Role of G-Proteins and GPCRs in Cardiovascular Pathologies
Abstract
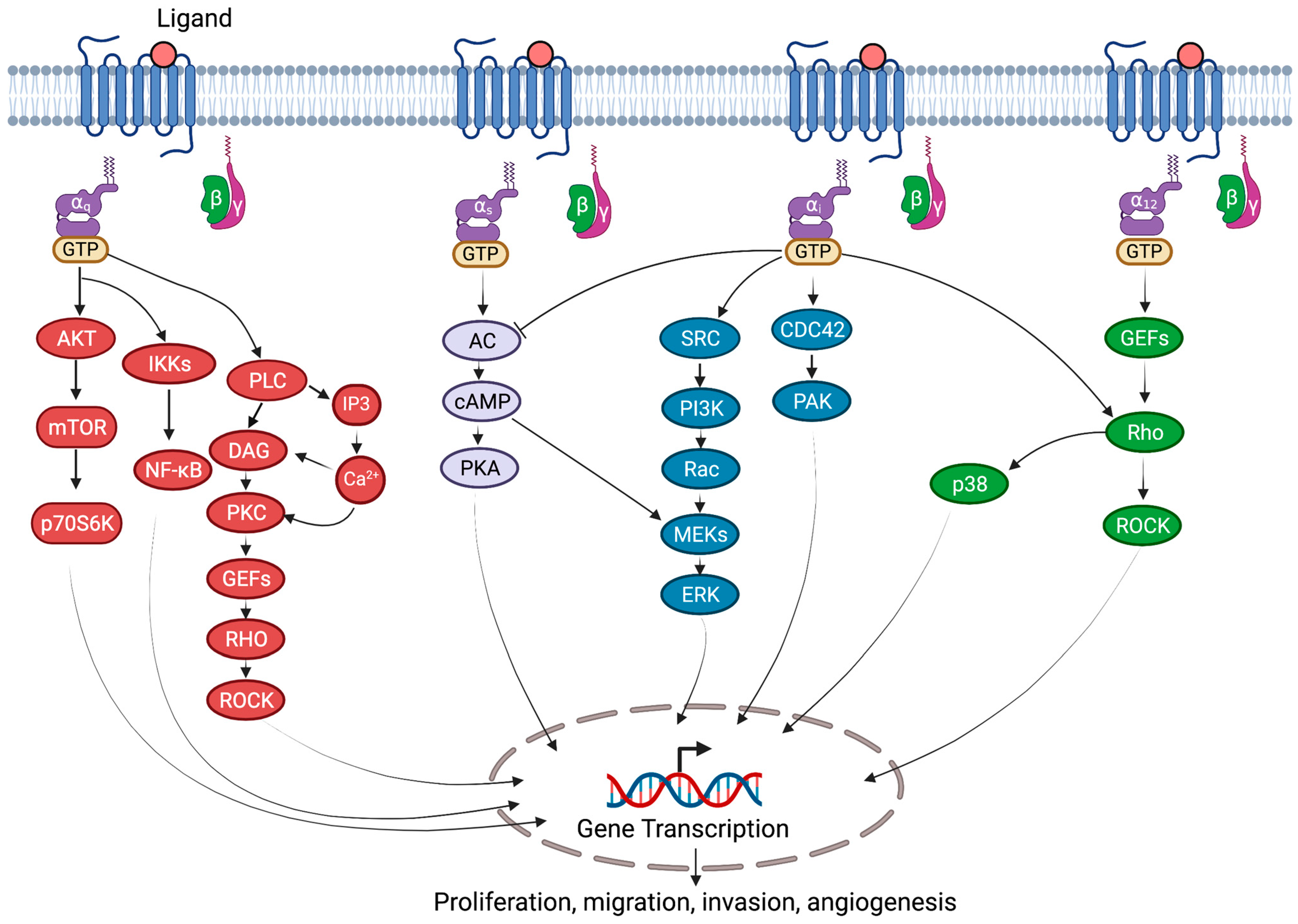
:1. Introduction
2. G-Proteins
3. Regulation of G-Proteins
3.1. General Mechanism
3.1.1. G-Protein Post-Translational Modification
G-Protein Phosphorylation
G-Protein Ubiquitination
G-Protein S-Nitrosylation
G-Protein Palmitoylation
3.1.2. GPCR Regulators
3.1.3. Non-GPCR Regulators
Ric-8 Proteins
GPR Domains
GBA Motif
RGS Proteins
4. Role of G-Proteins in Cardiovascular Diseases
4.1. Heart Failure
4.2. Myocardial Ischemia
4.3. Hypertension
4.4. Atherosclerosis
4.5. Stroke
4.6. Peripheral Artery Disease
4.7. Restenosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farley, A.; McLafferty, E.; Hendry, C. The cardiovascular system. Nurs. Stand. 2012, 27, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R. Determinants of cardiovascular disease and sequential decision-making for treatment among women: A Heckman’s approach. SSM Popul. Health 2019, 7, 100365. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The global burden of cardiovascular diseases and risk factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Saheera, S.; Krishnamurthy, P. Cardiovascular changes associated with hypertensive heart disease and aging. Cell Transplant. 2020, 29, 0963689720920830. [Google Scholar] [CrossRef]
- Jaén, R.I.; Val-Blasco, A.; Prieto, P.; Gil-Fernández, M.; Smani, T.; López-Sendón, J.L.; Delgado, C.; Boscá, L.; Fernández-Velasco, M. Innate immune receptors, key actors in cardiovascular diseases. Basic Transl. Sci. 2020, 5, 735–749. [Google Scholar] [CrossRef]
- Heldin, C.H.; Lu, B.; Evans, R.; Gutkind, J.S. Signals and receptors. Cold Spring Harb. Perspect. Biol. 2016, 8, a005900. [Google Scholar] [CrossRef] [Green Version]
- Tadevosyan, A.; Vaniotis, G.; Allen, B.G.; Hébert, T.E.; Nattel, S. G protein-coupled receptor signalling in the cardiac nuclear membrane: Evidence and possible roles in physiological and pathophysiological function. J. Physiol. 2012, 590, 1313–1330. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mystek, P.; Rysiewicz, B.; Gregrowicz, J.; Dziedzicka-Wasylewska, M.; Polit, A. Gγ and Gα identity dictate a G-protein heterotrimer plasma membrane targeting. Cells 2019, 8, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldham, W.M.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef]
- Gilman, A.G. G proteins and dual control of adenylyl cyclase. Cell 1984, 36, 577–579. [Google Scholar] [CrossRef]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, function, pharmacology, and therapeutic potential of the G protein, Gα/q, 11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, signaling, and physiological functions of g-proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gareri, C.; Rockman, H.A. G-protein–coupled receptors in heart disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef]
- Chi, X.; Wang, S.; Huang, Y.; Stamnes, M.; Chen, J.L. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 2013, 14, 7089–7108. [Google Scholar] [CrossRef]
- Tuteja, N. Signaling through G protein coupled receptors. Plant Signal. Behav. 2009, 4, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Canals, M.; Poole, D.P.; Veldhuis, N.A.; Schmidt, B.L.; Bunnett, N.W. G-protein–coupled receptors are dynamic regulators of digestion and targets for digestive diseases. Gastroenterology 2019, 156, 1600–1616. [Google Scholar] [CrossRef]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased signalling: From simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 2000, 21, 90–113. [Google Scholar] [CrossRef]
- Liccardo, F.; Luini, A.; Di Martino, R. Endomembrane-based signaling by GPCRs and G-proteins. Cells 2022, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Hein, P.; Bünemann, M. Coupling mode of receptors and G proteins. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Maggiolini, M. GPCRs and cancer. Acta Pharmacol. Sin. 2012, 33, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Chakravorty, D.; Assmann, S.M. G protein subunit phosphorylation as a regulatory mechanism in heterotrimeric G protein signaling in mammals, yeast, and plants. Biochem. J. 2018, 475, 3331–3357. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, H.G.; Campbell, S.L. Regulation of large and small G proteins by ubiquitination. J. Biol. Chem. 2019, 294, 18613–18623. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Létourneau, D.; Holleran, B.; Leduc, R.; Lavigne, P.; Lavoie, C. Gαs protein binds ubiquitin to regulate epidermal growth factor receptor endosomal sorting. Proc. Natl. Acad. Sci. USA 2017, 114, 13477–13482. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.L.; Luo, S.; Zhang, C.; Zhou, X.; Zhou, M.; Wang, J.; Kong, C.; Chen, J.; Lin, Z.; Tang, X.; et al. S-nitrosylation-mediated coupling of G-protein alpha-2 with CXCR5 induces Hippo/YAP-dependent diabetes-accelerated atherosclerosis. Nat. Commun. 2021, 12, 4452. [Google Scholar] [CrossRef]
- Szczepek, M.; Beyriere, F.; Hofmann, K.P.; Elgeti, M.; Kazmin, R.; Rose, A.; Bartl, F.J.; von Stetten, D.; Heck, M.; Sommer, M.E.; et al. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat. Commun. 2014, 5, 4801. [Google Scholar] [CrossRef] [Green Version]
- Milligan, G. The role of dimerisation in the cellular trafficking of G-protein-coupled receptors. Curr. Opin. Pharmacol. 2010, 10, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palczewski, K. Oligomeric forms of G protein-coupled receptors (GPCRs). Trends Biochem. Sci. 2010, 35, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Kofron, C.M.; Mende, U. Heterotrimeric G protein-mediated signaling and its non-canonical regulation in the heart. Life Sci. 2015, 129, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, S.J.; Inchausti, R.; Eaton, C.J.; Krystofova, S.; Borkovich, K.A. RIC8 is a guanine-nucleotide exchange factor for G-alpha subunits that regulates growth and development in Neurospora crassa. Genetics 2011, 189, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Papasergi, M.M.; Patel, B.R.; Tall, G.G. The G protein α chaperone Ric-8 as a potential therapeutic target. Mol. Pharmacol. 2015, 87, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.; Thomas, C.J.; Sprang, S.R.; Tall, G.G. Molecular chaperoning function of Ric-8 is to fold nascent heterotrimeric G protein α subunits. Proc. Natl. Acad. Sci. USA 2013, 110, 3794–3799. [Google Scholar] [CrossRef] [Green Version]
- Eppinga, R.N.; Hagemeijer, Y.; Burgess, S.; Hinds, D.A.; Stefansson, K.; Gudbjartsson, D.F.; van Veldhuisen, D.J.; Munroe, P.B.; Verweij, N.; van der Harst, P. Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat. Genet. 2016, 48, 1557–1563. [Google Scholar] [CrossRef]
- Munroe, P.B.; Tinker, A. Heritability of resting heart rate and association with mortality in middle-aged and elderly twins. Heart 2018, 104, 6–7. [Google Scholar] [CrossRef]
- den Hoed, M.; Eijgelsheim, M.; Esko, T.; Brundel, B.J.; Peal, D.S.; Evans, D.M.; Nolte, I.M.; Segrè, A.V.; Holm, H.; Handsaker, R.E.; et al. Identification of heart rateassociated loci and their effects on cardiac conduction and rhythm disorders. Nat. Genet. 2013, 45, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, S.; Nobles, M.; Tsisanova, E.; Ludwig, A.; Munroe, P.B.; Tinker, A. The role of resistance to inhibitors of cholinesterase 8b in the control of heart rate. Physiol. Genom. 2021, 53, 150–159. [Google Scholar] [CrossRef]
- Thomas, C.J.; Tall, G.G.; Adhikari, A.; Sprang, S.R. Ric-8A catalyzes guanine nucleotide exchange on G alphai1 bound to the GPR/GoLoco exchange inhibitor AGS3. J. Biol. Chem. 2008, 283, 23150–23160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aznar, N.; Midde, K.K.; Dunkel, Y.; Lopez-Sanchez, I.; Pavlova, Y.; Marivin, A.; Barbazán, J.; Murray, F.; Nitsche, U.; Janssen, K.P.; et al. Daple is a novel non-receptor GEF required for trimeric G protein activation in Wnt signaling. Elife 2015, 4, e07091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Marcos, M.; Ghosh, P.; Farquhar, M.G. GIV/Girdin transmits signals from multiple receptors by triggering trimeric G protein activation. J. Biol. Chem. 2015, 290, 6697–6704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Ear, J.; Midde, K.; Lopez-Sanchez, I.; Aznar, N.; Garcia-Marcos, M.; Kufareva, I.; Abagyan, R.; Ghosh, P. Structural basis for activation of trimeric Gi proteins by multiple growth factor receptors via GIV/Girdin. Mol. Biol. Cell 2014, 25, 3654–3671. [Google Scholar] [CrossRef]
- Marivin, A.; Maziarz, M.; Zhao, J.; DiGiacomo, V.; Calvo, I.O.; Mann, E.A.; Ear, J.; Blanco-Canosa, J.B.; Ross, E.M.; Ghosh, P.; et al. DAPLE protein inhibits nucleotide exchange on Gαs and Gαq via the same motif that activates Gαi. J. Biol. Chem. 2020, 295, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, M.; Ghosh, P.; Farquhar, M.G. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 3178–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, P. Heterotrimeric G proteins as emerging targets for network-based therapy in cancer: End of a long futile campaign striking heads of a Hydra. Aging 2015, 7, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Opakua, A.I.; Parag-Sharma, K.; DiGiacomo, V.; Merino, N.; Leyme, A.; Marivin, A.; Villate, M.; Nguyen, L.T.; de la Cruz-Morcillo, M.A.; Blanco-Canosa, J.B.; et al. Molecular mechanism of Gαi activation by non-GPCR proteins with a Gα-binding and activating motif. Nat. Commun. 2017, 8, 15163. [Google Scholar] [CrossRef] [Green Version]
- Jules, J.; Yang, S.; Chen, W.; Li, Y.P. Role of regulators of G protein signaling proteins in bone physiology and pathophysiology. Prog. Mol. Biol. Transl. Sci. 2015, 133, 47–75. [Google Scholar]
- Masuho, I.; Balaji, S.; Muntean, B.S.; Skamangas, N.K.; Chavali, S.; Tesmer, J.J.; Babu, M.M.; Martemyanov, K.A. A global map of G protein signaling regulation by RGS proteins. Cell 2020, 183, 503–521. [Google Scholar] [CrossRef]
- Hercule, H.C.; Tank, J.; Plehm, R.; Wellner, M.; Goncalves, A.C.D.C.; Gollasch, M.; Diedrich, A.; Jordan, J.; Luft, F.C.; Gross, V. Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp. Physiol. 2007, 92, 1014–1022. [Google Scholar] [CrossRef]
- Zhang, W.; Anger, T.; Su, J.; Hao, J.; Xu, X.; Zhu, M.; Gach, A.; Cui, L.; Liao, R.; Mende, U. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J. Biol. Chem. 2006, 281, 5811–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Tang, X.-H.; Li, X.-H.; Dai, H.-J.; Miao, R.-J.; Cai, J.-J.; Huang, Z.-J.; Chen, A.F.; Xing, X.-W.; Lu, Y.; et al. Regulator of G protein signalling 14 attenuates cardiac remodelling through the MEKERK1/2 signalling pathway. Basic Res. Cardiol. 2016, 111, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Mende, U. Regulators of G-protein signaling in the heart and their potential as therapeutic targets. Circ. Res. 2011, 109, 320–333. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.; Huang, J.; Fisher, R.A. RGS Proteins in Heart: Brakes on the Vagus. Front. Physiol. 2012, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.L.; Lassègue, B.; Griendling, K.K.; Ushio-Fukai, M.; Lyons, P.R.; Alexander, R.W. Specific regulation of RGS2 messenger RNA by angiotensin II in cultured vascular smooth muscle cells. Mol. Pharmacol. 2000, 57, 460–467. [Google Scholar] [CrossRef]
- Miao, R.; Lu, Y.; Xing, X.; Li, Y.; Huang, Z.; Zhong, H.; Huang, Y.; Chen, A.F.; Tang, X.; Li, H.; et al. Regulator of G-Protein Signaling 10 Negatively Regulates Cardiac Remodeling by Blocking Mitogen-Activated Protein Kinase-Extracellular Signal-Regulated Protein Kinase 1/2 Signaling. Hypertension 2016, 67, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, G.; Li, X.; Yang, Z.; Heubach, J.; Gaudio, S.; Khoury, C.; Ravens, U.; Greenwood, M.T. Beta-adrenergic receptor-mediated atrial specific up-regulation of RGS5. Life Sci. 2005, 76, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Hendriks-Balk, M.C.; Hajji, N.; van Loenen, P.B.; Michel, M.C.; Peters, S.L.; Alewijnse, A.E. Sphingosine-1-phosphate regulates RGS2 and RGS16 mRNA expression in vascular smooth muscle cells. Eur. J. Pharmacol. 2009, 606, 25–31. [Google Scholar] [CrossRef]
- Meyer, E.E.; Clancy, C.E.; Lewis, T.J. Dynamics of adrenergic signaling in cardiac myocytes and implications for pharmacological treatment. J. Theor. Biol. 2021, 519, 110619. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Y.; Bai, J.; Liu, J.Q.; Li, H.H. Angiotensin II stimulates the proliferation and migration of lymphatic endothelial cells through angiotensin type 1 receptors. Front. Physiol. 2020, 11, 560170. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Takada, S.; Furihata, T.; Nambu, H.; Kakutani, N.; Maekawa, S.; Mizushima, W.; Nakano, I.; Fukushima, A.; Yokota, T.; et al. Brain-derived neurotrophic factor improves impaired fatty acid oxidation via the activation of adenosine monophosphate-activated protein kinase-α-proliferator-activated receptor-r coactivator-1α signaling in skeletal muscle of mice with heart failure. Circ. Heart Fail. 2021, 14, e005890. [Google Scholar] [CrossRef]
- Lee, C.W.; Rivera, R.; Dubin, A.E.; Chun, J. LPA (4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J. Biol. Chem. 2007, 282, 4310–4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarbit, E.; Singh, I.; Peart, J.N.; Bivol, S.; Rose’Meyer, R.B. Increased release of serotonin from rat primary isolated adult cardiac myofibroblasts. Sci. Rep. 2021, 11, 20376. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Y.; Chen, J.; Dai, C.; Liu, D.; Pan, W.; Wang, L.; Fasae, M.B.; Sun, L.; Wang, L.; et al. Activation of M3 muscarinic acetylcholine receptors delayed cardiac aging by inhibiting the caspase-1/IL-1β signaling pathway. Cell. Physiol. Biochem. 2018, 49, 1208–1216. [Google Scholar] [CrossRef]
- Bohm, M.; Flesch, M.; Schnabel, P. Beta-adrenergic signal transduction in the failing and hypertrophied myocardium. J. Mol. Med. 1997, 75, 842–848. [Google Scholar]
- Stiles, G.L. Adrenergic receptor responsiveness and congestive heart failure. Am. J. Cardiol. 1991, 67, 13C–17C. [Google Scholar] [CrossRef]
- Sigmund, M.; Jakob, H.; Becker, H.; Hanrath, P.; Schumacher, C.; Eschenhagen, T.; Schmitz, W.; Scholz, H.; Steinfath, M. Effects of metoprolol on myocardial beta-adrenoceptors and Gi-alpha-proteins in patients with congestive heart failure. Eur. J. Clin. Pharmacol. 1996, 51, 127–132. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Gordon, E.A.; Wickman, K.; Velimirović, B.; Krapivinsky, L.; Clapham, D.E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel protein. Nature 1995, 374, 135–141. [Google Scholar] [CrossRef]
- Corey, S.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Number and stoichiometry of subunits in the native atrial G-protein-gated K+ channel, IKACh. J. Biol. Chem. 1998, 273, 5271–5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledonne, A.; Berretta, N.; Davoli, A.; Rizzo, G.R.; Bernardi, G.; Mercuri, N.B. Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons. Front. Syst. Neurosci. 2011, 5, 56. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhang, T.N.; Wen, R.; Liu, C.F.; Yang, N. Role of posttranslational modifications of proteins in cardiovascular disease. Oxid. Med. Cell Longev. 2022, 2022, 3137329. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Woo, A.Y.; Zhang, Y.; Cao, C.M.; Xiao, R.P. β-adrenergic receptor subtype signaling in the heart: From bench to the bedside. Curr. Top. Membr. 2011, 67, 191–204. [Google Scholar]
- Violin, J.D.; DeWire, S.M.; Yamashita, D.; Rominger, D.H.; Nguyen, L.; Schiller, K.; Whalen, E.J.; Gowen, M.; Lark, M.W. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J. Pharmacol. Exp. Ther. 2010, 335, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Boerrigter, G.; Lark, M.W.; Whalen, E.J.; Soergel, D.G.; Violin, J.D.; Burnett, J.C. Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: A novel therapeutic strategy for acute heart failure. Circ. Heart Fail. 2011, 4, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. Endocrinol. 2021, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.; Thomsen, A.R.B.; Irannejad, R. Emerging role of compartmentalized g protein-coupled receptor signaling in the cardiovascular field. ACS Pharmacol. Transl. Sci. 2020, 3, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Evora, P.R.; Nobre, F. The role of G-proteins in the pathophysiology of the cardiovascular diseases. Arq. Bras. Cardiol. 1999, 72, 209–229. [Google Scholar] [CrossRef] [Green Version]
- Will-Shaab, L.; Rosenthal, W.; Schultze, W.; Kutner, I. G-protein function in ischemic myocardium. Eur. Heart J. 1991, 12, 135–138. [Google Scholar] [CrossRef]
- Rauch, B.; Niroomand, F. Specific M2-receptor activation: An alternative to treatment with beta-blockers? Eur. Heart J. 1991, 12, 76–82. [Google Scholar] [CrossRef]
- Strasser, R.H.; Marquetant, R. Sensitization of the beta-adrenergic system in acute myocardial ischemia by a protein kinase C-dependent mechanism. Eur. Heart J. 1991, 12, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Mensah, G.A. Commentary: Hypertension phenotypes: The many faces of a silent killer. Ethn. Dis. 2019, 29, 545. [Google Scholar] [CrossRef]
- Nadar, S.; Blann, A.D.; Lip, G.Y. Endothelial dysfunction: Methods of assessment and application to hypertension. Curr. Pharm. Des. 2004, 10, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Cohn, H.I.; Pesant, S.; Eckhart, A.D. GPCR signalling in hypertension: Role of GRKs. Clin. Sci. 2008, 115, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, N.; Itoh, H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals 2009, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q.; Qiao, Y.N.; Zhang, C.H.; Peng, Y.J.; Chen, C.; Wang, P.; Gao, Y.Q.; Chen, C.; Chen, X.; Tao, T.; et al. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H584–H591. [Google Scholar] [CrossRef] [PubMed]
- Brinks, H.L.; Eckhart, A.D. Regulation of GPCR signaling in hypertension. Biochim. Biophys. Acta 2010, 1802, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- B Anand-Srivastava, M. Modulation of Gi proteins in hypertension: Role of angiotensin II and oxidative stress. Curr. Cardiol. Rev. 2010, 6, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Jensen, B.C.; Baker, A.J.; Simpson, P.C. Cardiac alpha1- adrenergic receptors: Novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol. Rev. 2014, 66, 308–333. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, D.T.; Turnbull, L.; Swigart, P.; O’Connell, T.D.; Simpson, P.C.; Baker, A.J. Abnormal myocardial contraction in alpha(1A)- and alpha(1B)-adrenoceptor double-knockout mice. J. Mol. Cell. Cardiol. 2003, 35, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N. The Vasodilator-Heart Failure Trials (V-HeFT). Mechanistic data from the VA cooperative studies. Introduction. Circulation 1993, 87, VI1–VI4. [Google Scholar] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Katugampola, S.D.; Kuc, R.E.; Maguire, J.J.; Davenport, A.P. G-protein-coupled receptors in human atherosclerosis: Comparison of vasoconstrictors (endothelin and thromboxane) with recently deorphanized (urotensin-II, apelin and ghrelin) receptors. Clin. Sci. 2002, 103, 171S–175S. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.M.; Zhang, Y.L.; Chen, D.Y.; Hong, L.J.; Han, F.; Liu, Q.B.; Jiang, J.J.; Lu, Y.M. Endothelial GPR124 exaggerates the pathogenesis of atherosclerosis by activating inflammation. Cell. Physiol. Biochem. 2018, 45, 547–557. [Google Scholar] [CrossRef]
- Zhou, Y.; Little, P.J.; Ta, H.T.; Xu, S.; Kamato, D. Lysophosphatidic acid and its receptors: Pharmacology and therapeutic potential in atherosclerosis and vascular disease. Pharmacol. Ther. 2019, 204, 107404. [Google Scholar] [CrossRef]
- Kots, A.Y.; Gumanova, N.G.; Akhmedzhanov, N.M.; Varentsov, S.I.; Gerasimova, C.I.; Bulargina, T.V.; Shakhov, Y.A. The GTP-binding regulatory proteins, Gs and Gi, are altered in erythrocyte membranes of patients with ischemic heart disease resulting from coronary atherosclerosis. Arterioscler. Thromb. 1993, 13, 1244–1251. [Google Scholar] [CrossRef] [Green Version]
- Forni, V.; Wuerzner, G.; Pruijm, M.; Burnier, M. Long-term use and tolerability of irbesartan for control of hypertension. Integr. Blood Press Control 2011, 4, 17. [Google Scholar] [PubMed] [Green Version]
- Li, D.; Scott, L.; Crambert, S.; Zelenin, S.; Eklöf, A.C.; Di Ciano, L.; Ibarra, F.; Aperia, A. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J. Am. Soc. Nephrol. 2012, 23, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, G.; Soergel, D.G.; Violin, J.D.; Lark, M.W.; Burnett, J.C., Jr. TRV120027, a novel β-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ. Heart Fail. 2012, 5, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.C.; Yu, D.; Shen, Y.; Wu, Y. G-Protein Coupled Receptors: Promising Targets for Antibody-Drug Conjugates. 2020. Available online: https://bioprocessintl.com/manufacturing/monoclonal-antibodies/gpcrs-promising-targets-for-antibody-drug-conjugates/ (accessed on 20 October 2022).
- Schalop, L.; Allen, J. GPCRs, Desirable Therapeutic Targets in Oncology. 2017. Available online: https://www.drugdiscoverytrends.com/gpcrs-desirable-therapeutic-targets-in-oncology/ (accessed on 20 October 2022).
- Giessler, C.; Dhein, S.; Ponicke, K.; Brodde, O.E. Muscarinic receptors in the failing human heart. Eur. J. Pharmacol. 1999, 375, 197–202. [Google Scholar] [CrossRef]
- Barandier, C.; Ming, X.F.; Yang, Z. Small G proteins as novel therapeutic targets in cardiovascular medicine. News Physiol. Sci. 2003, 18, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Frank, D.; Zlotnik, A.; Boyko, M.; Gruenbaum, B.F. The development of novel drug treatments for stroke patients: A review. Int. J. Mol. Sci. 2022, 23, 5796. [Google Scholar] [CrossRef]
- De Oliveira, P.G.; Ramos, M.L.; Amaro, A.J.; Dias, R.A.; Vieira, S.I. Gi/o-protein coupled receptors in the aging brain. Front. Aging Neurosci. 2019, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Vahidinia, Z.; Joghataei, M.T.; Beyer, C.; Karimian, M.; Tameh, A.A. G-protein-coupled receptors and ischemic stroke: A focus on molecular function and therapeutic potential. Mol. Neurobiol. 2021, 58, 4588–4614. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berumen, L.C.; Rodríguez, A.; Miledi, R.; García-Alcocer, G. Serotonin receptors in hippocampus. Sci. World J. 2012, 2012, 823493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, F.F.; Hasseldam, H.; Smith, M.N.; Rasmussen, R.S. Drug-induced hypothermia by 5HT1A agonists provide neuroprotection in experimental stroke: New perspectives for acute patient treatment. J. Stroke Cerebrovasc. Dis. 2014, 23, 2879–2887. [Google Scholar] [CrossRef]
- Ranjbar-Slamloo, Y.; Fazlali, Z. Dopamine and Noradrenaline in the Brain; Overlapping or Dissociate Functions? Front. Mol. Neurosci. 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevich, E.V.; Gainetdinov, R.R.; Gurevich, V.V. G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol. Res. 2016, 111, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, F.; Fouillioux, C.; Bolívar, A.; Simonovis, N.; Hernández-Hernández, R.; Armas-Hernandez, M.; Velasco, M. Dopamine, hypertension and obesity. J. Hum. Hypertens. 2002, 16, S13–S17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenningsson, P.; Nishi, A.; Fisone, G.; Girault, J.A.; Nairn, A.C.; Greengard, P. DARPP-32: An integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 269–296. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.T. Dopamine receptor agonists for Parkinson’s disease. Expert Opin. Investig. Drugs 2014, 23, 387–410. [Google Scholar] [CrossRef]
- Hazarika, S.; Annex, B.H. Biomarkers and genetics in peripheral artery disease. Clin. Chem. 2017, 63, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.R.; McGraw, C.; Robinson, A.S. The specificity of downstream signaling for A1 and A2AR does not depend on the C-terminus, despite the importance of this domain in downstream signaling strength. Biomedicines 2020, 8, 603. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine receptors and the heart: Role in regulation of coronary blood flow and cardiac electrophysiology. Handb. Exp. Pharmacol. 2009, 193, 161–188. [Google Scholar]
- Headrick, J.P.; Hack, B.; Ashton, K.J. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1797–H1818. [Google Scholar] [CrossRef] [Green Version]
- Guzman, L.A.; Mick, M.J.; Arnold, A.M.; Forudi, F.; Whitlow, P.L. Role of intimal hyperplasia and arterial remodeling after balloon angioplasty: An experimental study in the atherosclerotic rabbit model. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment. J. Thorac. Dis. 2016, 8, E1150. [Google Scholar] [CrossRef]
- McCrink, K.A.; Maning, J.; Vu, A.; Jafferjee, M.; Marrero, C.; Brill, A.; Bathgate-Siryk, A.; Dabul, S.; Koch, W.J.; Lymperopoulos, A. β-Arrestin2 Improves Post–Myocardial Infarction Heart Failure via Sarco (endo) plasmic Reticulum Ca2+-ATPase–Dependent Positive Inotropy in Cardiomyocytes. Hypertension 2017, 70, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A. GRK2 and β-arrestins in cardiovascular disease: Something old, something new. Am. J. Cardiovasc. Dis. 2011, 1, 126–137. [Google Scholar] [PubMed]
- Smith, J.S.; Rajagopal, S. The β-arrestins: Multifunctional regulators of G protein-coupled receptors. J. Biol. Chem. 2016, 291, 8969–8977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamel, I.A.; Palakkott, A.; Ashraf, A.; Iratni, R.; Ayoub, M.A. Interplay between angiotensin II type 1 receptor and thrombin receptor revealed by bioluminescence resonance energy transfer assay. Front. Pharmacol. 2020, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Smithwick, L.A.; Lefkowitz, R.J.; Koch, W.J. Targeting Gβγ signaling in arterial vascular smooth muscle proliferation: A novel strategy to limit restenosis. Proc. Natl. Acad. Sci. 1999, 96, 3945–3950. [Google Scholar] [CrossRef]

| GPCR | Ligand | Location | Reference |
|---|---|---|---|
| Adrenergic receptor | Norepinephrine | Cardiomyocytes | [60] |
| Angiotensin II receptor | Angiotensin II | Endothelial cells | [61] |
| Endothelin receptor | Endothelin I | Blood vessel | [62] |
| Adenosine receptor | Adenosine | Heart and brain | [63] |
| LPA receptor | Lysophosphatidic acid | Heart and brain | [64] |
| Serotonin receptor | Serotonin | Cardiac cells | [65] |
| Muscarinic receptors | Acetylcholine | Cardiac myocytes | [66] |
| Drug(s) | Condition | Target | Reference |
|---|---|---|---|
| Irbesartan (Avapro) | Hypertension | Angiotensin II | [101] |
| Losartan | Hypertension | Angiotensin II | [102] |
| Vasomera (PB1046), vasoactive intestinal peptide | Hypertension | VIP and PACAP receptor family: VIPR1, VIPR2 | [103] |
| Serelaxin | Heart failure | Relaxin receptor: RXFP1, RXFP2 | [103] |
| TRV120027 | Heart failure | Angiotensin II | [104] |
| Plozalizumab | Atherosclerosis | CCR2 | [105] |
| Alfuzosin, Terazosin | Hypertension | Adrenoreceptor: Alpha-1 | [106] |
| Clonidine, Bisoprolol, Betaxolol | Hypertension | Adrenoreceptor: Alpha-2 | [106] |
| Metoprolol, Atenolol | Hypertension | Adrenoreceptor: Beta-1 | [106] |
| Atropine, Isoproterenol Forskolin Acetylcholine | Heart rate reduction | Muscarinic receptors: Gαq (M1, M3, M5), Gαi (M2, M4) | [17,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Verma, S.K.; Singh, D.; Singh, N.K. Role of G-Proteins and GPCRs in Cardiovascular Pathologies. Bioengineering 2023, 10, 76. https://doi.org/10.3390/bioengineering10010076
Kaur G, Verma SK, Singh D, Singh NK. Role of G-Proteins and GPCRs in Cardiovascular Pathologies. Bioengineering. 2023; 10(1):76. https://doi.org/10.3390/bioengineering10010076
Chicago/Turabian StyleKaur, Geetika, Shailendra Kumar Verma, Deepak Singh, and Nikhlesh K. Singh. 2023. "Role of G-Proteins and GPCRs in Cardiovascular Pathologies" Bioengineering 10, no. 1: 76. https://doi.org/10.3390/bioengineering10010076
APA StyleKaur, G., Verma, S. K., Singh, D., & Singh, N. K. (2023). Role of G-Proteins and GPCRs in Cardiovascular Pathologies. Bioengineering, 10(1), 76. https://doi.org/10.3390/bioengineering10010076








