Radiotherapy Advances in Renal Disease—Focus on Renal Ischemic Preconditioning
Abstract
:1. Introduction
2. Renal Ischemia-Reperfusion
3. Renal Ischemic Preconditioning
4. Radiation-Induced Effects on the Kidney
5. Radiation-Based Therapy in Kidney Diseases
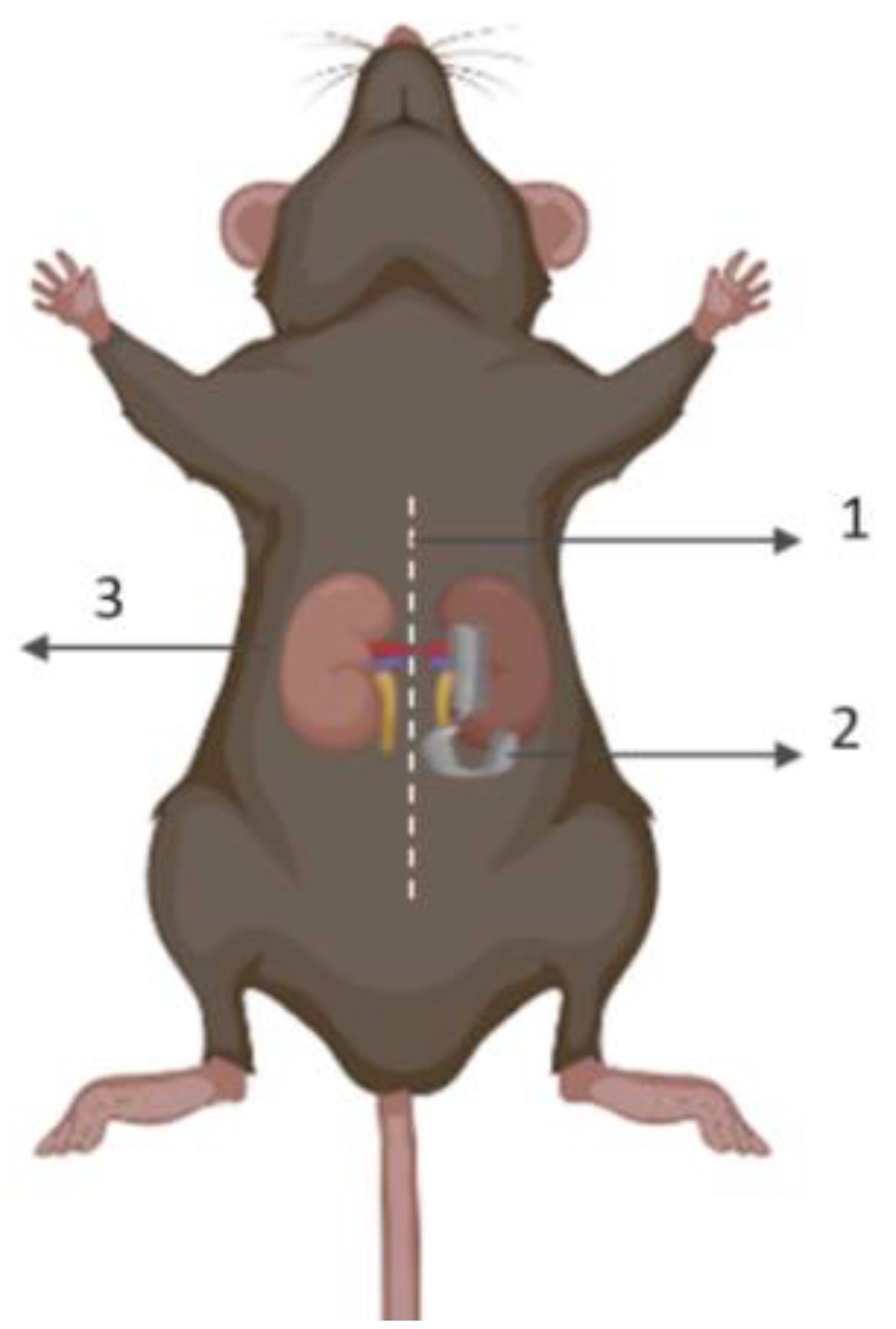
6. Irradiation Preconditioning against AKI
7. Kidney-Centered Irradiation Mediates IPC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havránková, R. Biological effects of ionizing radiation. Cas. Lek. Cesk. 2020, 159, 258–260. [Google Scholar]
- Tomonaga, M. Radiation injury to human body: Atomic bombs, Chernobyl and Fukushima. Rinsho Ketsueki 2011, 52, 1740–1747. [Google Scholar] [PubMed]
- Muirhead, C.R. Studies on the Hiroshima and Nagasaki survivors, and their use in estimating radiation risks. Radiat. Prot. Dosim. 2003, 104, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furdui, C.M. Ionizing Radiation: Mechanisms and Therapeutics. Antioxid. Redox Signal. 2014, 21, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.; Khan, I.; Simpson, W.; Prescott, G.; Townend, J.; Smith, W.; MacLeod, A. Incidence and Outcomes in Acute Kidney Injury: A Comprehensive Population-Based Study. J. Am. Soc. Nephrol. 2007, 18, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Muthuraman, A.; Jaggi, A.S.; Singh, N.; Grover, K.; Dhawan, R. Animal models of acute renal failure. Pharmacol. Rep. 2012, 64, 31–44. [Google Scholar] [CrossRef]
- Bonventre, J.J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Rowart, P.; Erpicum, P.; Detry, O.; Weekers, L.; Grégoire, C.; Lechanteur, C.; Briquet, A.; Beguin, Y.; Krzesinski, J.-M.; Jouret, F. Mesenchymal Stromal Cell Therapy in Ischemia/Reperfusion Injury. J. Immunol. Res. 2015, 2015, 602597. [Google Scholar] [CrossRef] [Green Version]
- Erpicum, P.; Rowart, P.; Poma, L.; Krzesinski, J.-M.; Detry, O.; Jouret, F. Administration of mesenchymal stromal cells before renal ischemia/reperfusion attenuates kidney injury and may modulate renal lipid metabolism in rats. Sci. Rep. 2017, 7, 8687. [Google Scholar] [CrossRef] [Green Version]
- Erpicum, P.; Rowart, P.; Defraigne, J.-O.; Krzesinski, J.-M.; Jouret, F. What we need to know about lipid-associated injury in case of renal ischemia-reperfusion. Am. J. Physiol. Physiol. 2018, 315, F1714–F1719. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Rossignol, P. Cardiovascular Consequences of Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; Bruss, Z.S.; Sehdev, J.S. Physiology, Renal Blood Flow and Filtration. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schrier, R.W.; Wang, W.; Poole, B.; Mitra, A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J. Clin. Investig. 2004, 114, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Dong, Z. Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am. J. Physiol. Physiol. 2012, 303, F1487–F1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Clef, N.; Verhulst, A.; D’Haese, P.C.; Vervaet, B.A. Unilateral Renal Ischemia-Reperfusion as a Robust Model for Acute to Chronic Kidney Injury in Mice. PLoS ONE 2016, 11, e0152153. [Google Scholar] [CrossRef] [Green Version]
- Khbouz, B.; Lallemand, F.; Cirillo, A.; Rowart, P.; Legouis, D.; Sounni, N.E.; Noël, A.; De Tullio, P.; de Seigneux, S.; Jouret, F. Kidney-targeted irradiation triggers renal ischemic preconditioning in mice. Am. J. Physiol. Physiol. 2022, 323, F198–F211. [Google Scholar] [CrossRef]
- Jouret, F.; Leenders, J.; Poma, L.; Defraigne, J.-O.; Krzesinski, J.-M.; de Tullio, P. Nuclear Magnetic Resonance Metabolomic Profiling of Mouse Kidney, Urine and Serum Following Renal Ischemia/Reperfusion Injury. PLoS ONE 2016, 11, e0163021. [Google Scholar] [CrossRef] [Green Version]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Bonventre, J.V. Kidney ischemic preconditioning. Curr. Opin. Nephrol. Hypertens. 2002, 11, 43–48. [Google Scholar] [CrossRef]
- Park, K.M.; Chen, A.; Bonventre, J.V. Prevention of Kidney Ischemia/Reperfusion-induced Functional Injury and JNK, p38, and MAPK Kinase Activation by Remote Ischemic Pretreatment. J. Biol. Chem. 2001, 276, 11870–11876. [Google Scholar] [CrossRef]
- Erpicum, P.; Krzesinski, J.-M.; Jouret, F. Place de l’AMP-activated protein kinase dans le préconditionnement ischémique rénal. Néphrologie Thérapeutique 2014, 10, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Khbouz, B.; Rowart, P.; Poma, L.; Dahlke, E.; Bottner, M.; Stokes, M.; Bolen, G.; Rahmouni, S.; Theilig, F.; Jouret, F. The genetic deletion of the Dual Specificity Phosphatase 3 (DUSP3) attenuates kidney damage and inflammation following ischaemia/reperfusion injury in mouse. Acta Physiol. 2022, 234, e13735. [Google Scholar] [CrossRef] [PubMed]
- Yakulov, T.A.; Todkar, A.P.; Slanchev, K.; Wiegel, J.; Bona, A.; Groß, M.; Scholz, A.; Hess, I.; Wurditsch, A.; Grahammer, F.; et al. CXCL12 and MYC control energy metabolism to support adaptive responses after kidney injury. Nat. Commun. 2018, 9, 3660. [Google Scholar] [CrossRef] [Green Version]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Datta, P.K. TGF-beta1 production in radiation nephropathy: Role of angiotensin II. Int. J. Radiat. Biol. 1999, 75, 473–479. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, M.; Kim, E.J. Involvement of Klotho, TNF-α and ADAMs in radiation-induced senescence of renal epithelial cells. Mol. Med. Rep. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Scharpfenecker, M.; Floot, B.; Russell, N.S.; Coppes, R.P.; Stewart, F.A. Endoglin haploinsufficiency attenuates radiation-induced deterioration of kidney function in mice. Radiother. Oncol. 2013, 108, 464–468. [Google Scholar] [CrossRef]
- Kruse, J.J.C.M.; Floot, B.G.J.; Poele, J.A.M.T.; Russell, N.S.; Stewart, F.A. Radiation-Induced Activation of TGF-β Signaling Pathways in Relation to Vascular Damage in Mouse Kidneys. Radiat. Res. 2009, 171, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Abou Daher, A.; Francis, M.; Azzam, P.; Ahmad, A.; Eid, A.A.; Fornoni, A.; Marples, B.; Zeidan, Y.H. Modulation of radiation-induced damage of human glomerular endothelial cells by SMPDL3B. FASEB J. 2020, 34, 7915–7926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skliarenko, J.; Warde, P. Practical and clinical applications of radiation therapy. Medicine 2016, 44, 15–19. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.-W.; Park, K.M. Increased superoxide formation induced by irradiation preconditioning triggers kidney resistance to ischemia-reperfusion injury in mice. Am. J. Physiol. Physiol. 2009, 296, F1202–F1211. [Google Scholar] [CrossRef] [Green Version]
- Aunapuu, M.; Pechter, Ü.; Gerskevits, E.; Marjamägi, M.-M.; Suuroja, S.; Arend, A.; Kolts, I.; Kühnel, W.; Ots, M. Low-dose radiation modifies the progression of chronic renal failure. Ann. Anat.-Anat. Anz. 2004, 186, 277–282. [Google Scholar] [CrossRef]
- Aunapuu, M.; Arend, A.; Ots, M.; Pilmane, M. Cell proliferation and apoptosis in Wistar rat kidney after renal mass ablation and low-dose irradiation. Medicina 2010, 46, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Nazneen, A.; Taguchi, T.; Razzaque, M.S. Low-Dose Local Kidney Irradiation Inhibits Progression of Experimental Crescentic Nephritis by Promoting Apoptosis. Am. J. Nephrol. 2008, 28, 555–568. [Google Scholar] [CrossRef]
- El-Sonbaty, S.M.; Moawed, F.; Elbakry, M.M.M. Amphora algae with low-level ionizing radiation exposure ameliorate D-galactosamine-induced inflammatory impairment in rat kidney. Environ. Toxicol. 2021, 36, 451–459. [Google Scholar] [CrossRef]
- Cheng, J.; Li, F.; Wang, G.; Guo, W.; Huang, S.; Wang, B.; Li, C.; Jiang, Q.; Cai, L.; Cui, J. Optimal LDR to Protect the Kidney From Diabetes: Whole-Body Exposure to 25 mGy X-rays Weekly for 8 Weeks Efficiently Attenuates Renal Damage in Diabetic Mice. Dose-Response 2018, 16. [Google Scholar] [CrossRef]
- Nomura, T.; Li, X.-H.; Ogata, H.; Sakai, K.; Kondo, T.; Takano, Y.; Magae, J. Suppressive Effects of Continuous Low-Dose-Rate γ Irradiation on Diabetic Nephropathy in Type II Diabetes Mellitus Model Mice. Radiat. Res. 2011, 176, 356–365. [Google Scholar] [CrossRef]
- Taylor, K.; Lemon, J.A.; Phan, N.; Boreham, D.R. Low-dose radiation from 18F-FDG PET does not increase cancer frequency or shorten latency but reduces kidney disease in cancer-prone Trp53+/− mice. Mutagenesis 2014, 29, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Lu, X.; Cong, W.; Xing, X.; Tan, Y.; Li, Y.; Li, X.; Jin, L.; Wang, X.; Dong, J.; et al. Multiple low-dose radiation prevents type 2 diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PLoS ONE 2014, 9, e92574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.-P.; Chen, S.-C.; Liu, Y.-H.; Chen, C.-F.; Hour, T.-C. Postconditioning with far-infrared irradiation increases heme oxygenase-1 expression and protects against ischemia/reperfusion injury in rat testis. Life Sci. 2013, 92, 35–41. [Google Scholar] [CrossRef]
- Lakyová, L.; Toporcer, T.; ScD, V.T.; Sabo, J.; Radoňak, J. Low-level laser therapy for protection against skeletal muscle damage after ischemia-reperfusion injury in rat hindlimbs. Lasers Surg. Med. 2010, 42, 825–832. [Google Scholar] [CrossRef]
- Verhaegen, F.; Granton, P.; Tryggestad, E. Small animal radiotherapy research platforms. Phys. Med. Biol. 2011, 56, R55–R83. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, J.; Zhong, Y.; Shen, C.; Jia, X. Design and experimental validation of a unilateral magnet for MRI-guided small animal radiation experiments. J. Magn. Reson. 2021, 332, 107062. [Google Scholar] [CrossRef]
- Chiu, T.D.; Arai, T.J.; Iii, J.C.; Jiang, S.B.; Mason, R.P.; Stojadinovic, S. MR-CBCT image-guided system for radiotherapy of orthotopic rat prostate tumors. PLoS ONE 2018, 13, e0198065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallemand, F.; Chiver, I.; dos Santos, E.B.; Ball, G.F.; Balthazart, J. Repeated assessment of changes in testes size in canaries by X-ray computer tomography. Gen. Comp. Endocrinol. 2021, 310, 113808. [Google Scholar] [CrossRef]
- Lallemand, F.; Leroi, N.; Blacher, S.; Bahri, M.A.; Balteau, E.; Coucke, P.; Noël, A.; Plenevaux, A.; Martinive, P. Tumor Microenvironment Modifications Recorded with IVIM Perfusion Analysis and DCE-MRI after Neoadjuvant Radiotherapy: A Preclinical Study. Front. Oncol. 2021, 11, 784437. [Google Scholar] [CrossRef]


| Drugs | Protein Targets | Pathways |
|---|---|---|
| Cyclosporine; FK-506 Mesenchymal stem cells (MSC); Adenosine; Apyrase; and Catecholamines | MAPK GPCR | Inflammation |
| Erythropoıetin; Isoflurane | HIF-1a | Hypoxia |
| L-carnitine; Lithium; Danshen; N-acetylcysteine; and Spermine NONOate | Free radicals; NOS | Oxidative stress |
| Metformin; AICAR; and Hemin | AMPK Hmox1 inducer | Metabolism |
| Disease Model | Radiation | Site | Dose | Time | Aspect | Tendency | Reference |
|---|---|---|---|---|---|---|---|
| Adult Wistar rats (CKD) | Γ | Left kidney | 3 Gy | 2 w | Function (urinary proteins excretion rate, awake systolic blood pressure, and SCr) Morphology MCP-1 | ↑ ↑ ↓ | [36] |
| Adult Wistar rats (CKD) | Γ | Left kidney | 3 Gy | 1/2 w | Function (Urinary proteins, systolic blood pressure, and SCr) Morphology Apoptosis (TUNEL) | ↑ ↑ ↓ | [37] |
| Inbred male Sprague- Dawley rats (Crescentic nephritis) | X | Local bilateral kidneys | 0.5 Gy | 1/2/3/4 w | Function (SCr) Morphology PCNA ED-1 Apoptosis (Caspase 3/7 and TUNEL) | ↑ ↑ ↓ ↑ | [38] |
| Adult male C57BL/6 mice (I/R induced AKI) | X | Whole body | 8 Gy | 1 w | Function (BUN and SCr) Morphology Oxidative stress (MnSOD and HSP27) | ↑ ↑ ↑ | [35] |
| Adult male Swiss albino rats (D-GalN-induced renal damage) | Γ | Whole body | 0.25 Gy | N/A | Antioxidant activities (CuZnSOD and GPx) Lipid peroxidation level Function Inflammation (TNF-a and NF-KB) Morphology Nrf-2 gene | ↑ ↓ ↑ ↓ ↑ ↓ | [39] |
| Adult male C57BL/6J mice (Type I diabetes) | X | Whole body | 12.5 or 25 mGy | 4/8 w | Function (SCr and urinary microalbumin) Morphology (PAS staining) Nitrosative damage (3-NT and 4-HNE) Renal fibrosis (Col IV and fibronectin) | ↑ ↑ ↓ ↓ | [40] |
| Adult male C57BL/6 mice (I/R-induced AKI) | X | Local bilateral kidneys | 8 Gy | 2 w | Function (BUN and SCr) Morphology (PAS staining) Inflammation (Cd11b; F4/80) Angiogenesis (PECAM1) Oxidative stress (HSP70; HO1) | ↑ ↑ ↓ ↑ ↑ | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khbouz, B.; Gu, S.; Pinto Coelho, T.; Lallemand, F.; Jouret, F. Radiotherapy Advances in Renal Disease—Focus on Renal Ischemic Preconditioning. Bioengineering 2023, 10, 68. https://doi.org/10.3390/bioengineering10010068
Khbouz B, Gu S, Pinto Coelho T, Lallemand F, Jouret F. Radiotherapy Advances in Renal Disease—Focus on Renal Ischemic Preconditioning. Bioengineering. 2023; 10(1):68. https://doi.org/10.3390/bioengineering10010068
Chicago/Turabian StyleKhbouz, Badr, Shiyang Gu, Tiago Pinto Coelho, François Lallemand, and François Jouret. 2023. "Radiotherapy Advances in Renal Disease—Focus on Renal Ischemic Preconditioning" Bioengineering 10, no. 1: 68. https://doi.org/10.3390/bioengineering10010068







