Abstract
Hyperlipidemia is increasing in prevalence and is highly correlated with cardiovascular disease (CVD). Lipid-lowering medications prevent CVD but may not be suitable when the side effects are intolerable or hypercholesterolemia is too severe. Double-filtration plasmapheresis (DF) has shown its therapeutic effect on hyperlipidemia, but its side effects are not yet known. We enrolled 45 adults with hyperlipidemia in our study. The sera before and two weeks after DF were evaluated, and we also analyzed perfluorochemicals to see if DF could remove these lipophilic toxins. After DF, all lipid profile components (total cholesterol, triglycerides, high-density lipoprotein [HDL], and low-density lipoprotein [LDL]) had significantly decreased. Leukocyte counts increased while platelet levels decreased, which may have been caused by the puncture wound from DF and consumption of platelets during the process. As for uremic toxins and inflammation, levels of C-reactive protein, uric acid, and alanine transaminase (ALT) all decreased, which may be related to the removal of serum perfluorooctane sulfonate (PFOS) and improvement of renal function. The total cholesterol/HDL ratio and triglycerides were significantly higher in the diabetes mellitus (DM) group at baseline but did not significantly differ after DF. In conclusion, DF showed potential for improving inflammation and removing serum lipids and PFOS in adults with hyperlipidemia.
1. Introduction
Hyperlipidemia’s prevalence is increasing in response to changing dietary habits as part of the modern lifestyle. This rising prevalence has correlated with higher rates of cardiovascular disease (CVD) and stroke. [1]
Many lipid-lowering agents have shown a therapeutic effect on hyperlipidemia. However, medication’s effects in certain populations are still unsatisfactory. For patients with familial hyperlipidemia, for example, serum triglyceride levels are too high to be controlled solely by medication [2]. These patients may need dialysis therapy to remove serum lipids during childhood [3]. Some patients cannot tolerate the side effects of medication. Statins may cause rhabdomyolysis and muscle soreness, which may contribute to poor compliance. Furthermore, statins may increase the incidence of new-onset diabetes [4]. Clinically, it is not uncommon for patients receiving a statin or other lipid-lowering agent to fail to meet the therapeutic target [5].
Many studies have shown patients with chronic kidney disease (CKD) to be refractory to statin treatment, such as the 4D [6] and AURORA trials [7]. For patients undergoing hemodialysis, statin use does not improve survival rates, although statins may lower LDL levels, as reported by the Cochrane Database of Systematic Reviews [8]. On the other hand, the mortality rate of patients with CKD is higher than that of the general population [9].
For the reasons mentioned above, the need for other lipid-lowering methods is indicated, such as anion exchange resin or double filtration (DF) [10]. However, how double filtration influences hyperlipidemia and levels of persistent organic pollutants (POPs), most of which are lipophilic and hydrophobic, is still unclear. Furthermore, DM patients have a higher risk of CVD, [11] so we compared the demographic biochemical profiles between DM and non-DM patients before and after DF in order to find whether DF may influence the risk factors of CVD.
1.1. Persistent Organic Pollutants: Perfluorochemicals (PFCs)
Perfluorochemicals (PFCs) are hydrophobic persistent organic pollutants. These environmental hormones are widely used in leather, textiles, and food packaging because of their stability and water-repelling properties. Once ingested through food or water, these compounds are carried by serum protein into the human body and have a long half-life [12]. PFCs form an eight-carbon perfluoroalkyl chain; all hydrogen atoms are replaced by fluorine atoms with strong bonds, contributing to PFCs’ stability against heat and ability to avoid degradation [13]. However, PFCs are toxic to the liver, immune system, and embryos [14,15]. Bioconcentration means that toxins are concentrated at much higher levels inside living creatures than in the environment, and biomagnification means that toxins produce more harmful effects on humans because humans are higher on the food chain [14].
Our study targets are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) because they are among the toxins with the highest concentrations in humans [16]. Compared with other toxins, PFCs’ properties and metabolism are not fully understood [17]. A large epidemiologic study showed a positive correlation between serum PFC and serum LDL [18]. According to previous studies, the half-life of PFOA is 3.8 years, and the half-life of PFOS is 5.4 years [19]. These concentration of PFCs in these patients with normal kidney function is about normal range in Taiwan, which is about 5–10 ng/mL. [12]
1.2. Double Filtration (DF)
Hemodialysis (HD) is the main blood purification method worldwide. However, it can only remove small and mainly water-soluble molecules. The removal of large hydrophobic molecules by HD is not satisfactory [12,20].
Double filtration (DF) purifies blood by first separating serum and blood cells, and then removing large particles from the serum [1]. DF only removes particles larger than serum albumin after serum is separated from blood. The procedure returns the remaining filtered serum back into patients to limit blood volume loss [21]. Both filters are made of polyvinyl alcohol and have different pore sizes.
Currently, no study has evaluated the serum levels of persistent organic pollutants such as PFCs in patients with metabolic syndrome or hyperlipidemia. As a result, the serum concentrations of perfluorochemicals are still unknown, as is whether PFCs can be removed by the abovementioned methods. The effects of toxin removal on inflammation are also worthy of investigation.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
We enrolled adults with hyperlipidemia and metabolic syndrome who poorly tolerated the available medications or were medically refractory. After informed consent, the participants had double filtration once, with serum taken before and two weeks after the therapy. We checked the serum lipid profile, inflammatory markers, and the environmental toxins PFOA and PFOS.
We included patients aged 18 to 90 who were diagnosed as hyperlipidemic with triglycerides (TG) > 200 mg/dL or LDL > 160 mg/dL for more than three months. The exclusion criteria were age < 18 years, pregnancy or breastfeeding status, thrombocytopenia (platelet count < 100,000/mm3), or bleeding risk. Diabetes mellitus was defined as hemoglobin A1c (HbA1c) > 6.5%.
Also excluded from the study were patients with renal failure who had received a transplant or were undergoing hemodialysis or peritoneal dialysis, patients receiving chemotherapy for a malignancy, those who had a blood transfusion in the past two weeks or intravenous medication (such as a lipid-based nutritional supplement, propofol, dopamine, methotrexate, fluorouracil, vancomycin, prednisolone, furosemide, or cyclosporine), and those who were unwilling to sign the study agreement. A total of 45 patients were included.
Demographic and clinical data such as gender, comorbidity of diabetes mellitus, and age were obtained from medical records, and laboratory parameters were gathered prior to treatment. Blood samples were collected at a teaching hospital in northern Taiwan before and two weeks after DF. Serum complete blood count and differential count (CBC/DC) were checked, as was biochemical profile (lipid profile, liver function, renal function, uric acid, sugar, hemoglobin A1c, and high-sensitivity C-reactive protein (hsCRP). The hemogram auto-analyzer was SYSMEX XE2100 (Sysmex, Kobe, Japan). The biochemical parameters were determined by ADVIAR 1800 Chemistry System, Siemens, Germany. Our study used isotope dilution high-performance liquid chromatography coupled with mass spectrometry (IDLCMS) to quantify the level. The methods are listed in Appendix A.1 [18].
2.2. Double Filtration (DF) Protocol
The DF session was carried out on a Plasauto with a Plasmaflo OP-08W + Cascadeflo EC30W (Asahi Kasei Medical, Tokyo, Japan). The sieving coefficients of the different filters are shown in Appendix A.2. The two filters differed in pore size; the first was larger, mainly for blood cell separation. The second filter with smaller pores was used to filter larger molecules in serum, such as lipoprotein [21]. Plasma rejection during the DF session varied from 5 to 15% of the treated plasma volume as a function of the secondary filter transmembrane pressure. The total time of each session was around 1–2 h. The main material for the first and second filter was polyvinyl alcohol.
The volume of plasma treated was equal to 1.5 times the plasma volume, calculated using the Kaplan formula: PV = (0.065 × weight (kg)) × (1 − hematocrit). Blood flow was drained at 60–100 mL/min via an 18-gauge needle from one brachial vein and returned to the other brachial vein. Plasma separation was ~25% of maximum blood-flow rate. Extracorporeal anticoagulation was based on heparin, with 2000–3000 units used at the beginning of treatment and 20–40 units/kg/h used during treatment.
2.3. Statistical Analysis
Continuous data were expressed as the mean ± standard deviation. Statistical analyses were performed using SPSS 20.0 for Windows (web version). The paired t-test was employed to compare differences before and after DF. The χ2 test was conducted for categorical variables, and the t-test was used for continuous variables. Distributions of continuous variables in groups were expressed as means ± standard deviations. Non-normally distributed data were expressed as medians and interquartile ranges. A p-value < 0.05 was considered statistically significant (indicated with an asterisk in the figures and tables). The sample size was determined based on an effect size to detect differences in different groups. If we permitted a 5% chance of a type I error (α = 0.05), with a power of 90%, and assumed the differences among the target values before and after DF were at least equal to the standard deviation, then approximately 21 patients would be required; 45 patients were enrolled and completed the study.
3. Results
As mentioned previously, 45 patients completed the study. Demographic data are listed in Table 1. Of the patients, 11 had DM, and 34 did not. No sex or age differences were noted for these two groups, except that DM patients had a much higher body weight and higher body mass index (BMI). The differences in laboratory profiles before and after DF are listed in Table 2.

Table 1.
Demographic data of participants.

Table 2.
Biochemical profile before and after DF, and linear regression of changed values with PFOS.
3.1. DF Effect on Lipid Profile
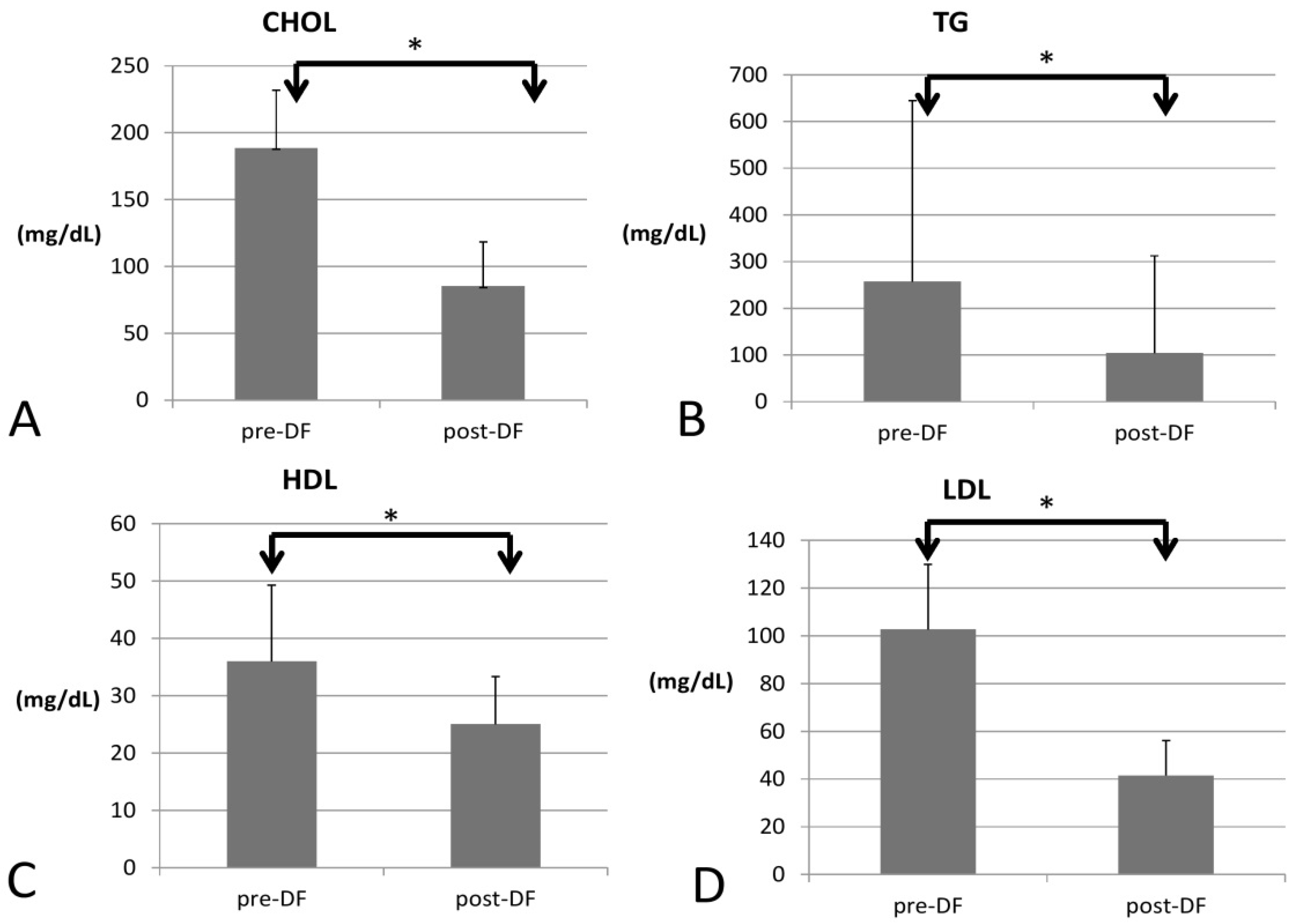
All lipid profile components were significantly lower after DF, including total cholesterol (188 to 85 mg/dL, p < 0.001), triglycerides (TG, 257 to 104 mg/dL, p < 0.001), high-density lipoprotein (HDL, 35 to 25 mg/dL, p < 0.001), low-density lipoprotein (LDL, 102 to 41 mg/dL, p < 0.001), and the marker of coronary artery disease (CAD): total cholesterol/HDL (5.77 to 3.3, p < 0.001, Figure 1).
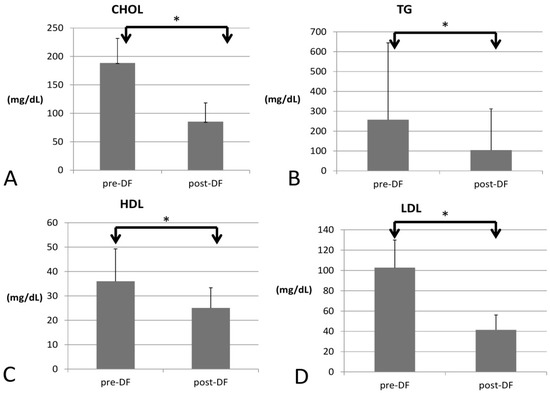
Figure 1.
Changes in the lipid profile, including (A) total cholesterol (CHOL), (B) triglycerides (TG), (C) high-density lipoprotein (HDL), and (D) low-density lipoprotein (LDL) before and after DF (all showed significant decrease). (* for p < 0.05).
3.2. DF Effect on Complete Blood Count (CBC)
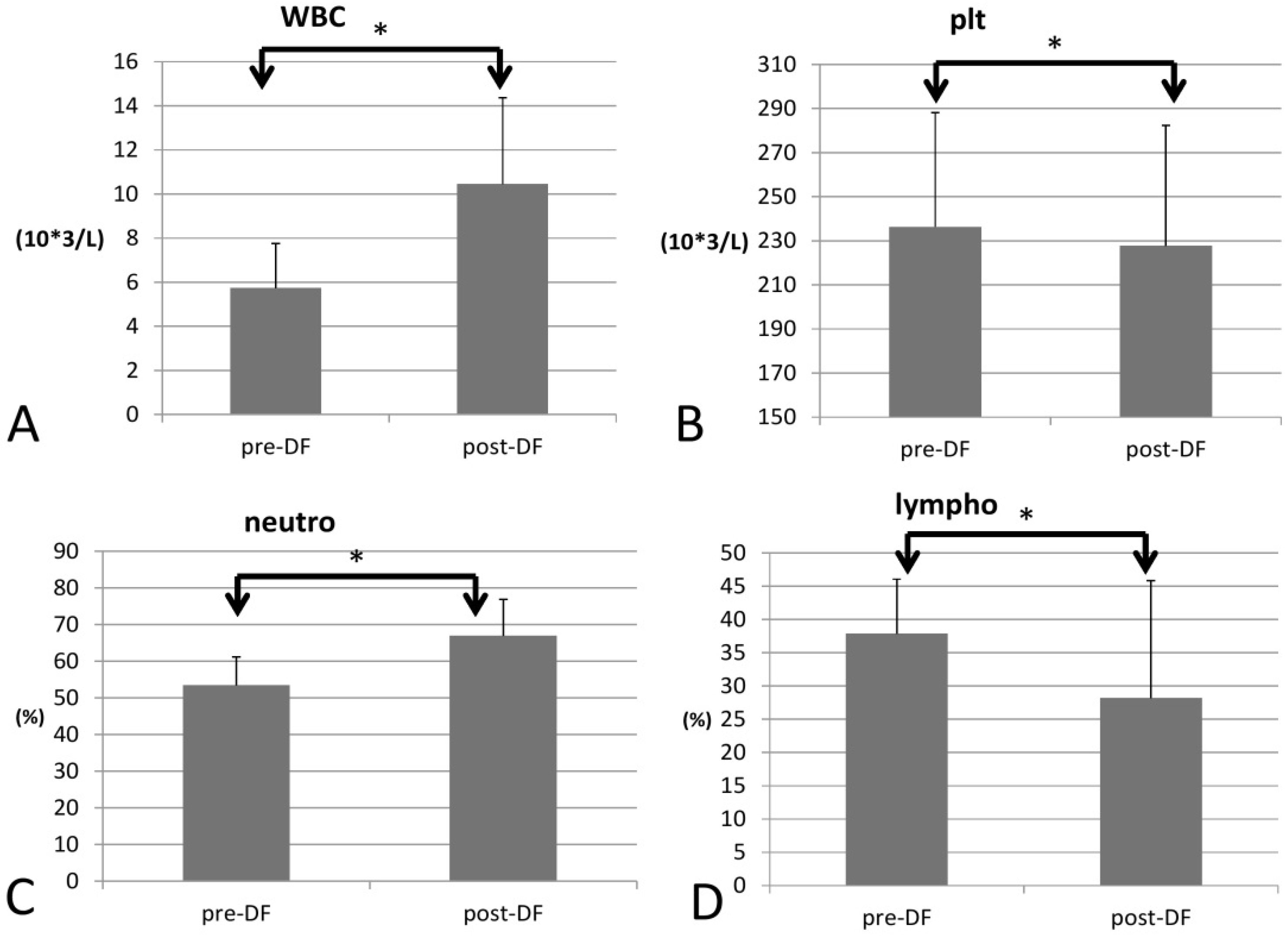
Regarding blood cell count, white blood cell (WBC) count increased significantly (p = 0.033), as did the red blood cell count (RBC), Hb, hematocrit (Hct), and mean corpuscular hemoglobin (MCH) (p < 0.001 for all four components) For the differential count (DC), neutrophils rose significantly (53 to 66%, p < 0.001), while platelet decreased (p = 0.022) and all other types of WBCs decreased after DF (lymphocytes, p = 0.001; monocytes, p = 0.006; eosinophils, p < 0.001; and basophils, p = 0.004, Figure 2).
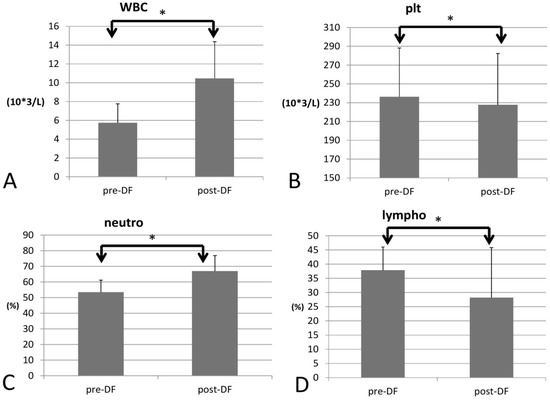
Figure 2.
Changes in complete blood count (CBC) with increase of WBC (A) and decrease of platelet (B) and differential count (DC) with increase of neutrophil (C) and decrease of lymphocyte (D) before and after DF. (* for p < 0.05).
3.3. DF Effect on Liver Function, Uric Acid, Inflammation, and PFOS
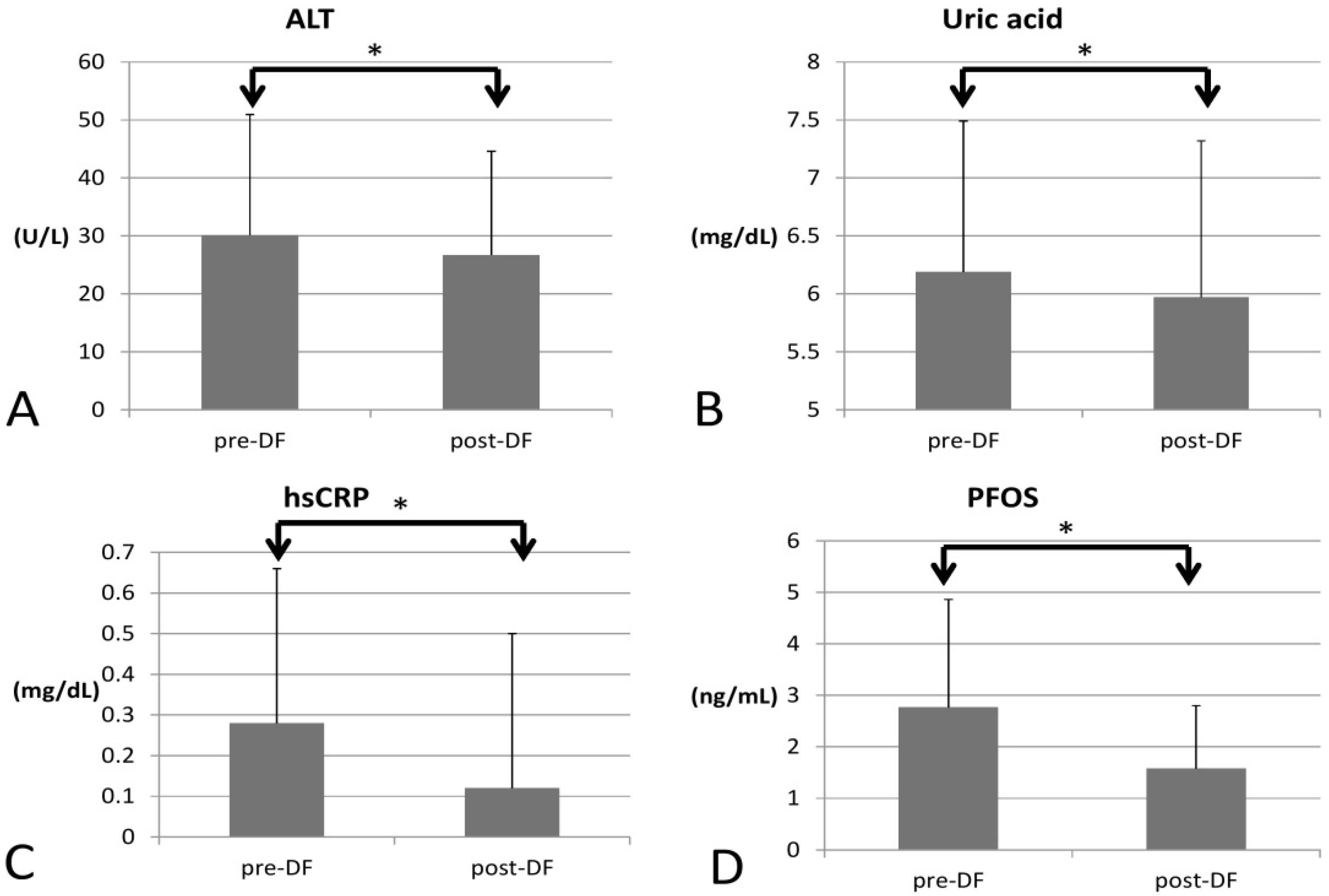
In terms of inflammation, CRP decreased significantly (0.28 to 0.12, p < 0.001), so the inflammation likely subsided (Figure 3).
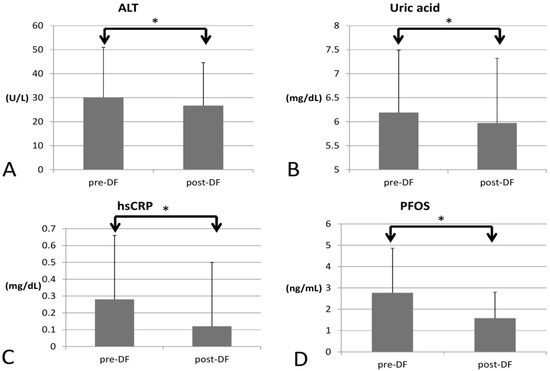
Figure 3.
Changes in inflammatory marker, PFOS, uric acid, and liver function before and after DF (all showed significant decrease). (A) alanine transaminase (ALT), (B) Uric acid (C) hsCRP (D) perfluorooctane sulfonate (PFOS). (* for p < 0.05).
As for environmental toxins, PFOS decreased significantly (2.774 to 1.605, p = 0.016) and could be removed by DF. However, PFOA was not significantly decreased (3.704 to 3.717, p = 0.847). Serum creatinine significantly decreased (0.87 to 0.81 p = 0.005), implying better renal function and hence higher estimated glomerular filtration rate (eGFR, 102 to 115 mL/min, p = 0.027). Uric acid (6.19 to 5.97 mg/dL, p = 0.012) and ALT (30 to 26 U/L, p < 0.001) both decreased significantly, which may have resulted from an improvement in renal and liver function after toxins were removed. Over the two-week process, there were no significant changes in AST (p = 0.729), HbA1c (p = 0.929), or insulin (p = 0.933).
3.4. Comparison of DF Effect on DM and Non-DM Patients
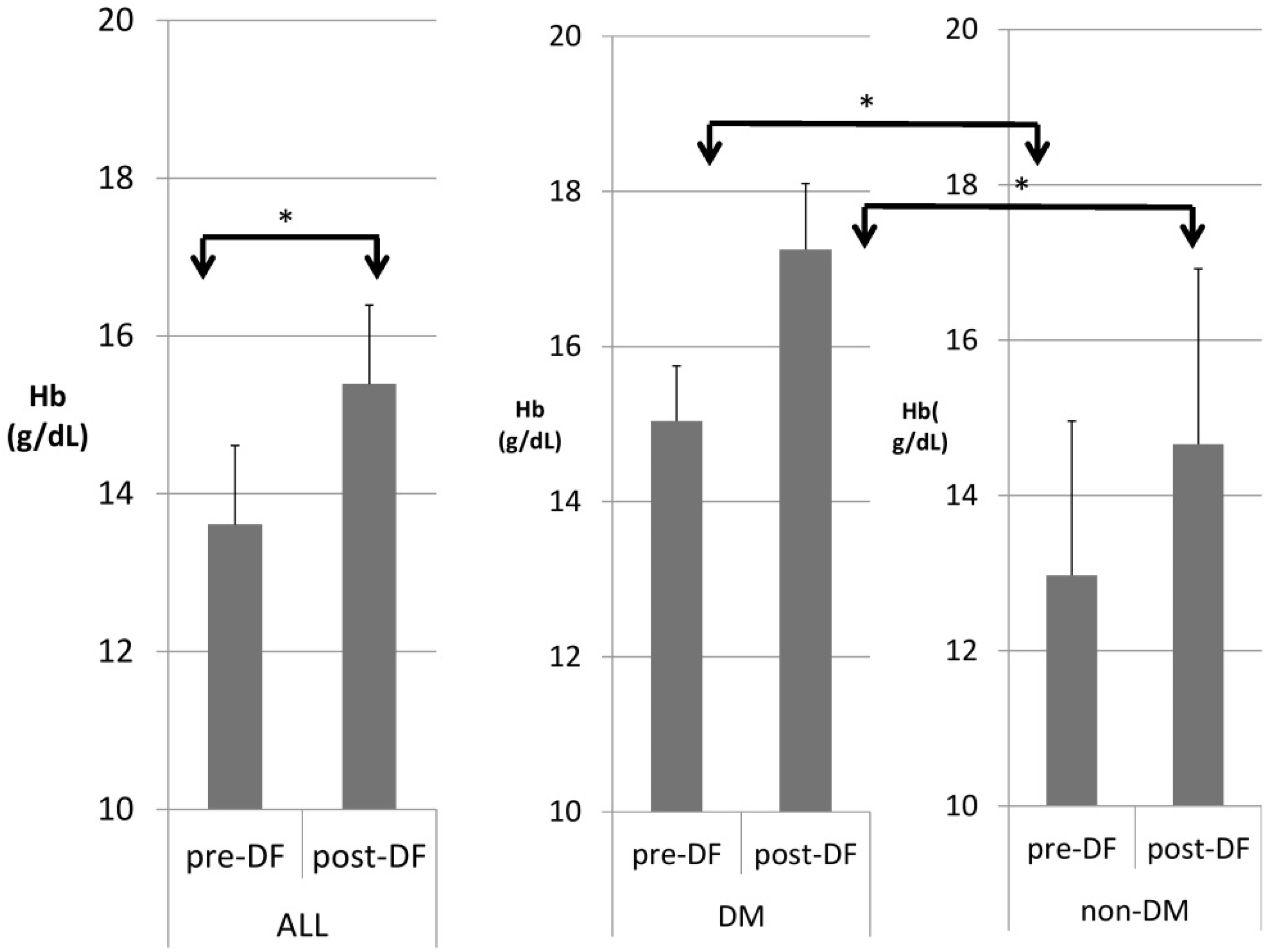
Table 3 compares patients with and without DM. DM patients were heavier in body weight and had higher hemoglobin (Figure 4), hematocrit, serum glucose, HbA1c, insulin, ALT, and triglycerides, but significantly lower HDL and LDL compared with nondiabetic patients. A similar pattern was found after DF except for TG and T chol/HDL.

Table 3.
Comparison of DM (n = 11) vs. non-DM (n = 31) patients before and after DF.
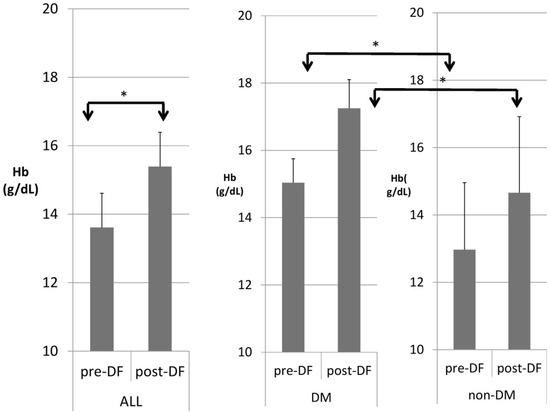
Figure 4.
Subgroup comparison of DM and non-DM patients in hemoglobin (Hb). (* for p < 0.05).
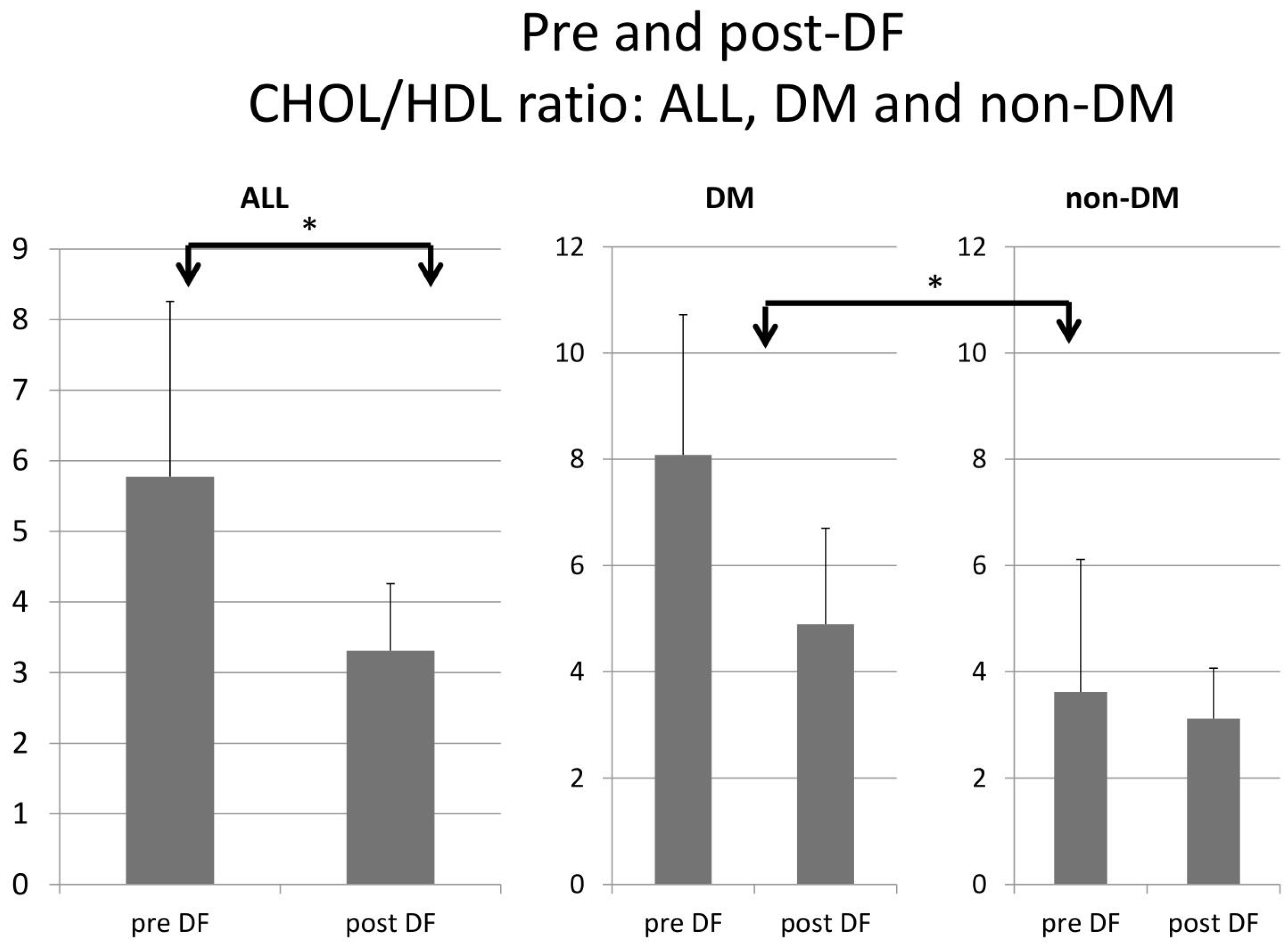
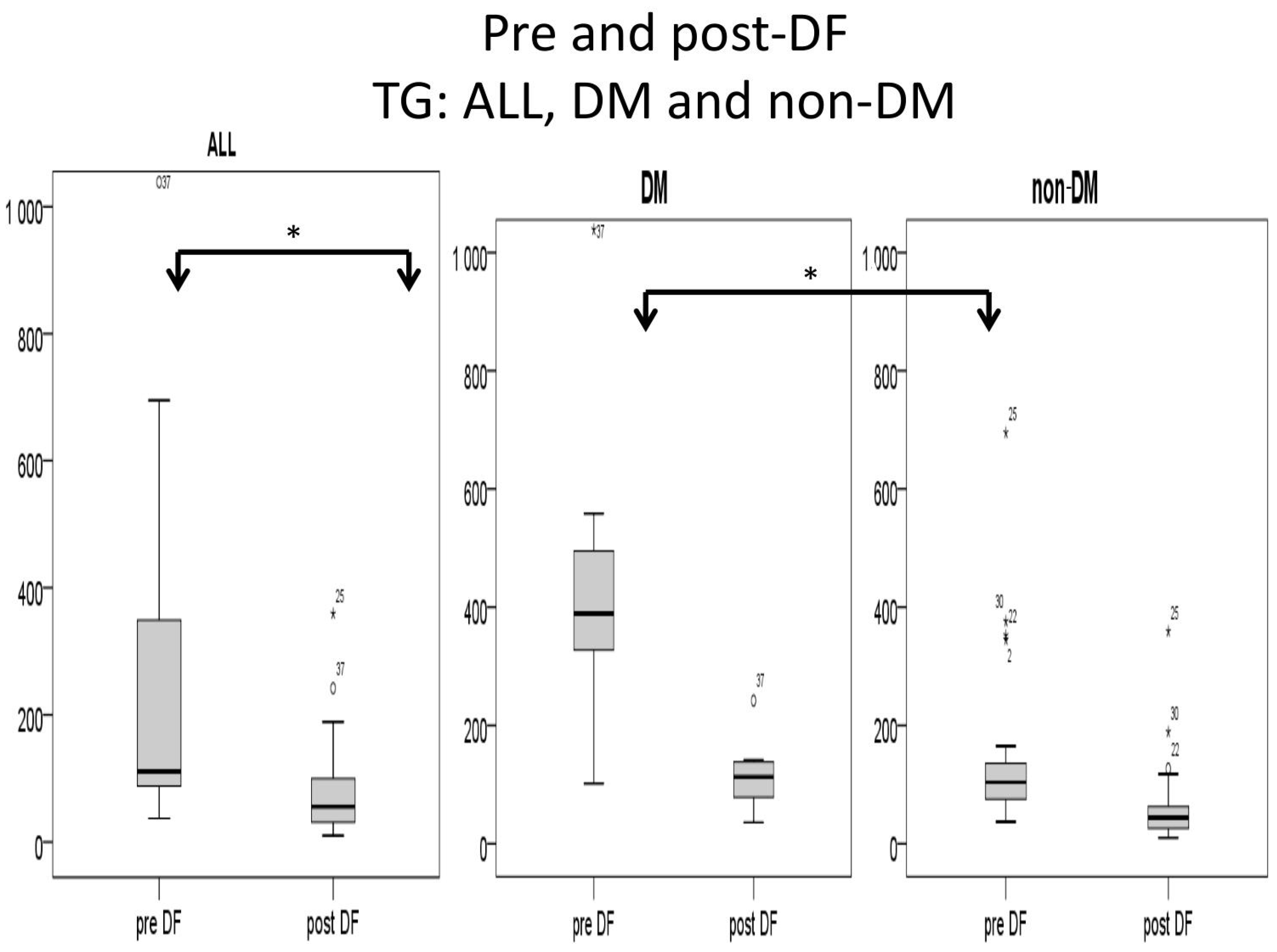
Higher TG and total cholesterol/HDL ratio were noted in the DM group before DF, but the difference became insignificant after DF (Figure 5 and Figure 6).
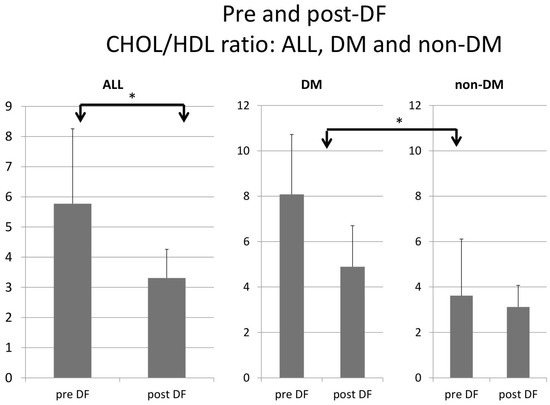
Figure 5.
Subgroup comparison of DM and non-DM patients in total cholesterol to HDL ratio. (* for p < 0.05).
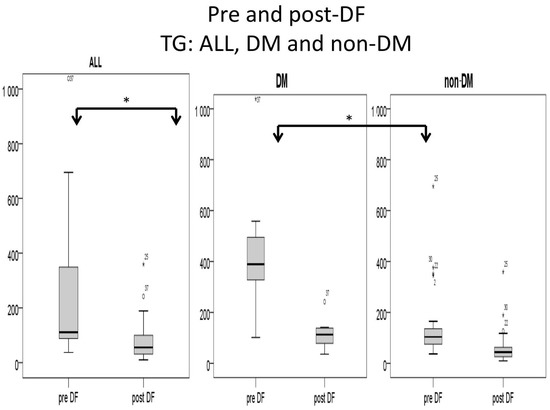
Figure 6.
Subgroup comparison of triglyceride (TG) levels (mg/dL) for DM and non-DM patients. (* for p < 0.05).
4. Discussion
WBC and neutrophil counts were significantly elevated, most likely due to the active stress induced by the puncture of vessels. DF required one puncture hole in a vein of each arm, with one site indicated for draining blood and the other site for returning the filtrated blood. DF may also concentrate the blood, activate WBC proliferation, and result in higher hematocrit (Table 2). The only blood cell type to decrease was platelets, which significantly dropped after DF because of the consumption of coagulation factor during DF [21].
Lower LDL may contribute to reduced inflammation via less arthrosclerosis. All lipid profile components were decreased after DF, such as total cholesterol, triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). This implies that DF removed all the lipid profile components and decreased the ratio of total cholesterol/HDL, which may further lower the risk of CVD [22]. Lower LDL may also mean lower inflammation and better physical status and mood [23].
Creatinine was significantly decreased (0.87 to 0.81, p = 0.005). The reductions in inflammation and PFOS may contribute to better renal function and lower serum creatinine [12]. By removing environmental toxins (PFOS), DF may further improve renal and liver function, with decreased serum uric acid levels and ALT. Previous studies have shown that higher PFOS levels may induce an increase in uric acid and liver function markers [24]. Improved liver function (ALT) may have resulted from the removal of PFC [20].
The decrease in PFOS was highly correlated with the change in PFOA (p = 0.008). This relationship may imply that the two are removed by the same mechanism. The change in LDL, on the other hand, was not correlated with the change in PFOS, even though these two factors both decreased. Our hypothesis is that the removal of PFCs and lipids probably occurs via different mechanisms [20]. Meanwhile, higher WBCs may indicate higher blood viscosity (p = 0.03) and cause poor removal of PFOS due to poorer clearance [25].
For the DM versus non-DM comparison, diabetes patients were heavier in body weight, with higher hemoglobin and hematocrit, which may indicate an elevated risk of cardiovascular diseases due to an increased thrombosis risk (Table 1 and Table 3). Serum glucose, HbA1c, and insulin levels were higher in diabetic patients compared with nondiabetic patients, as expected. Higher liver function test results (ALT) and triglycerides (TG) were also noted in the diabetic group, which might relate to glycogen formation in the liver [26]. However, lower HDL and LDL also were noted in the DM group. The lipid profiles before and after DF, triglycerides, and T chol/HDL were higher in the DM group before DF, but the difference became insignificant after DF (Figure 4 and Figure 5). This finding may indicate similar CVD risks between both groups after DF [22]. Whether diabetes patients may benefit more from DF still needs more investigation.
Despite the small number of participants and short experimental period, we reached some interesting findings. Serum PFCs can be successfully removed by DF. Whether DF can remove other lipid-soluble toxins and whether PFOA can be removed under repetitive DF remain unclear. The percentage of each DF session for PFCs still needs to be verified.
Other blood purification procedures are worthy of future investigation. Through plasma exchange with the transfusion of plasma from healthy donors, disease-causing factors may be removed, such as autoantibodies in autoimmune diseases [27]. Hemoperfusion is an add-on procedure that lets blood flow into a hydrophobic filter (which normally contains active charcoal) before hemodialysis [28]. The target particles for removal are absorbable toxins. The procedure has proven to be beneficial in certain conditions, such as medication-related suicidal intoxication [29]. Further investigations of different dialysis modalities’ ability to remove environmental toxins are indicated.
5. Conclusions
This is the first study to show that DF may remove serum PFOS, with improved renal function, liver function, and inflammatory status.
Author Contributions
Conceptualization, W.-S.L., K.-H.T. and H.-H.T.; methodology, H.-H.T.; software, C.-H.L.; validation, C.-Y.T., S.-Y.L. and T.-Y.L.; formal analysis, W.-S.L.; investigation, W.-S.L.; resources, T.-Y.L.; data curation, H.-H.T.; writing—original draft preparation, W.-S.L.; writing—review and editing, A.C.T.; visualization, H.-T.W. and W.-S.L.; supervision, C.-C.L.; project administration, C.-C.L.; funding acquisition, W.-S.L. and C.-Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taipei City Hospital (TCHIRB-11103030-E) and TCHIRB 11106016.
Institutional Review Board Statement
All patients provided written informed consent to participate. Institutional Review Board (IRB)/Ethics Committee approval was obtained before the trial began, and the study was conducted in full compliance with the Declaration of Helsinki. The study was conducted and approved by Taipei City Hospital, Taipei, Taiwan. (TCHIRB-11103030-E) 28 April 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Measurement of PFOA and PFOS by Isotope Dilution High Performance Liquid Chromatography Coupled with Mass Spectrometry (IDLCMS)
All blood samples were stored before analysis at –80 °C. The method for analysis of PFCs was as following: The frozen serum was thawed at 4 °C and vortex mixed for 30 s to reach homogeneity. A serum sample of 50 µL in a polypropylene centrifuge tube was then vortexed with 50 µL of 1% formic acid (pH 2.4) for 30 s. Afterwards, 1 µL of 10 µg/mL internal standard solution (13C4-PFOA and 13C4-PFOS, Wellington Laboratories Inc., Guelph, ON, Canada) and 40 µL of acetonitrile were added to each sample before further vortexing. These samples were sonicated for 20 min and centrifuged at 18,000× g for 20 min. These supernatants were collected and filtered through a 0.22 µm polyether sulfone syringe filter to a screw cap vial.
In this study, the LC-MS/MS system used comprised an Agilent 1100 series system (Agilent Tech., Santa Clara, CA, USA), coupled to a Finnigan TSQ Quantum Discovery Max spectrometer system (Thermo Electron Corporation, Breda, The Netherlands). The electron spray ionization source was in negative ion mode. LC-MS/MS and isotope dilution were carried out simultaneously for quantification of PFC.
A sample of serum (5 µL) was injected onto a 2.0 × 150 mm Capcell Pak® 3 µm C18 column (Shiseido Co., Tokyo, Japan). The mobile phases consisted of 10 mM ammonium acetate in water (A) and pure acetonitrile (B), delivered at a constant flow rate of 0.2 mL/min. After the injection, the mobile phase was kept for 3 min at 30% B. Then, the gradient of B was increased to 65% in 3 min. Later, the gradient of B was gradually increased 100% in 5 min to 100% B, where it was kept for 7 min. The column was set at 30% B for 1.5 min.
Optimized mass spectrometry parameters are listed below: spray ion voltage 3000 V, capillary temperature 210 °C, sheath gas pressure 10 arbitrary units, auxiliary gas pressure 5 arbitrary units, ion sweep gas pressure 4 arbitrary units, collision gas pressure 1.0 mTorr, and dwell time 100 msec. The detection was carried out in selective reaction monitoring (SRM) mode. The collision energy (V) and SRM transitions monitored were as follows: 10 V, m/z 413→369 for PFOA; 12 V, m/z 417→372 for 13C4-PFOA; 40 V, m/z 499→80 for PFOS; 40V, m/z 503→80 for 13C4-PFOS. We used LC-MS/MS to quantify PFOA (m/z 413→369), PFOS (m/z 499→80) as the main particles for PFOA and PFOS for further analysis. [10]
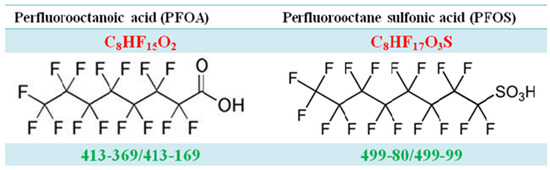
Figure A1.
The molecular structure of PFOA and PFOS.
Figure A1.
The molecular structure of PFOA and PFOS.
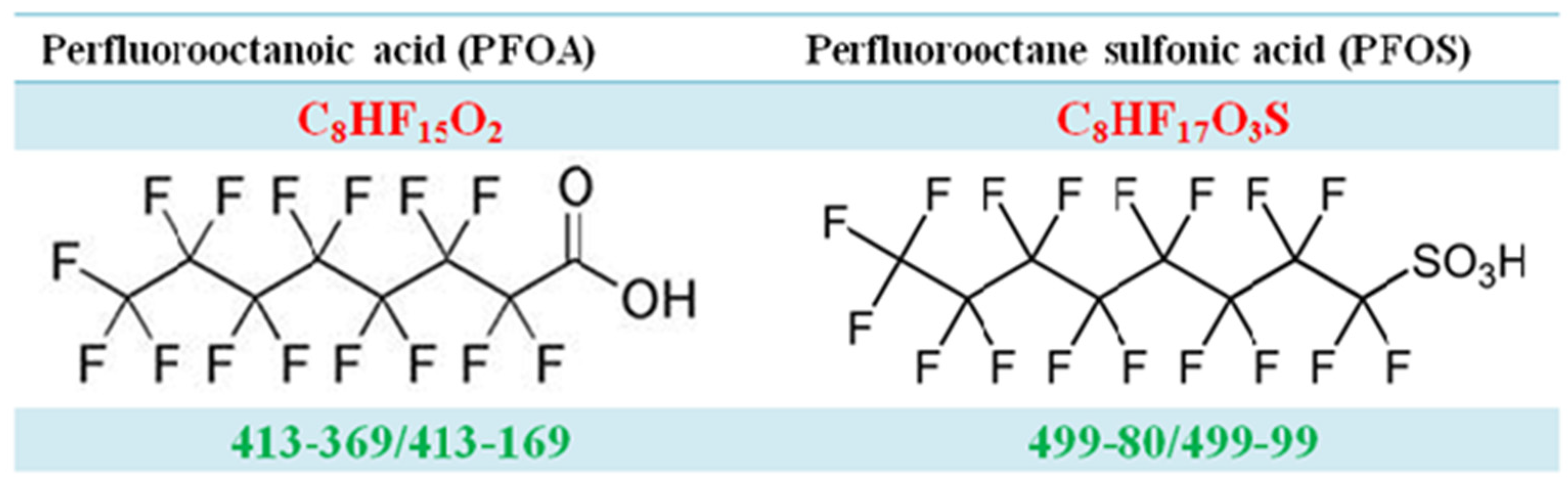
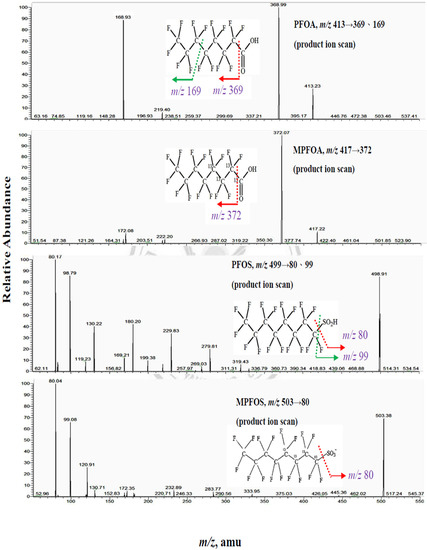
Figure A2.
The product ion scans of PFOA and PFOS.
Figure A2.
The product ion scans of PFOA and PFOS.
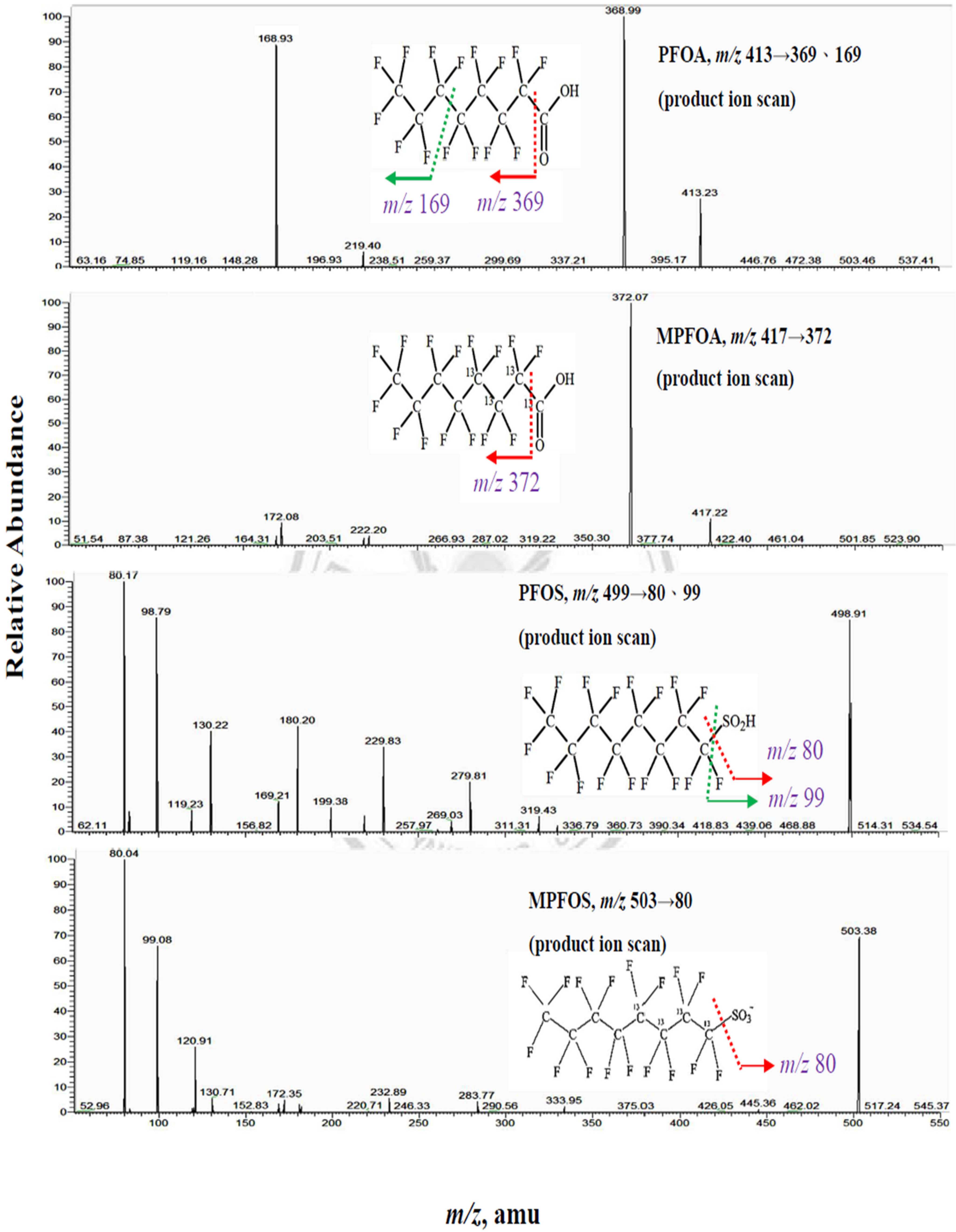
Appendix A.2. Process for Double Filtration Plasmaphresis (DFPP), Which Means the Same as Double Filtration (DF) in Our Manuscript (Copyright: Asahi Kasei Medical, Tokyo, Japan)
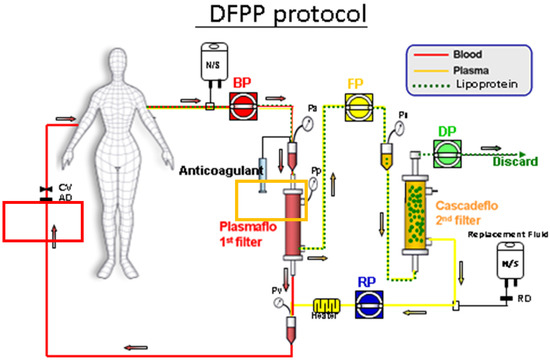
Figure A3.
The position of 1st filter and 2nd filter, both made of polyvinyl alcohol. 1st filter: For removing blood cells from blood and serum, was directed to 2nd filter. 2nd filter: For removing serum lipoprotein from serum and clean filtrate, was returned (after mixing with blood cells) into human body.
Figure A3.
The position of 1st filter and 2nd filter, both made of polyvinyl alcohol. 1st filter: For removing blood cells from blood and serum, was directed to 2nd filter. 2nd filter: For removing serum lipoprotein from serum and clean filtrate, was returned (after mixing with blood cells) into human body.
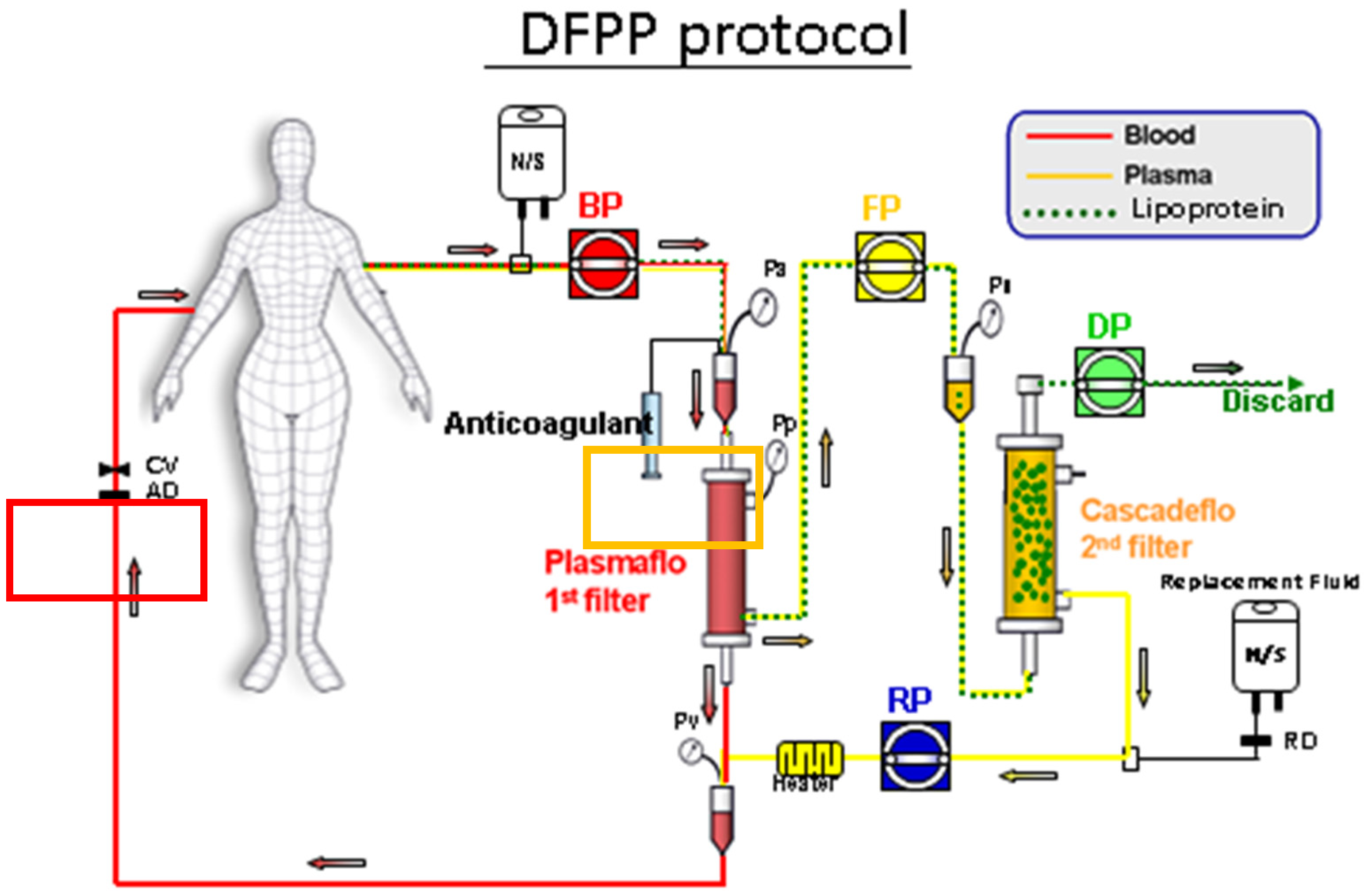
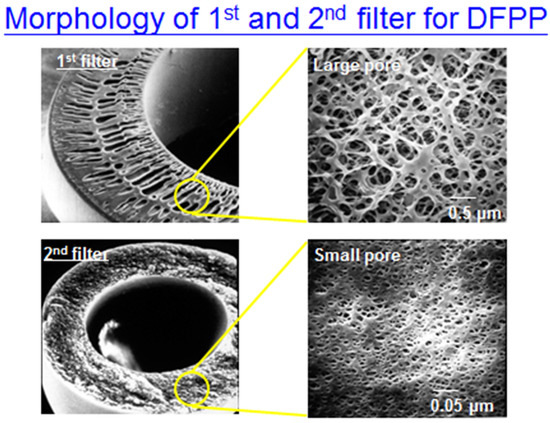
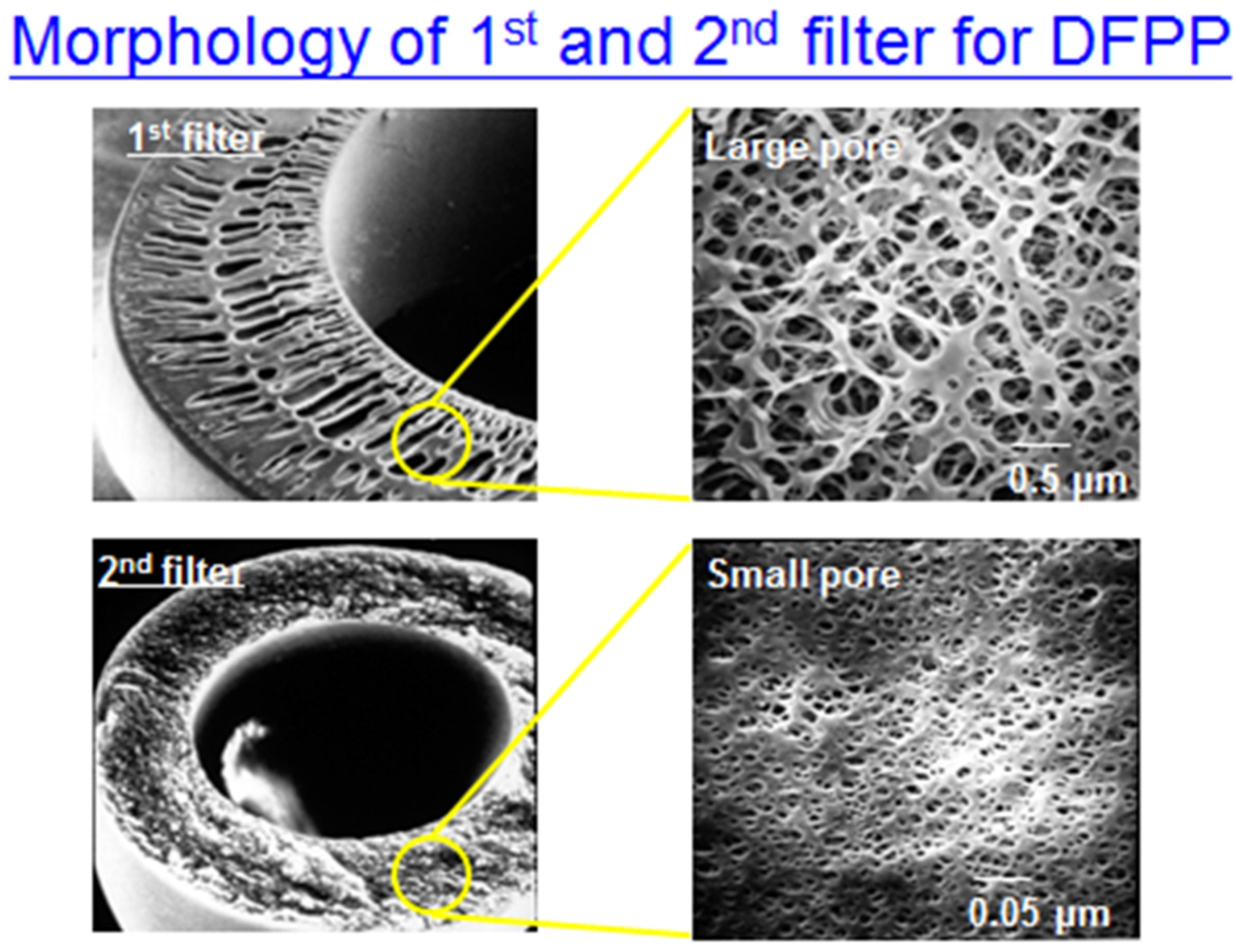
Figure A4.
The morphology of 1st filter and 2nd filter (1st filter with larger pores compared to 2nd filter).
Figure A4.
The morphology of 1st filter and 2nd filter (1st filter with larger pores compared to 2nd filter).
References
- Gotto, A.M., Jr. Treatment of hyperlipidemia. Am. J. Cardiol. 1986, 57, G11–G16. [Google Scholar] [CrossRef]
- Fernandez-Fuertes, L.F.; Tapia Martin, M.; Nieves Pla, I.; Novoa Mogollon, F.J.; Diaz Cremades, J. Low-density lipoprotein apheresis using double filtration plasmapheresis: 27-month use in a child with homozygous familial hypercholesterolemia. Ther. Apher. Dial. 2010, 14, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.L.; Wu, M.J.; Shu, K.H.; Tsai, S.F. Long-Term Follow-Up of a Homozygous Familial Hypercholesterolemic Patient Receiving Regular Double Filtration Plasmapheresis-Case Report and Literature Review. Blood Purif. 2016, 41, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Szczesniak, A.; Kolodziejczak, M.; Gorny, B.; Kubica, J.; Suryapranata, H. Statins and risk of new-onset diabetes mellitus: Is there a rationale for individualized statin therapy? Am. J. Cardiovasc. Drugs 2014, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Brinton, E.A. Does the addition of fibrates to statin therapy have a favorable risk to benefit ratio? Curr. Atheroscler. Rep. 2008, 10, 25–32. [Google Scholar] [CrossRef]
- Wanner, C.; Schmidt, K.R.; Krane, V. Results of the 4D study: Ten years of follow-up? Clin. Exp. Nephrol. 2014, 18, 274–277. [Google Scholar] [CrossRef]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Johnson, D.W.; Perkovic, V.; Nigwekar, S.U.; Hegbrant, J.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane. Database Syst. Rev. 2013, 12, CD004289. [Google Scholar] [CrossRef]
- Buemi, M.; Floccari, F.; Nostro, L.; Campo, S.; Caccamo, C.; Sturiale, A.; Aloisi, C.; Giacobbe, M.S.; Frisina, N. Statins in the prevention of cardiovascular events in patients with renal failure. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 7–13. [Google Scholar]
- Demetriou, K.; H’Maltezou, E.; Pierides, A.M. Familial homozygous hypercholesterolemia: Effective long-term treatment with cascade double filtration plasmapheresis. Blood Purif. 2001, 19, 308–313. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Qiao, X.; Duo, Y.; Xu, J.; Peng, Z.; Zhang, J.; Chen, Y.; Nie, X.; Sun, Q.; et al. ALT/AST as an Independent Risk Factor of Gestational Diabetes Mellitus Compared with TG/HDL-C. Int. J. Gen. Med. 2022, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Lai, Y.T.; Chan, H.L.; Li, S.Y.; Lin, C.C.; Liu, C.K.; Tsou, H.H.; Liu, T.Y. Associations between perfluorinated chemicals and serum biochemical markers and performance status in uremic patients under hemodialysis. PLoS ONE 2018, 13, e0200271. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.A.; Arbuckle, T.E.; Lye, E.; Legrand, M.; Fisher, M.; Langlois, R.; Fraser, W. Reporting results of human biomonitoring of environmental chemicals to study participants: A comparison of approaches followed in two Canadian studies. J. Epidemiol. Community Health 2011, 65, 191–198. [Google Scholar] [CrossRef]
- Fromme, H.; Tittlemier, S.A.; Völkel, W.; Wilhelm, M.; Twardella, D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [Google Scholar] [CrossRef] [PubMed]
- Powley, C.R.; Michalczyk, M.J.; Kaiser, M.A.; Buxton, L.W. Determination of perfluorooctanoic acid (PFOA) extractable from the surface of commercial cookware under simulated cooking conditions by LC/MS/MS. Analyst 2005, 130, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 189–195. [Google Scholar] [CrossRef]
- Renner, R. Growing concern over perfluorinated chemicals. Environ. Sci. Technol. 2001, 35, 154A–160A. [Google Scholar] [CrossRef]
- Frisbee, S.J.; Shankar, A.; Knox, S.S.; Steenland, K.; Savitz, D.A.; Fletcher, T.; Ducatman, A.M. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 Health Project. Arch. Pediatr. Adolesc. Med. 2010, 164, 860–869. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Liu, W.S.; Chan, H.L.; Lai, Y.T.; Lin, C.C.; Li, S.Y.; Liu, C.K.; Tsou, H.H.; Liu, T.Y. Dialysis Membranes Influence Perfluorochemical Concentrations and Liver Function in Patients on Hemodialysis. Int. J. Environ. Res. Public Health 2018, 15, 2574. [Google Scholar] [CrossRef]
- Naciri Bennani, H.; Marlu, R.; Terrec, F.; Motte, L.; Seyve, L.; Chevallier, E.; Malvezzi, P.; Jouve, T.; Rostaing, L.; Noble, J. How to improve clotting factors depletion in double-filtration plasmapheresis. J. Clin. Apher. 2021, 36, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Min, Y.; Qi, B.; Zhu, C.M.; Chen, J.H.; Deng, G.X.; Wang, Y.; Li, J.F.; Li, R.S. Causal effect between total cholesterol and HDL cholesterol as risk factors for chronic kidney disease: A mendelian randomization study. BMC Nephrol. 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Teng, H.W.; Lai, Y.T.; Li, S.Y.; Lin, C.C.; Yang, A.C.; Chan, H.L.; Hsieh, Y.H.; Lin, C.F.; Hsu, F.Y.; et al. Statins Reduces the Risk of Dementia in Patients with Late-Onset Depression: A Retrospective Cohort Study. PLoS ONE 2015, 10, e0137914. [Google Scholar] [CrossRef]
- Steenland, K.; Tinker, S.; Shankar, A.; Ducatman, A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ. Health Perspect. 2010, 118, 229–233. [Google Scholar] [CrossRef]
- Zarkovic, M.; Kwaan, H.C. Correction of hyperviscosity by apheresis. Semin. Thromb. Hemost. 2003, 29, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Sidhu, D.; Snyder, E.L.; Tormey, C.A. Two approaches to the clinical dilemma of treating TTP with therapeutic plasma exchange in patients with a history of anaphylactic reactions to plasma. J. Clin. Apher. 2017, 32, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T.; Inoue, T.; Yoh, K.; Shin, J.; Fujita, Y.; Yoshiya, K.; Kim, J.I.; Sakai, R.; Sekita, K.; Goto, T.; et al. Effectiveness of beta(2)-microglobulin adsorption column in treating dialysis-related amyloidosis: A multicenter study. Blood Purif. 2011, 32, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Uchita, K.; Orita, H.; Kamimura, M.; Oda, M.; Hasegawa, H.; Kobata, H.; Fukunishi, M.; Shimazaki, M.; Akizawa, T.; et al. Effect of beta(2)-microglobulin adsorption column on dialysis-related amyloidosis. Kidney Int. 2003, 64, 1522–1528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).