Transcranial Magnetic Stimulation Measures, Pyramidal Score on Expanded Disability Status Scale and Magnetic Resonance Imaging of Corticospinal Tract in Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Clinical Disease-Related Assessment Measures
2.3. Radiological Magnetic Resonance Imaging (MRI) Assessment and Image Evaluation
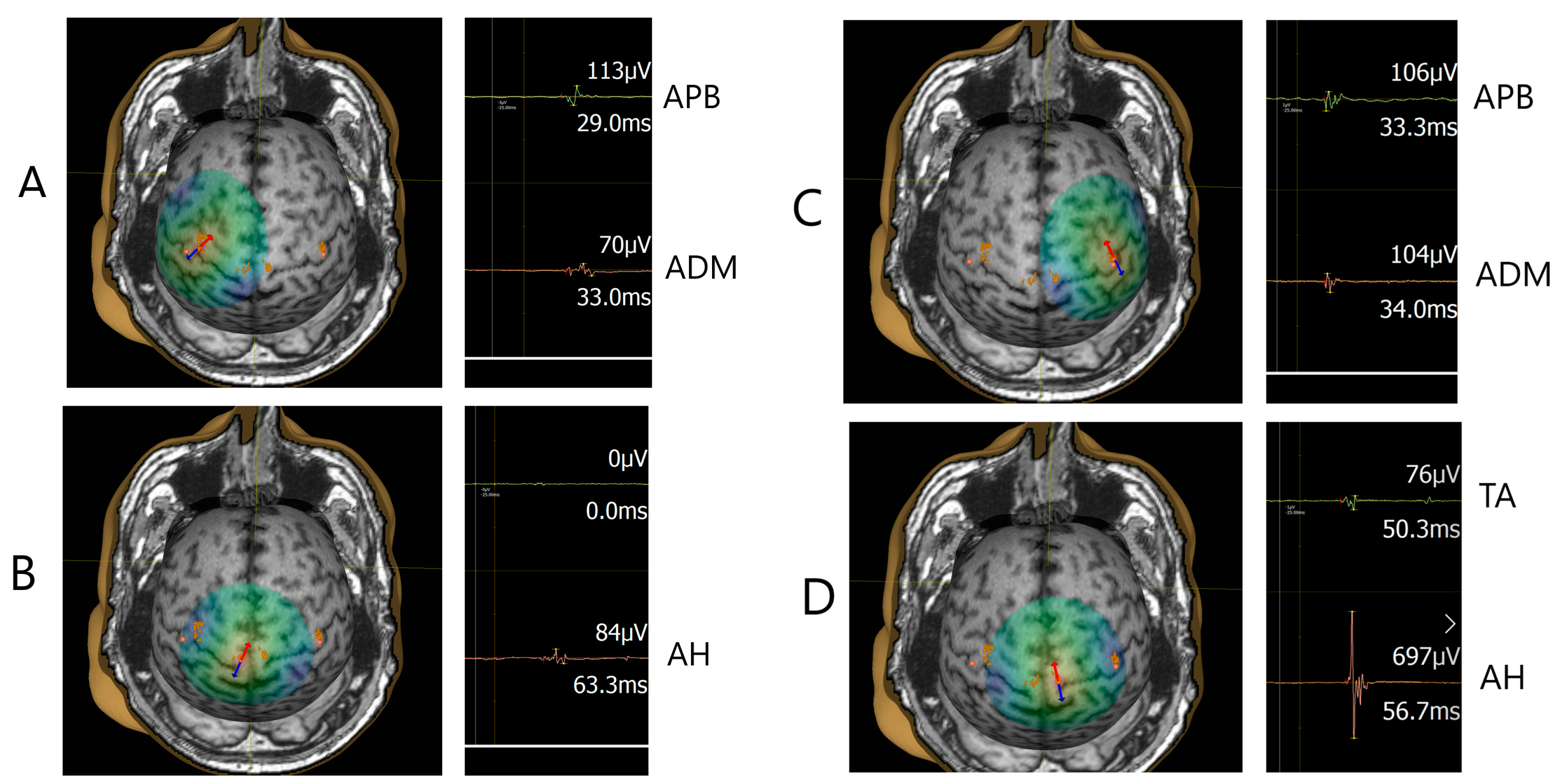
2.4. Navigated Transcranial Magnetic Stimulation (TMS) Procedure
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
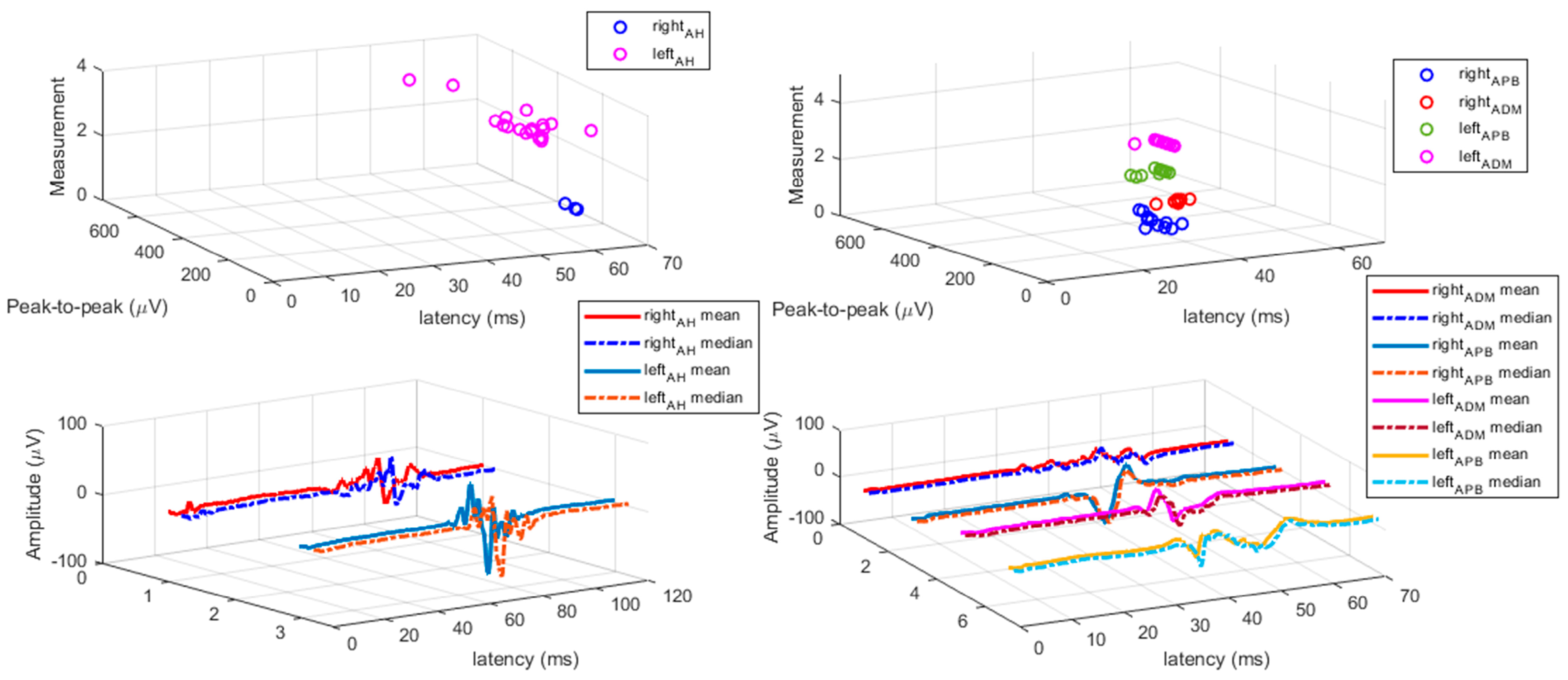
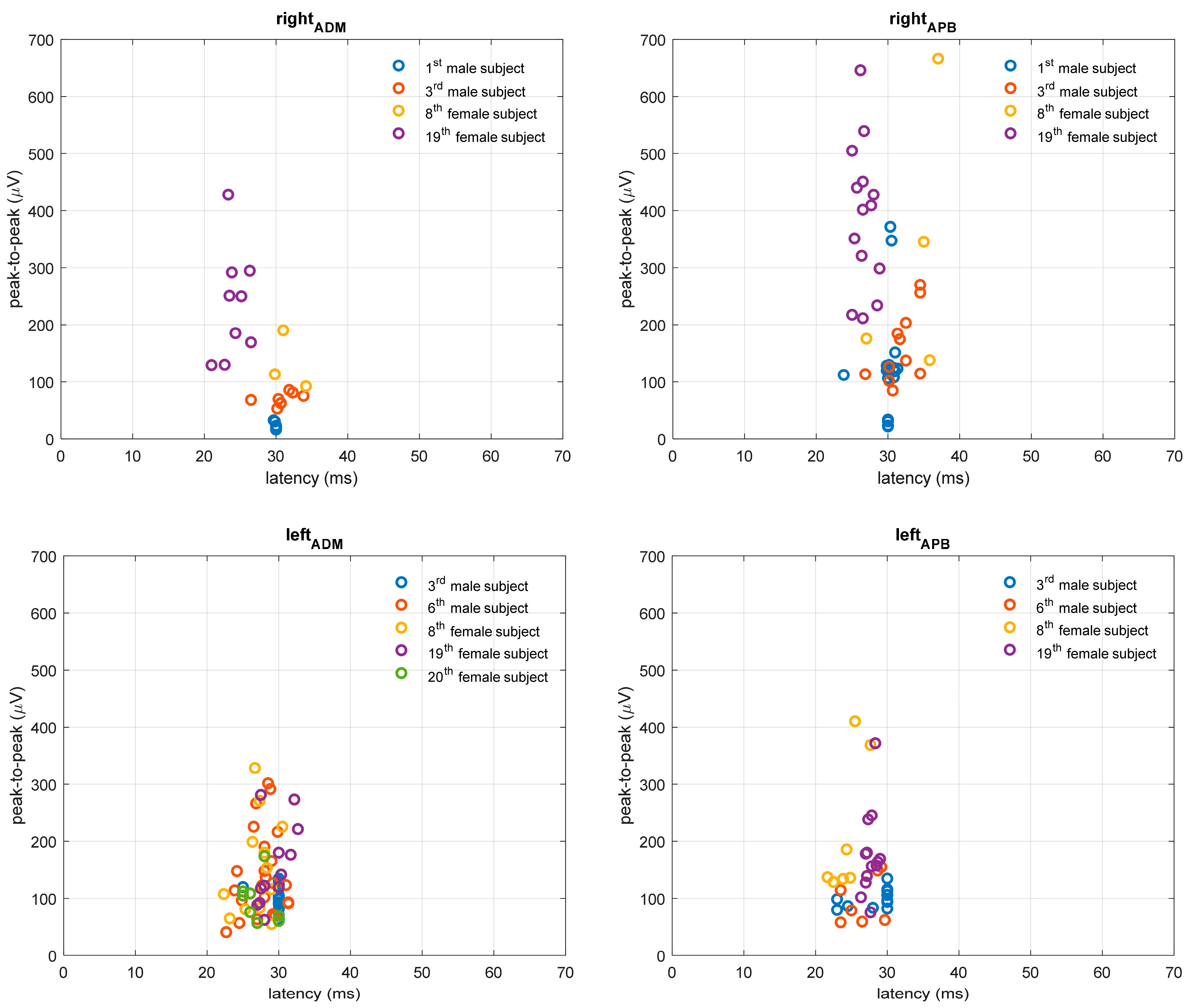
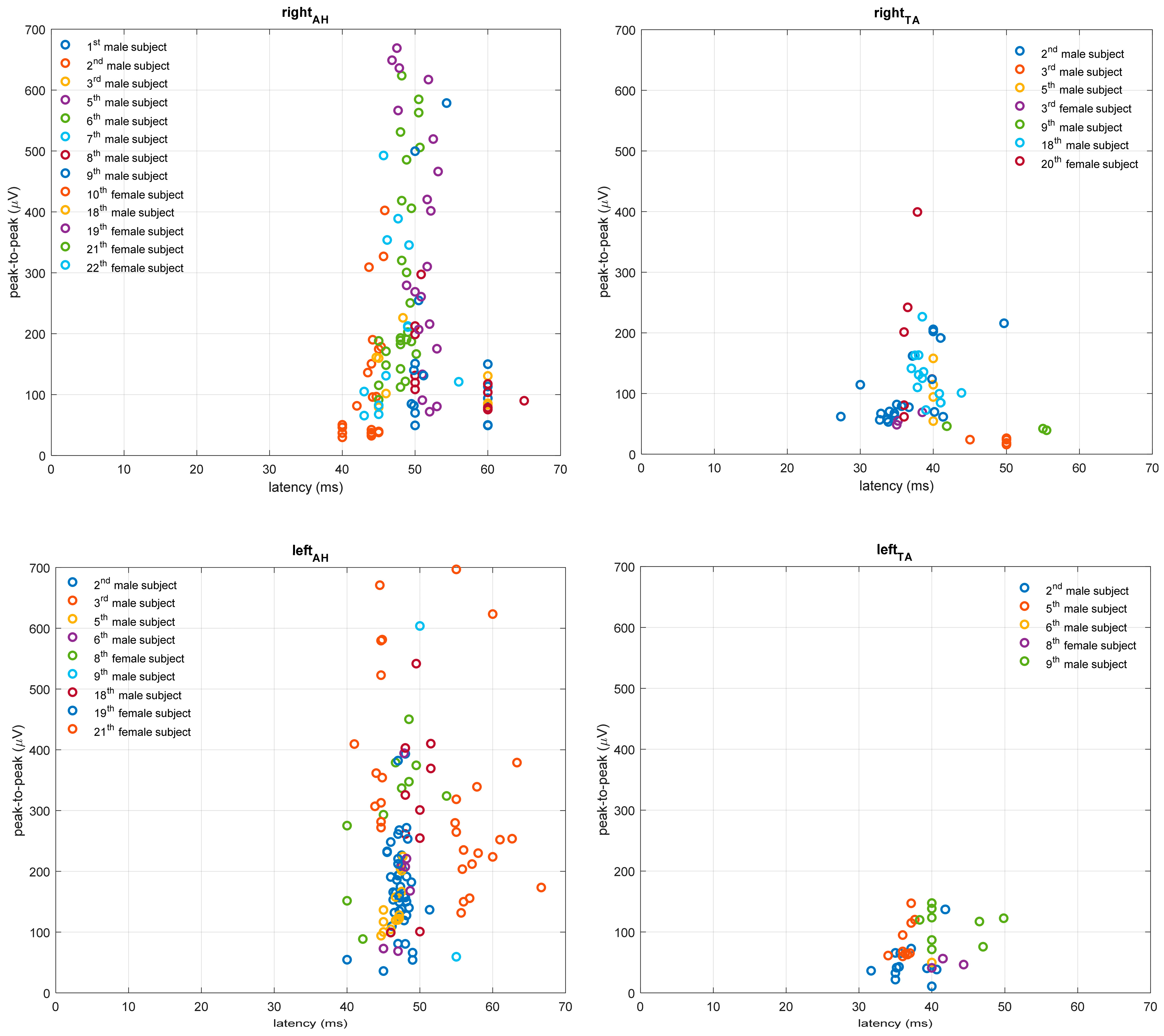
3.2. TMS Measures
3.3. TMS, EDSS, and MRI Correspondence Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassmann, H.; Brück, W.; Lucchinetti, C. Heterogeneity of multiple sclerosis pathogenesis: Implications for diagnosis and therapy. Trends Mol. Med. 2001, 7, 115–121. [Google Scholar] [CrossRef]
- Pitteri, M.; Romualdi, C.; Magliozzi, R.; Monaco, S.; Calabrese, M. Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult. Scler. J. 2017, 23, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Brück, W.; Chard, D.; Fazekas, F.; Geurts, J.J.; Enzinger, C.; Hametner, S.; Kuhlmann, T.; Preziosa, P.; Rovira, A.; et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2019, 18, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Li, R. Cellular immunology of relapsing multiple sclerosis: Interactions, checks, and balances. Lancet Neurol. 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Pisa, M.; Chieffo, R.; Giordano, A.; Gelibter, S.; Comola, M.; Comi, G.; Leocani, L. Upper limb motor evoked potentials as outcome measure in progressive multiple sclerosis. Clin. Neurophysiol. 2020, 131, 401–405. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Buttari, F.; Gilio, L.; Paolis, N.; Fresegna, D.; Centonze, D.; Lezzi, E. Inflammation and Corticospinal Functioning in Multiple Sclerosis: A TMS Perspective. Front. Neurol. 2020, 11, 566. [Google Scholar] [CrossRef]
- Hardmeier, M.; Schindler, C.; Kuhle, J.; Fuhr, P. Validation of Quantitative Scores Derived From Motor Evoked Potentials in the Assessment of Primary Progressive Multiple Sclerosis: A Longitudinal Study. Front. Neurol. 2020, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Palm, U.; Ayache, S.S. Editorial: Corticospinal Excitability in Patients With Multiple Sclerosis. Front. Neurol. 2021, 11, 635612. [Google Scholar] [CrossRef] [PubMed]
- Neva, J.L.; Lakhani, B.; Brown, K.E.; Wadden, K.P.; Mang, C.S.; Ledwell, N.H.M.; Borich, M.R.; Vavasour, I.M.; Laule, C.; Traboulsee, A.L.; et al. Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behav. Brain Res. 2016, 297, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mamoei, S.; Hvid, L.G.; Boye Jensen, H.; Zijdewind, I.; Stenager, E.; Dalgas, U. Neurophysiological impairments in multiple sclerosis-Central and peripheral motor pathways. Acta Neurol. Scand. 2020, 142, 401–417. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksenet, J.L.; et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Triggs, W.J.; Calvanio, R.; Macdonell, R.A.; Cros, D.; Chiappa, K.H. Physiological motor asymmetry in human handedness: Evidence from transcranial magnetic stimulation. Brain Res. 1994, 636, 270–276. [Google Scholar] [CrossRef]
- Eisen, A.A.; Shtybel, W. Clinical experience with transcranial magnetic stimulation. Muscle Nerve 1990, 13, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Macdonell, R.A.; Donnan, G.A. Magnetic cortical stimulation in acute spinal cord injury. Neurology 1995, 45, 303–306. [Google Scholar] [CrossRef]
- Claus, D. Central motor conduction: Method and normal results. Muscle Nerve 1990, 13, 1125–1132. [Google Scholar] [CrossRef]
- Cantone, M.; Lanza, G.; Vinciguerra, L.; Puglisi, V.; Ricceri, R.; Fisicaro, F.; Vagli, C.; Bella, R.; Ferri, R.; Pennisi, G.; et al. Age, Height, and Sex on Motor Evoked Potentials: Translational Data From a Large Italian Cohort in a Clinical Environment. Front. Hum. Neurosci. 2019, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Osei-Lah, A.D.; Mills, K.R. Optimising the detection of upper motor neuron function dysfunction in amyotrophic lateral sclerosis-a transcranial magnetic stimulation study. J. Neurol. 2004, 251, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Screening questionnaire before TMS: An update. Clin. Neurophysiol. 2011, 122, 1686. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–14452. [Google Scholar] [CrossRef]
- Preston, D.C.; Shapiro, B.E. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9781455726721. [Google Scholar]
- Šoda, J.; Rogić Vidaković, M.; Lorincz, J.; Jerković, A.; Vujović, I. Novel Latency Estimation Algorithm of Motor Evoked Potential Signals. IEEE Access 2020, 8, 193356–193374. [Google Scholar] [CrossRef]
- Chard, D.; Trip, S.A. Resolving the clinico-radiological paradox in multiple sclerosis. F1000Research 2017, 6, 1828. [Google Scholar] [CrossRef] [PubMed]
- Lapucci, C.; Schiavi, S.; Signori, A.; Sbragia, E.; Bommarito, G.; Cellerino, M.; Uccelli, A.; Inglese, M.; Roccatagliata, L.; Pardini, M. The role of disconnection in explaining disability in multiple sclerosis. Eur. Radiol. Exp. 2022, 6, 23. [Google Scholar] [CrossRef]
- McFarland, H.; Barkhof, F.; Antel, J.; Miller, D. The role of MRI as a surrogate outcome measure in multiple sclerosis. Mult. Scler. J. 2002, 8 (Suppl. 1), 40–51. [Google Scholar] [CrossRef] [PubMed]
- Boneschi, F.M.; Rovaris, M.; Comi, G.; Filippi, M. The use of magnetic resonance imaging in multiple sclerosis: Lessons learned from clinical trials. Mult. Scler. J. 2004, 10, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Moassefi, M.; Elham Barati, E.; Ali Sahraian, M.; Aghajani, F.; Fattahi, M.R. Correlation between the clinical disability and T1 hypointense lesions’ volume in cerebral magnetic resonance imaging of multiple sclerosis patients: A systematic review and meta-analysis. CNS Neurosci. Ther. 2021, 27, 1268–1280. [Google Scholar] [CrossRef]
- Yperman, J.; Popescu, V.; Van Wijmeersch, B.; Becker, T.; Peeters, L.M. Motor evoked potentials for multiple sclerosis, a multiyear follow-up dataset. Sci. Data 2022, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Neumann, J.; Kaufmann, J.; Heidel, J.; Stadler, E.; Sweeney-Reed, C.; Sailer, M.; Schreiber, S. Loss of corticospinal tract integrity in early MS disease stages. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e399. [Google Scholar] [CrossRef]
- Kerbrat, A.; Gros, C.; Badji, A.; Bannier, E.; Galassi, F.; Combès, B.; Chouteau, R.; Labauge, P.; Ayrignac, X.; Carra-Dalliere, C.; et al. Multiple sclerosis lesions in motor tracts from brain to cervical cord: Spatial distribution and correlation with disability. Brain 2020, 143, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Victoria Fernández, V. The Use of Motor-Evoked Potentials in Clinical Trials in Multiple Sclerosis. J. Clin. Neurophysiol. 2021, 38, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.D.; Ivry, B.; Bowen, J.D.; Cheng, E.M.; Dobson, R.; Goodin, D.S.; Lechner-Scott, J.; Kappos, L.; Galea, I. A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult. Scler. J. 2016, 22, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Šoda, J.; Pavelin, S.; Vujović, I.; Rogić Vidaković, M. Assessment of Motor Evoked Potentials in Multiple Sclerosis. Sensors 2023, 23, 497. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Characteristics | N | % | Mean (SD) Median (IQR) |
|---|---|---|---|
| Mean age in years | 41.6 (8.8) | ||
| Female/Male | 14/9 | 60.9/39.1 | |
| Education (elementary school/high school/undergraduate university study/graduate university study) Right hand dominance (%) | 2/17/1/3 21 | 8.7/73.9/4.4/13.0 91.3 | |
| Mean height (cm) | 175 (10.2) | ||
| Mean weight (kg) | 77.9 (19.0) | ||
| Mean BMI (kg/m^2) | 25.1 (3.7) | ||
| Disease-related characteristics | |||
| Mean age at onset of MS in years | 31.7 (10.9) | ||
| Mean MS disease duration in years | 9.3 (5.7) | ||
| Mean number of relapses from MS diagnosis | 4.0 (3.7) | ||
| Mean Teriflunomide intake duration in years | 3.6 (1.6) | ||
| Other chronic diseases, not MS (yes/no) | 10/13 | 43.5/56.5 | |
| Median EDSS score | 2.5 (3.5) | ||
| Median EDSS functional pyramidal score | 2.0 (3) | ||
| Median EDSS functional pyramidal score right leg | 1.0 (2.5) | ||
| Median EDSS functional pyramidal score left leg | 1.0 (2.5) | ||
| Median EDSS functional pyramidal score right arm | 0 (0) | ||
| Median EDSS functional pyramidal score left arm | 0 (0) |
| TMS | Altered MEP (N = 15) | Non-Altered MEP (N = 8) | All pwMS subjects (N = 23) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Measure | M | SD | Absent MEP (%) | M | SD | Absent MEP (%) | M | SD | Absent MEP (%) | |
| LEFT hemisphere stimulation | APB | RMT % | 41.4 | 10.2 | 0 | 38.0 | 7.60 | 0 | 40.2 | 9.4 | 0 |
| APB | MEP L (ms) | 25.14 | 4.1 | 0 | 22.0 | 1.42 | 0 | 24.0 | 3.7 | 0 | |
| APB | MEP A (µV) | 318.9 | 192.7 | 0 | 195.6 | 88.65 | 0 | 276.0 | 172.4 | 0 | |
| ADM | RMT % | 44.5 | 13.7 | 0 | 38.0 | 6.97 | 0 | 42.2 | 12.1 | 0 | |
| ADM | MEP L (ms) | 24.7 | 3.5 | 0 | 22.2 | 1.03 | 0 | 23.9 | 3.1 | 0 | |
| ADM | MEP A (µV) | 167.1 | 102.0 | 0 | 154.3 | 52.12 | 0 | 162.7 | 86.7 | 0 | |
| TA | RMT % | 85.6 | 15.6 | 6.67 | 62.8 | 28.09 | 0 | 81.1 | 15.5 | 4.3 | |
| TA | MEP L (ms) | 39.3 | 7.8 | 33.33 | 27.3 | 11.29 | 0 | 36.0 | 7.0 | 21.7 | |
| TA | MEP A (µV) | 95.3 | 52.3 | 33.33 | 95.4 | 70.49 | 0 | 101.5 | 54.2 | 21.7 | |
| AH | RMT % | 84.0 | 15.0 | 0 | 67.1 | 13.21 | 0 | 78.1 | 16.3 | 0 | |
| AH | MEP L (ms) | 49.0 | 5.0 | 6.67 | 43.2 | 1.83 | 0 | 46.9 | 5.0 | 4.3 | |
| AH | MEP A (µV) | 208.9 | 141.1 | 6.67 | 331.4 | 109.50 | 0 | 253.5 | 141.3 | 4.3 | |
| RIGHT hemisphere stimulation | APB | RMT % | 49.8 | 16.4 | 0 | 41.6 | 6.1 | 0 | 47.0 | 14.1 | 0 |
| APB | MEP L (ms) | 24.5 | 3.1 | 6.6 | 22.6 | 1.2 | 0 | 23.8 | 2.7 | 4.3 | |
| APB | MEP A (µV) | 178.4 | 83.4 | 6.6 | 339.1 | 373.9 | 0 | 232.0 | 229.1 | 4.3 | |
| ADM | RMT % | 52.2 | 16.6 | 0 | 42.5 | 5.9 | 0 | 48.8 | 14.4 | 0 | |
| ADM | MEP L(ms) | 25.1 | 2.9 | 0 | 22.1 | 1.2 | 0 | 24.0 | 2.8 | 0 | |
| ADM | MEP A (µV) | 210.3 | 134.1 | 0 | 192.3 | 164.9 | 0 | 204.1 | 142.0 | 0 | |
| TA | RMT % | 89.5 | 11.7 | 0 | 65.1 | 11.8 | 0 | 82.5 | 16.0 | 0 | |
| TA | MEP L (ms) | 35.5 | 4.6 | 26.6 | 32.1 | 1.0 | 0 | 34.2 | 4.0 | 17.3 | |
| TA | MEP A (µV) | 147.9 | 184.9 | 26.6 | 134.8 | 80.3 | 0 | 143.0 | 150.7 | 17.3 | |
| AH | RMT % | 87.2 | 12.9 | 6.6 | 63.8 | 10.8 | 0 | 78.7 | 16.5 | 4.3 | |
| AH | MEP L (ms) | 46.6 | 5.2 | 20.0 | 42.2 | 0.7 | 0 | 44.8 | 4.5 | 13.0 | |
| AH | MEP A (µV) | 260.1 | 114.8 | 20.0 | 557.2 | 336.8 | 0 | 378.9 | 267.8 | 13.0 | |
| LEFT Hemisphere Stimulation | RIGHT Hemisphere Stimulation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | APB | ADM | TA | AH | APB | ADM | TA | AH | |||||
| Measure | RMT (%) | MEP L (ms) | RMT (%) | MEP L (ms) | MEP L (ms) | MEP L (ms) | RMT (%) | MEP L (ms) | RMT (%) | MEP L (ms) | MEP L (ms) | MEP L (ms) | |
| Current study All pwMS subjects N = 23 | M | 40.2 | 24.0 | 42.2 | 23.9 | 36.0 | 46.9 | 47.0 | 23.8 | 48.8 | 24.0 | 34.2 | 44.8 |
| SD | 9.4 | 3.70 | 12.1 | 3.1 | 7.0 | 5.0 | 14.1 | 2.7 | 14.4 | 2.8 | 4.0 | 4.5 | |
| Current study pwMS –altered MEP N = 15 | M | 41.4 | 25.1 | 44.5 | 24.7 | 39.3 | 49.0 | 49.8 | 24.5 | 52.2 | 25.1 | 35.5 | 46.6 |
| SD | 10.2 | 4.1 | 13.7 | 3.5 | 7.8 | 5.0 | 16.4 | 3.1 | 16.6 | 2.9 | 4.6 | 5.2 | |
| Published studies Non-clinical sample | Triggs et al. [14] | Eisen and Shtybel [15] | Macdonell and Donnan [16] | Claus [17] | Cantone et al. [18] | Osei-Lah and Mills [19] | Triggs et al. [14] | Eisen and Shtybel [15] | Macdonell and Donnan [16] | Claus [17] | Cantone et al. [18] | Osei-Lah and Mills [19] | |
| M | 41.6 | 20.2 | 49 | 19.7 | 25.5 | 39.1 | 38.5 | 20.2 | 49 | 19.7 | 26.5 | 39.1 | |
| SD | 7 | 1.6 | 8 | 1.0 | 2.2 | 2.5 | 6 | 1.6 | 8 | 1.0 | 2.2 | 2.5 | |
| N | 30 | 150 | 20 | 54 | 487 | 20 | 30 | 150 | 20 | 54 | 487 | 20 | |
| Comparisons with the current study All pwMS subjects | t | −0.5 | 4.94 | −2.18 | 6.34 | 6.12 | 6.52 | 2.70 | 6.14 | −0.04 | 7.15 | 7.64 | 4.96 |
| df | 39 | 23 | 38 | 24 | 16 | 32 | 28 | 23 | 35 | 24 | 15 | 30 | |
| p | 0.33 | <0.001 | 0.04 * | <0.001 | <0.001 | <0.001 | 0.01 * | <0.001 | 0.40 | <0.001 | <0.001 | <0.001 | |
| Comparisons with the current study pwMS-TMS altered finding | t | −0.05 | 4.61 | −1.12 | 5.55 | 5.26 | 6.84 | 2.59 | 5.11 | 0.70 | 7.00 | 6.12 | 4.67 |
| df | 21 | 14 | 21 | 15 | 8 | 18 | 16 | 14 | 19 | 15 | 9 | 14 | |
| p | 0.39 | <0.001 | 0.21 | <0.001 | <0.001 | <0.001 | 0.02 * | <0.001 | 0.30 | <0.001 | <0.001 | <0.001 | |
| pwMS (N = 23) | McDonald | Corticospinal Tract | Corticospinal Tract Right | Corticospinal Tract Left | |
|---|---|---|---|---|---|
| Altered MEP | 1. | 44 | 3 | 2 | 1 |
| 2. | 38 | 3 | 2 | 1 | |
| 3. | 14 | 4 | 2 | 2 | |
| 4. | 16 | 3 | 0 | 3 | |
| 5. | 23 | 2 | 0 | 2 | |
| 6. | 5 | 1 | 0 | 1 | |
| 7. | 49 | 3 | 2 | 1 | |
| 8. | 33 | 4 | 2 | 2 | |
| 9. | 6 | 2 | 2 | 0 | |
| 10. | 13 | 2 | 1 | 1 | |
| 11. | 8 | 1 | 0 | 1 | |
| 12. | 50 | 2 | 0 | 2 | |
| 13. | 50 | 2 | 1 | 1 | |
| 14. | 20 | 6 | 3 | 3 | |
| 15. | 13 | 4 | 3 | 1 | |
| Non -altered MEP | 16. | 19 | 0 | 0 | 0 |
| 17. | 90 | 0 | 0 | 0 | |
| 18. | 18 | 0 | 0 | 0 | |
| 19. | 10 | 0 | 0 | 0 | |
| 20. | 42 | 5 | 2 | 3 | |
| 21. | 34 | 0 | 0 | 0 | |
| 22. | 8 | 0 | 0 | 0 | |
| 23. | 12 | 0 | 0 | 0 | |
| M | 26.73 | 2.04 | |||
| SD | 20.54 | 1.79 | |||
| pwMS (N = 23) | RIGHT LEG | LEFT LEG | RIGHT ARM | LEFT ARM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMS | MRI | EDSS | TMS | MRI | EDSS | TMS | MRI | EDSS | TMS | MRI | EDSS | |
| 1. | 1 | 3 | 3 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 |
| 2. | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 3 | 0 |
| 3. | 1 | 2 | 3 | 1 | 2 | 1 | 1 | 2 | 3 | 1 | 2 | 3 |
| 4. | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| 5. | 1 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 0 |
| 6. | 1 | 0 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 0 |
| 7. | 1 | 1 | 2.5 | 1 | 2 | 2.5 | 1 | 1 | 1 | 1 | 2 | 1 |
| 8. | 1 | 2 | 3.5 | 1 | 2 | 3.5 | 0 | 2 | 0 | 0 | 2 | 0 |
| 9. | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| 10. | 1 | 1 | 3 | 0 | 1 | 3 | 0 | 1 | 0 | 0 | 1 | 0 |
| 11. | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| 12. | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 0 |
| 13. | 1 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 1 | 1 | 2 |
| 14. | 1 | 4 | 0 | 1 | 4 | 0 | 0 | 4 | 0 | 0 | 4 | 0 |
| 15. | 1 | 1 | 2 | 1 | 3 | 3 | 0 | 1 | 1 | 0 | 3 | 1 |
| 16. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17. | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18. | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20. | 0 | 2 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 3 | 0 |
| 21. | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 22. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 23. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TMS % of the corresponding classification * | 78% | 70% | 78% | 83% | ||||||||
| MRI % of the corresponding classification * | 65% | 65% | 61% | 57% | ||||||||
| χ2 | 0.8 | 0 | 1.125 | 3.125 | ||||||||
| p | 0.298 | 1 | 0.214 | 0.047 * | ||||||||
| pwMS (N = 23) | Right Leg | Left Leg | Right Arm | Left Arm | EDSS General/Pyramid Score | EDSS General | EDSS Pyramid | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TMS | MRI | TMS | MRI | TMS | MRI | TMS | MRI | ||||
| 1. | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3.5 | 3 |
| 2. | 1 | 0 | 1 | 3 | 0 | 0 | 0 | 3 | 1 | 1.5 | 1 |
| 3. | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 3.5 | 3 |
| 4. | 1 | 0 | 1 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 5. | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 2.5 | 2 |
| 6. | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 2.5 | 2 |
| 7. | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 3.5 | 2.5 |
| 8. | 1 | 2 | 1 | 2 | 0 | 2 | 0 | 2 | 1 | 4 | 3.5 |
| 9. | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | 3 |
| 10. | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 3.5 | 3 |
| 11. | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 12. | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3.5 | 3 |
| 13. | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 3.5 | 3 |
| 14. | 1 | 4 | 1 | 4 | 0 | 4 | 0 | 4 | 0 | 0 | 0 |
| 15. | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 3 | 1 | 4 | 3 |
| 16. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 |
| 18. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 |
| 19. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20. | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 3 | 0 | 0 | 0 |
| 21. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.5 | 1 |
| 22. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 23. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Corresponding classification | 74% | 61% | 61% | 57% | 44% | 61% | 48% | 57% | |||
| χ2 | 1.88 | 0.38 | 0 | 0 | |||||||
| p | 0.17 | 0.54 | 1 | 1 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogić Vidaković, M.; Ćurković Katić, A.; Pavelin, S.; Bralić, A.; Mikac, U.; Šoda, J.; Jerković, A.; Mastelić, A.; Dolić, K.; Markotić, A.; et al. Transcranial Magnetic Stimulation Measures, Pyramidal Score on Expanded Disability Status Scale and Magnetic Resonance Imaging of Corticospinal Tract in Multiple Sclerosis. Bioengineering 2023, 10, 1118. https://doi.org/10.3390/bioengineering10101118
Rogić Vidaković M, Ćurković Katić A, Pavelin S, Bralić A, Mikac U, Šoda J, Jerković A, Mastelić A, Dolić K, Markotić A, et al. Transcranial Magnetic Stimulation Measures, Pyramidal Score on Expanded Disability Status Scale and Magnetic Resonance Imaging of Corticospinal Tract in Multiple Sclerosis. Bioengineering. 2023; 10(10):1118. https://doi.org/10.3390/bioengineering10101118
Chicago/Turabian StyleRogić Vidaković, Maja, Ana Ćurković Katić, Sanda Pavelin, Antonia Bralić, Una Mikac, Joško Šoda, Ana Jerković, Angela Mastelić, Krešimir Dolić, Anita Markotić, and et al. 2023. "Transcranial Magnetic Stimulation Measures, Pyramidal Score on Expanded Disability Status Scale and Magnetic Resonance Imaging of Corticospinal Tract in Multiple Sclerosis" Bioengineering 10, no. 10: 1118. https://doi.org/10.3390/bioengineering10101118
APA StyleRogić Vidaković, M., Ćurković Katić, A., Pavelin, S., Bralić, A., Mikac, U., Šoda, J., Jerković, A., Mastelić, A., Dolić, K., Markotić, A., Đogaš, Z., & Režić Mužinić, N. (2023). Transcranial Magnetic Stimulation Measures, Pyramidal Score on Expanded Disability Status Scale and Magnetic Resonance Imaging of Corticospinal Tract in Multiple Sclerosis. Bioengineering, 10(10), 1118. https://doi.org/10.3390/bioengineering10101118








