]
- EAD. Groundwater Atlas of Abu Dhabi Emirate; Groundwater Wells and Soil Salinity Mapping of Abu Dhabi Emirate Project, Environment Agency: Abu Dhabi, UAE, 2018. [Google Scholar]
- American Public Health Association. Standard Method for Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; 1220p. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Wolters Kluwer, LWW: Philadelphia, PA, USA, 1954; Volume 78. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters; U.S. Dept. of Agriculture: Washington, DC, USA, 1995.
- Raghunath, H.M. Ground Water: Hydrogeology, Ground Water Survey and Pumping Tests, Rural Water Supply and Irrigation Systems, 2nd ed.; New Age International (p) ltd Publishers: New Delhi, India, 1987. [Google Scholar]
- Kelley, W.P.; Brown, S.M.; Liebig, G.F., Jr. Chemical effects of saline irrigation water on soils. Soil Sci. 1940, 49, 95–108. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial variability: Its documentation, accommodation and implication to soil surveys. In Soil Spatial Variability; Nielsen, D.R., Bouma, J., Eds.; Purdon: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- WHO. Guidelines for Drinking Water Quality, 3rd ed.; Recommendations; WHO: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Hem, J.D.; Durum, W.H. Solubility and occurrence of lead in surface water. J. Am. Water Works Assoc. 1973, 65, 562–568. [Google Scholar] [CrossRef]
- Tijani, M. Hydrogeochemical assessment of groundwater in Moro area, Kwara State, Nigeria. Environ. Geol. 1994, 24, 194–202. [Google Scholar] [CrossRef]
- Ahmed, A.; Clark, I. Groundwater flow and geochemical evolution in the Central Flinders Ranges, South Australia. Sci. Total Environ. 2016, 572, 837–851. [Google Scholar] [CrossRef]
- Khalifa, M.A. Geohydrology and the Source of Flowing Well Water in Al-Neima Area, Al, Abu Dhabi Emirate, UAE; National Drilling Company and United States Geological Survey: Abu Dhabi, UAE, 2003; Volume 9. [Google Scholar]
- Edition, F. Guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- WHO. The Guidelines for Drinking-Water Quality, 4th ed.; WHO Library Cataloguing: Geneva, Switzerland, 2011. [Google Scholar]
- Scholler, H. Qualitative Evaluation of Groundwater Resources; Methods and techniques of groundwater investigations and development; UNESCO: Paris, France, 1965; pp. 54–83. [Google Scholar]
- Sulin, V.A. Basis of Classification of Natural Waters; USSR Academy of Science: Moscow, Russia, 1948. (in Russian) [Google Scholar]
- Datta, P.S.; Tyagi, S.K. Major ion chemistry of groundwater in Delhi area: Chemical weathering processes and groundwater flow regime. J. Geol. Soc. India 1996, 47, 88–179. [Google Scholar]
- Bandara, J.M.R.S.; Wijewardena, H.V.P.; Bandara, Y.M.A.Y.; Jayasooriya, R.G.P.T.; Rajapaksha, H. Pollution of River Mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP, Sri Lanka. Environ. Geochem. Health Kew 2011, 33, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Carolyn, K. Lead Contamination in Our Environment, Online Magazine Environmental Quality in the 21st Century; Yale-New Haven Institute: New Haven, CT, USA, 1997. [Google Scholar]
- Rajappa, B.; Manjappa, S.; Puttaiah, E.T. Monitoring of heavy metal concentration in groundwater of Hakinaka Taluk, India. Contemp. Eng. Sci. 2010, 3, 90–183. [Google Scholar]
- Foster, S.; Pulido-Bosch, A.; Vallejos, Á.; Molina, L.; Llop, A.; MacDonald, A.M. Impact of irrigated agriculture on groundwater-recharge salinity: A major sustainability concern in semi-arid regions. Hydrogeol. J. 2018, 26, 2781–2791. [Google Scholar] [CrossRef]









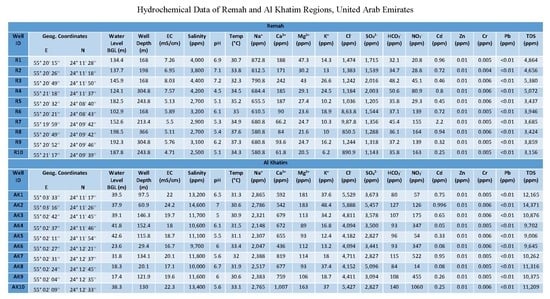
| Remah | |||||
|---|---|---|---|---|---|
| Parameters | Min | Max | Mean | CV | WHO [41] |
| EC (mS/cm) | 4.7 | 8 | 6.2 | 18% | 0.78–31.2 |
| Salinity (mg/L) | 2500 | 4400 | 3350 | 20% | — |
| TDS (mg/L) | 3156 | 5380 | 4148 | 18% | 500–1500 |
| pH | 4.5 | 7.2 | 5.9 | 15% | 6.5–8.5 |
| Na (mg/L) | 581 | 873 | 695 | 14% | 200 |
| Cl (mg/L) | 851 | 1474 | 1163 | 19% | 200–600 |
| Ca (mg/L) | 62 | 242 | 137 | 45% | 75–200 |
| Mg (mg/L) | 21 | 47 | 29 | 29% | 30–150 |
| K (mg/L) | 6.2 | 27 | 15 | 42% | 10 |
| SO4 (mg/L) | 1143 | 2016 | 1513 | 20% | 200–400 |
| HCO3 (mg/L) | 32 | 51 | 39 | 15% | 300 |
| NO3 (mg/L) | 21 | 164 | 96 | 60% | 45 |
| Water Depth (m) | 103 | 198 | 156 | 20% | — |
| Al Khatim | |||||
| EC (mS/cm) | 17 | 24 | 19.8 | 12% | 0.78–31.2 |
| Salinity (mg/L) | 9700 | 14,600 | 11,770 | 13% | — |
| TDS (mg/L) | 9006 | 14,371 | 10,893 | 13% | 500–1500 |
| pH | 5.02 | 7.03 | 6 | 10% | 6.5–8.5 |
| Na (mg/L) | 2047 | 2865 | 2453 | 11% | 200 |
| Cl (mg/L) | 4094 | 5888 | 4730 | 13% | 200–600 |
| Ca (mg/L) | 436 | 1007 | 684 | 22% | 75–200 |
| Mg (mg/L) | 89 | 183 | 125 | 28% | 30–150 |
| K (mg/L) | 12 | 48 | 27 | 45% | 10 |
| SO4 (mg/L) | 2827 | 5457 | 3632 | 24% | 200–400 |
| HCO3 (mg/L) | 80 | 140 | 104 | 17% | 300 |
| NO3 (mg/L) | 14 | 1060 | 316 | 95% | 45 |
| Water Depth (m) | 17 | 42 | 33 | 30% | — |
| Remah | |||||
|---|---|---|---|---|---|
| Parameter | Min | Max | Mean | CV | WHO [47] |
| Cd (mg/L) | 0.25 | 0.96 | 0.6275 | 69.89% | 0.003 |
| Zn (mg/L) | 0.005 | 0.009 | 0.0073 | 20.41% | 5 |
| Cr (mg/L) | 0.004 | 0.006 | 0.0053 | 12.1% | 0.05 |
| Pb (mg/L) | <0.01 | <0.01 | |||
| Al Khatim | |||||
| Cd (mg/L) | 0.052 | 0.996 | 0.4293 | 78.8% | 0.003 |
| Cr (mg/L) | 0.005 | 0.006 | 0.0055 | 9.12% | 0.05 |
| Zn (mg/L) | 0.007 | 0.009 | 0.0082 | 19.4% | 5 |
| Pb (mg/L) | <0.01 | <0.01 | |||
| Well ID | Na% | Salinity Hazard EC (µS/cm) | SAR | MH (%) | KI (mEq/L) |
|---|---|---|---|---|---|
| R1 | 74% | 7260 | 14.7 | 29.5 | 2.8 |
| R2 | 76% | 6950 | 15 | 22.8 | 3.2 |
| R3 | 69% | 8030 | 12.3 | 22.8 | 2.2 |
| R4 | 72% | 7570 | 12.3 | 20.8 | 2.5 |
| R5 | 71% | 5130 | 11.8 | 19.6 | 2.4 |
| R6 | 80.7% | 5890 | 14.8 | 30.4 | 4.1 |
| R7 | 84.8% | 5500 | 18.1 | 38.3 | 5.5 |
| R8 | 81% | 5110 | 14.6 | 30 | 4.2 |
| R9 | 81.7% | 5760 | 16.1 | 30.5 | 4.4 |
| R10 | 84.1% | 4710 | 16.3 | 35.6 | 5.3 |
| AK1 | 74% | 22,000 | 26.3 | 33.8 | 2.8 |
| AK2 | 74.3% | 24,200 | 26.3 | 36.0 | 2.9 |
| AK3 | 70% | 19,700 | 21.7 | 21.7 | 2.3 |
| AK4 | 70% | 18,000 | 20.6 | 18.1 | 2.3 |
| AK5 | 71% | 18,700 | 22.3 | 19.1 | 2.5 |
| AK6 | 74% | 16,700 | 22.6 | 30 | 2.9 |
| AK7 | 67% | 20,100 | 20.7 | 18.8 | 2.1 |
| AK8 | 73% | 17,100 | 24.0 | 18.6 | 2.6 |
| AK9 | 69% | 19,600 | 21.4 | 18.9 | 2.2 |
| AK10 | 65% | 22,300 | 21.3 | 21.2 | 1.9 |
| Minimum | 65.5% | 4710 | 11.8 | 18.1 | 1.9 |
| Maximum | 84.8% | 24,200 | 26.3 | 38.3 | 5.5 |
| Mean | 74.1% | 13,015.5 | 18.7 | 25.8 | 3.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Q.; Kalbus, E.; Alshamsi, D.M.; Mohamed, M.M.; Liaqat, M.U. Hydrochemical Analysis of Groundwater in Remah and Al Khatim Regions, United Arab Emirates. Hydrology 2019, 6, 60. https://doi.org/10.3390/hydrology6030060
Khan Q, Kalbus E, Alshamsi DM, Mohamed MM, Liaqat MU. Hydrochemical Analysis of Groundwater in Remah and Al Khatim Regions, United Arab Emirates. Hydrology. 2019; 6(3):60. https://doi.org/10.3390/hydrology6030060
Chicago/Turabian StyleKhan, Qasim, Edda Kalbus, Dalal M. Alshamsi, Mohamed M. Mohamed, and Muhammad U. Liaqat. 2019. "Hydrochemical Analysis of Groundwater in Remah and Al Khatim Regions, United Arab Emirates" Hydrology 6, no. 3: 60. https://doi.org/10.3390/hydrology6030060
APA StyleKhan, Q., Kalbus, E., Alshamsi, D. M., Mohamed, M. M., & Liaqat, M. U. (2019). Hydrochemical Analysis of Groundwater in Remah and Al Khatim Regions, United Arab Emirates. Hydrology, 6(3), 60. https://doi.org/10.3390/hydrology6030060