Systematic Myostatin Expression Screening Platform for Identification and Evaluation of Myogenesis-Related Phytogenic in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Extract Preparation and Cells Transfection
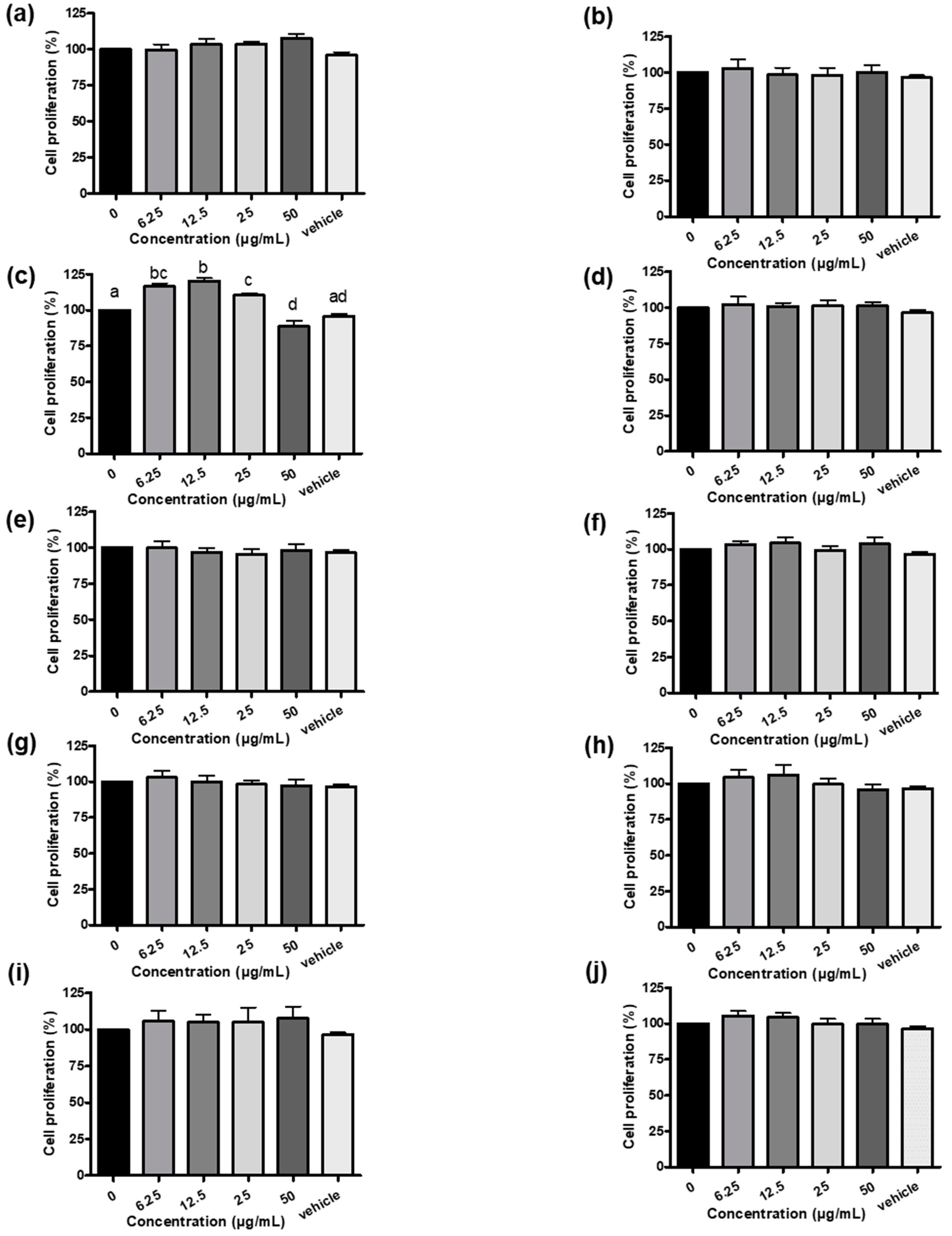
2.3. MTT Assay
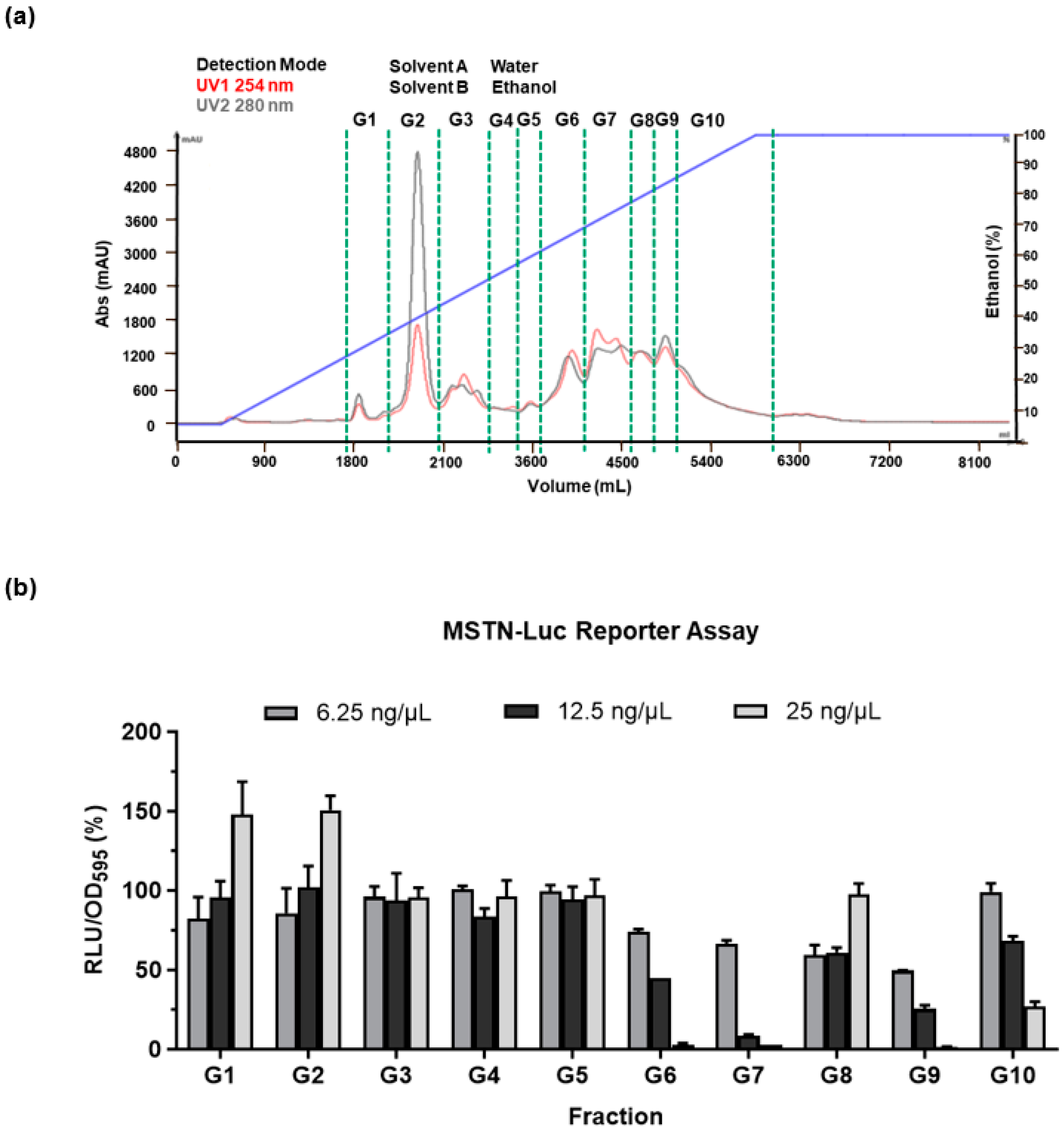
2.4. Luciferase Assays
2.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. GUE Preparation
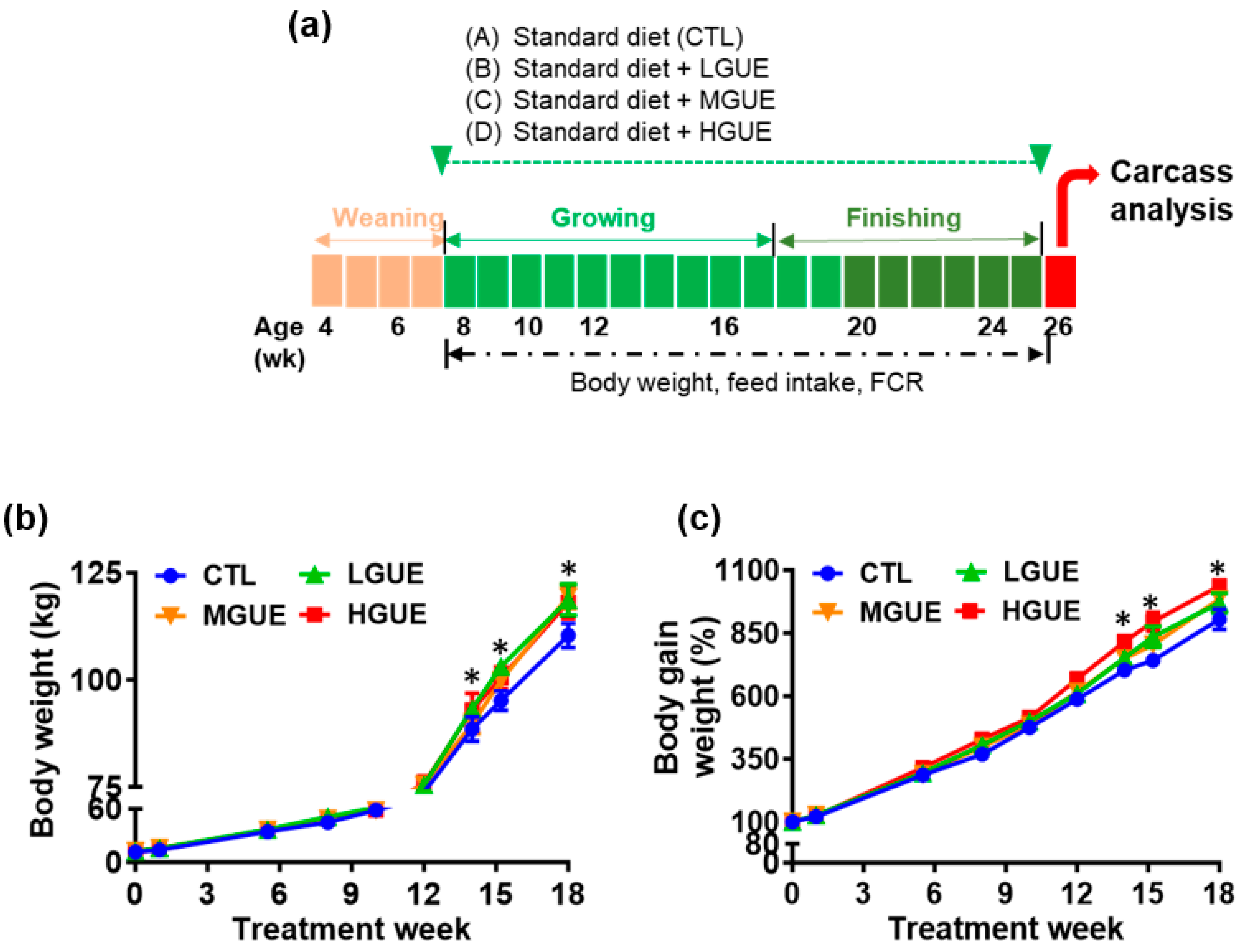
2.7. Animal Study
2.8. Pyrosequencing and Data Analysis
2.9. Statistics
3. Results
3.1. Establishment of Systematic Rodent Myoblast Screen Platform and Phytogenic Screening
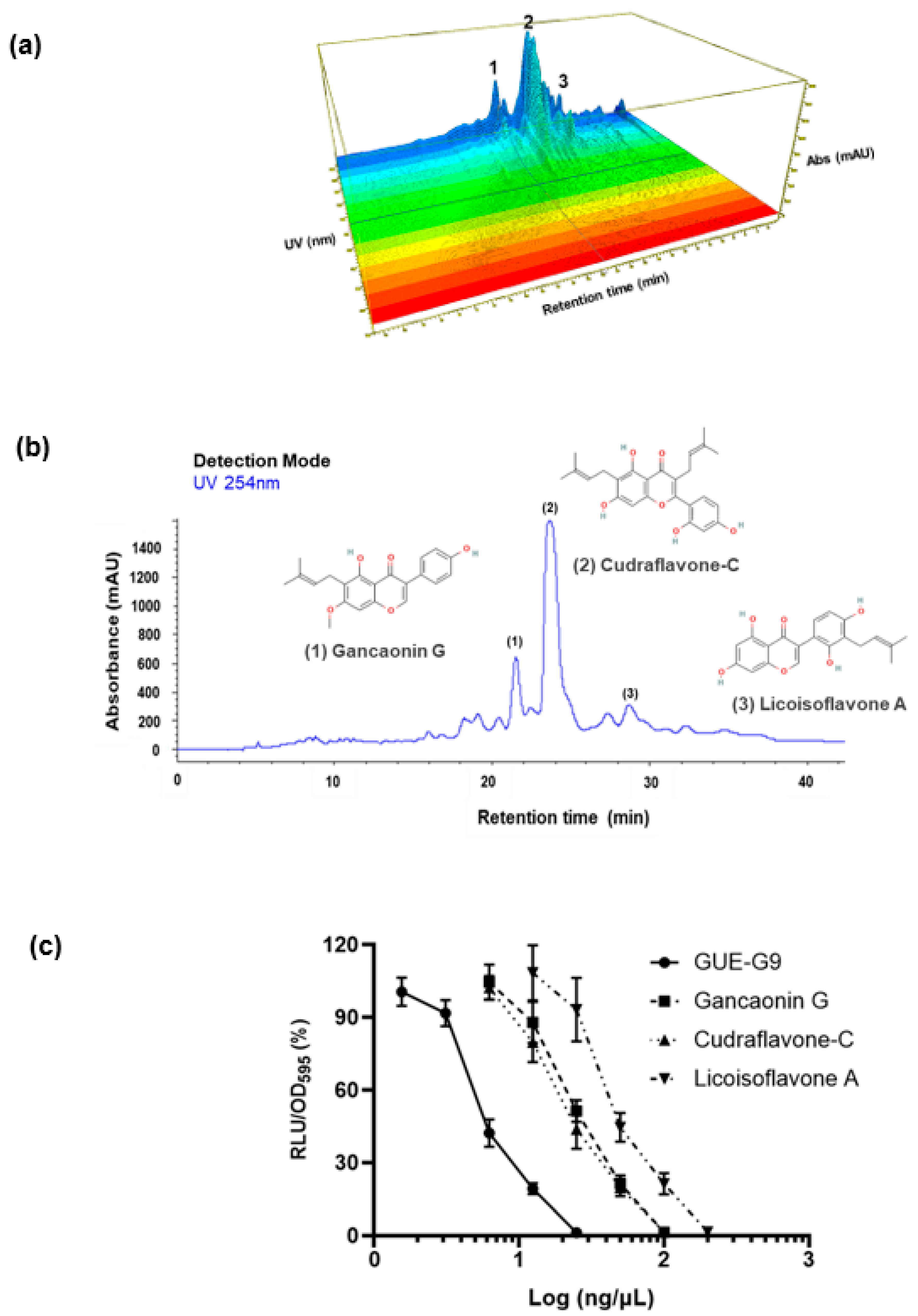
3.2. Characterization and Identification of Active Fractions in GUE
3.3. Effect of GUE as a Feed Additive on Growth Performance in Growth-Finishing Pigs
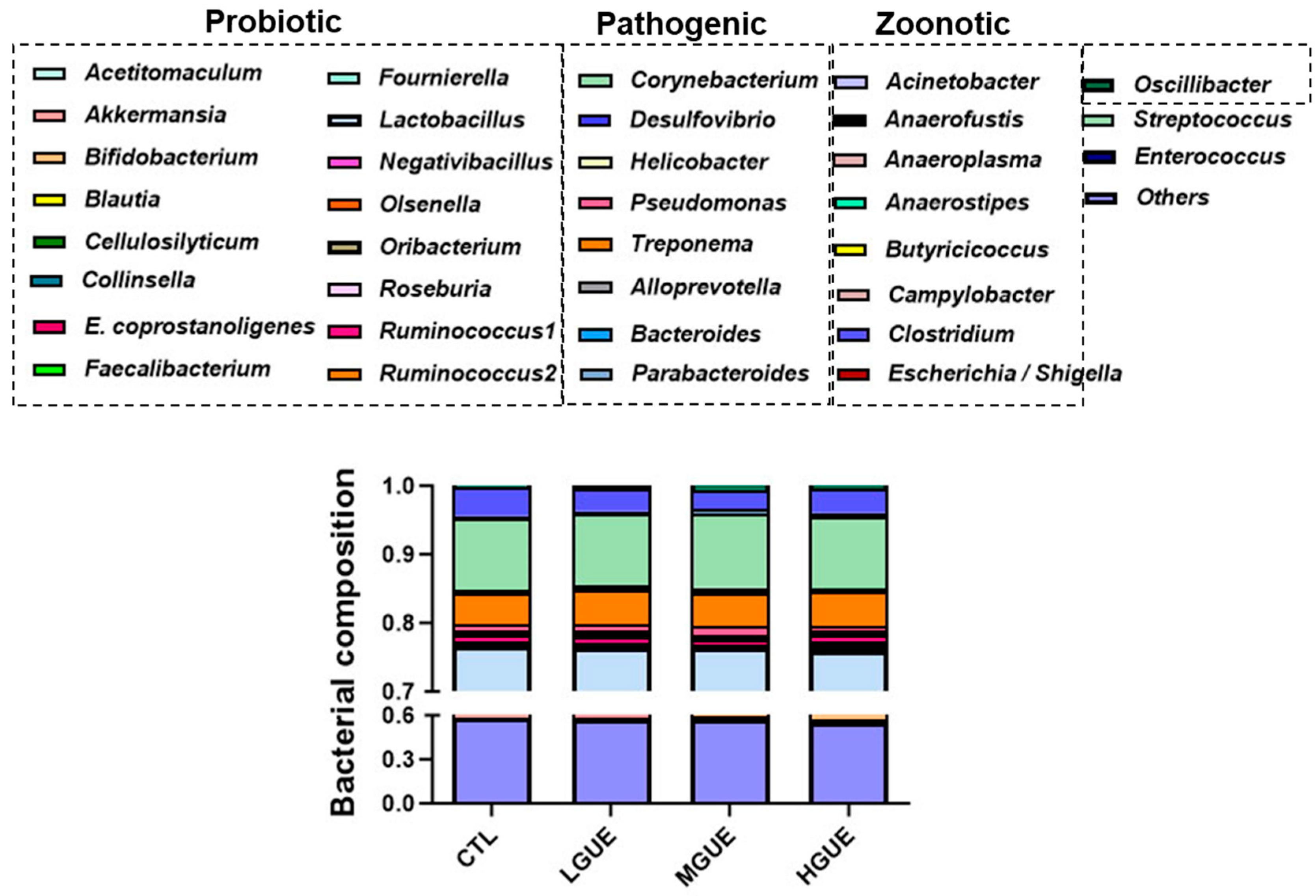
3.4. Effect of GUE as a Feed Additive on Gut Microbiota in Growth-Finishing Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.C.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell 2019, 74, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology that Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef]
- Niranjan, D.; Sridhar, N.; Chandra, U.; Manjunatha, S.; Borthakur, A.; Vinuta, M.; Mohan, B. Recent perspectives of growth promoters in livestock: An overview. J. Livest. Sci. 2023, 14, 53–64. [Google Scholar] [CrossRef]
- Elliott, C.T.; Crooks, S.R.; McEvoy, J.G.; McCaughey, W.J.; Hewitt, S.A.; Patterson, D.; Kilpatrick, D. Observations on the effects of long-term withdrawal on carcass composition and residue concentrations in clenbuterol-medicated cattle. Vet. Res. Commun. 1993, 17, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Moseley, W.M.; Paulissen, J.B.; Goodwin, M.C.; Alaniz, G.R.; Claflin, W.H. Recombinant bovine somatotropin improves growth performance in finishing beef steers. J. Anim. Sci. 1992, 70, 412–425. [Google Scholar] [CrossRef]
- Martínez-Navarro, J.F. Food poisoning related to consumption of illicit β-agonist in liver. Lancet 1990, 336, 1311. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Thomas, A.; Schiwy-Bochat, K.-H.; Geyer, H.; Thevis, M.; Glenewinkel, F.; Rothschild, M.A.; Andresen-Streichert, H.; Juebner, M. Death after misuse of anabolic substances (clenbuterol, stanozolol and metandienone). Forensic. Sci. Int. 2019, 303, 109925–109931. [Google Scholar] [CrossRef]
- Skoupá, K.; Šťastný, K.; Sládek, Z. Anabolic Steroids in Fattening Food-Producing Animals—A Review. Animals 2022, 12, 2115. [Google Scholar] [CrossRef]
- Hirpessa, B.B.; Ulusoy, B.H.; Hecer, C. Hormones and hormonal anabolics: Residues in animal source food, potential public health impacts, and methods of analysis. J. Food Qual. 2020, 2020, 5065386. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Sana, U.; Khan, R.U.; Alagawany, M.; et al. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/Extracts Countering Viral Pathogens—Current Knowledge and Future Prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Kim, I.H. Efficacy of Phytogenic Feed Additive on Performance, Production and Health Status of Monogastric Animals—A Review. Ann. Anim. Sci. 2017, 17, 929–948. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Mnisi, C.M.; Mlambo, V.; Gila, A.; Matabane, A.N.; Mthiyane, D.M.N.; Kumanda, C.; Manyeula, F.; Gajana, C.S. Antioxidant and Antimicrobial Properties of Selected Phytogenics for Sustainable Poultry Production. Appl. Sci. 2023, 13, 99. [Google Scholar] [CrossRef]
- Basiouni, S.; Tellez-Isaias, G.; Latorre, J.D.; Graham, B.D.; Petrone-Garcia, V.M.; El-Seedi, H.R.; Yalçın, S.; El-Wahab, A.A.; Visscher, C.; May-Simera, H.L.; et al. Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Vet. Sci. 2023, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.; Grześkowiak, Ł.; Martinez-Vallespin, B.; Dietz, H.; Zentek, J. Porcine and Chicken Intestinal Epithelial Cell Models for Screening Phytogenic Feed Additives—Chances and Limitations in Use as Alternatives to Feeding Trials. Microorganisms 2022, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Lee, S.-J. Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 2004, 20, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Kambadur, R.; Sharma, M.; Smith, T.P.; Bass, J.J. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997, 7, 910–915. [Google Scholar] [CrossRef]
- Grisolia, A.; D’Angelo, G.; Porto Neto, L.; Siqueira, F.; Garcia, J.F. Myostatin (GDF8) single nucleotide polymorphisms in Nellore cattle. Genet. Mol. Res. 2009, 8, 822–830. [Google Scholar] [CrossRef]
- Dilger, A.C.; Gabriel, S.; Kutzler, L.; McKeith, F.; Killefer, J. The myostatin null mutation and clenbuterol administration elicit additive effects in mice. Animal 2010, 4, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.S.; Thomas, M.; Forbes, D.; Watson, T.; Kambadur, R.; Sharma, M. Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am. J. Physiol.-Cell Physiol. 2004, 287, C1031–C1040. [Google Scholar] [CrossRef]
- Yeh, J.Y.; Cheng, L.C.; Ou, B.R.; Whanger, D.P.; Chang, L.W. Differential influences of various arsenic compounds on glutathione redox status and antioxidative enzymes in porcine endothelial cells. Cell. Mol. Life Sci. 2002, 59, 1972–1982. [Google Scholar] [CrossRef]
- Tahara, S.; Orihara, S.; Ingham, J.L.; Mizutani, J. Seventeen isoflavonoids from Lupinus albus roots. Phytochemistry 1989, 28, 901–911. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.-Y. Antibacterial Compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Dej-adisai, S.; Meechai, I.; Puripattanavong, J.; Kummee, S. Antityrosinase and antimicrobial activities from Thai medicinal plants. Arch. Pharm. Res. 2014, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.Y.; Chen, W.C.; Ou, B.R.; Yeh, J.Y.; Cheng, Y.H.; Tsng, P.H.; Hsu, M.H.; Tsai, M.S.; Liang, Y.C. Simultaneous detection of multiple pathogens by multiplex PCR coupled with DNA biochip hybridization. Lab. Anim. 2018, 52, 186–195. [Google Scholar] [CrossRef]
- Branscheid, W.; Dobrowolski, A.; Sack, E. Simplification of the EC reference method for the full dissection of pig carcasses. Fleischwirtschaft 1990, 70, 565–567. [Google Scholar]
- Salehian, B.; Mahabadi, V.; Bilas, J.; Taylor, W.E.; Ma, K. The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism 2006, 55, 1239–1247. [Google Scholar] [CrossRef]
- Qin, J.; Du, R.; Yang, Y.-Q.; Zhang, H.-Q.; Li, Q.; Liu, L.; Guan, H.; Hou, J.; An, X.-R. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 2013, 94, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; SalinRaj, P.; Athira, R.; Soumya, R.S.; Raghu, K.G. Cerium nanoparticles synthesized using aqueous extract of Centella asiatica: Characterization, determination of free radical scavenging activity and evaluation of efficacy against cardiomyoblast hypertrophy. RSC Adv. 2015, 5, 21074–21083. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Yamashita, Y.; Kishida, H.; Nakagawa, K.; Ashida, H. Licorice flavonoid oil enhances muscle mass in KK-Ay mice. Life Sci. 2018, 205, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Tang, L.; Zhang, J.; Zhang, K. Chemical composition of three polysaccharides from Gynostemma pentaphyllum and their antioxidant activity in skeletal muscle of exercised mice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 479–485. [Google Scholar] [CrossRef]
- Yoon, H.J.; Bang, M.-H.; Kim, H.; Imm, J.-Y. Improvement of palmitate-induced insulin resistance in C2C12 skeletal muscle cells using Platycodon grandiflorum seed extracts. Food Biosci. 2018, 25, 61–67. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Wu, J.; Yang, Y.; Zeng, X.; Lv, X.; Cui, L.; Yao, W.; Liu, Y. Preventive effects of Polygonum multiflorum on glucocorticoid-induced osteoporosis in rats. Exp. Ther. Med. 2017, 14, 2445–2460. [Google Scholar] [CrossRef] [PubMed]
- Parry, O.; Okwuasaba, F.; Ejike, C. Skeletal muscle relaxant action of an aqueous extract of Portulaca oleracea in the rat. J. Ethnopharmacol. 1987, 19, 247–253. [Google Scholar] [CrossRef]
- Jung, M.-H.; Song, M.-C.; Bae, K.; Kim, H.S.; Kim, S.H.; Sung, S.H.; Ye, S.K.; Lee, K.H.; Yun, Y.-P.; Kim, T.-J. Sauchinone attenuates oxidative stress-induced skeletal muscle myoblast damage through the down-regulation of ceramide. Biol. Pharm. Bull. 2011, 34, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Amaro, C.A.B.; González-Cortazar, M.; Herrera-Ruiz, M.; Román-Ramos, R.; Aguilar-Santamaría, L.; Tortoriello, J.; Jiménez-Ferrer, E. Hypoglycemic and Hypotensive Activity of a Root Extract of Smilax aristolochiifolia, Standardized on N-trans-Feruloyl-Tyramine. Molecules 2014, 19, 11366–11384. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, H.; Gao, Y.; Meng, Y.; Zhao, X.-X.; Pan, S.-N. Effects of dandelion extract on the proliferation of rat skeletal muscle cells and the inhibition of a lipopolysaccharide-induced inflammatory reaction. Chin. Med. J. 2018, 131, 1724–1731. [Google Scholar] [CrossRef]
- Recharla, N.; Park, S.; Kim, M.; Kim, B.; Jeong, J.Y. Protective effects of biological feed additives on gut microbiota and the health of pigs exposed to deoxynivalenol: A review. J. Anim. Sci. Technol. 2022, 64, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Lee, Y.-S. Myostatin inhibitors: Panacea or predicament for musculoskeletal disorders? J. Bone. Metab. 2020, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Amabile, M.I.; Rossi Fanelli, F.; Muscaritoli, M. Novel therapeutic options for cachexia and sarcopenia. Expert Opin. Biol. Ther. 2016, 16, 1239–1244. [Google Scholar] [CrossRef]
- Lee, S.-J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Ryan, D.H. Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice? J. Obes. Metab. Syndr. 2021, 30, 196–208. [Google Scholar] [CrossRef]
- Joulia, D.; Bernardi, H.; Garandel, V.; Rabenoelina, F.; Vernus, B.; Cabello, G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 2003, 286, 263–275. [Google Scholar] [CrossRef]
- Thomas, M.; Langley, B.; Berry, C.; Sharma, M.; Kirk, S.; Bass, J.; Kambadur, R. Myostatin, a Negative Regulator of Muscle Growth, Functions by Inhibiting Myoblast Proliferation. J. Biol. Chem. 2000, 275, 40235–40243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shou, J.-W.; Li, X.-Y.; Zhao, Z.-X.; Fu, J.; He, C.-Y.; Feng, R.; Ma, C.; Wen, B.-Y.; Guo, F. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 2017, 70, 72–84. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, L.; Zhang, X.; Ding, Y.; Li, H.; Yang, Y.; Zhang, A.; Li, Y.; Lv, S.; Zhang, J. Prevention and treatment of chronic heart failure through traditional Chinese medicine: Role of the gut microbiota. Pharmacol. Res. 2020, 151, 104552–104560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Zhao, P.; Shao, Q.; Ma, Y.; Bai, D.; Liao, C.; He, L.; Huang, S.; Wang, X. Effects of dietary Glycyrrhiza polysaccharide supplementation on growth performance, intestinal antioxidants, immunity and microbiota in weaned piglets. Anim. Biotechnol. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Zuo, G.; Lee, S.K.; Lim, S.S. Acute and subchronic toxicity study of nonpolar extract of licorice roots in mice. Food Sci. Nutr. 2020, 8, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]


| Treatment group | CTL | LGUE | MGUE | HGUE |
|---|---|---|---|---|
| Initial body weight (kg) | 11.71 ± 1.22 | 12.62 ± 1.62 | 12.89 ± 1.18 | 11.40 ± 1.18 |
| Final body weight (kg) | 110.38 ± 6.29 a | 118.68 ± 8.12 b | 119.48 ± 6.54 b | 118.17 ± 10.91 b |
| Body weight gain (kg) | 98.67 ± 7.38 a | 106.06 ± 7.48 b | 106.59 ± 6.43 b | 106.77 ± 9.83 b |
| ADG (kg/day) | 0.78 ± 0.06 a | 0.84 ± 0.06 b | 0.85 ± 0.05 b | 0.85 ± 0.08 b |
| ADFI (kg/day) | 2.00 ± 0.57 | 2.00 ± 0.42 | 2.00 ± 0.75 | 1.98 ± 0.82 |
| TFI (kg) | 257.31 ± 7.91 | 256.92 ± 8.32 | 257.17 ± 6.11 | 255.16 ± 5.43 |
| FCR | 2.61 a | 2.42 b | 2.41 b | 2.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, B.-R.; Hsu, M.-H.; Haung, L.-Y.; Lin, C.-J.; Kuo, L.-L.; Tsai, Y.-T.; Chang, Y.-C.; Lin, W.-Y.; Huang, T.-C.; Wu, Y.-C.; et al. Systematic Myostatin Expression Screening Platform for Identification and Evaluation of Myogenesis-Related Phytogenic in Pigs. Bioengineering 2023, 10, 1113. https://doi.org/10.3390/bioengineering10101113
Ou B-R, Hsu M-H, Haung L-Y, Lin C-J, Kuo L-L, Tsai Y-T, Chang Y-C, Lin W-Y, Huang T-C, Wu Y-C, et al. Systematic Myostatin Expression Screening Platform for Identification and Evaluation of Myogenesis-Related Phytogenic in Pigs. Bioengineering. 2023; 10(10):1113. https://doi.org/10.3390/bioengineering10101113
Chicago/Turabian StyleOu, Bor-Rung, Ming-Hua Hsu, Ling-Ya Haung, Chuan-Ju Lin, Li-Li Kuo, Yu-Ting Tsai, Yu-Chia Chang, Wen-Yuh Lin, Tsung-Chien Huang, Yun-Chu Wu, and et al. 2023. "Systematic Myostatin Expression Screening Platform for Identification and Evaluation of Myogenesis-Related Phytogenic in Pigs" Bioengineering 10, no. 10: 1113. https://doi.org/10.3390/bioengineering10101113






