Biomechanical Variability and Usability of a Novel Customizable Fracture Fixation Technique
Abstract
:1. Introduction
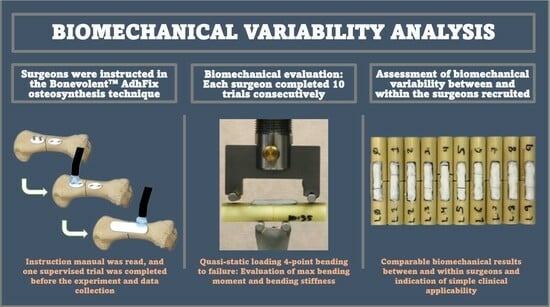
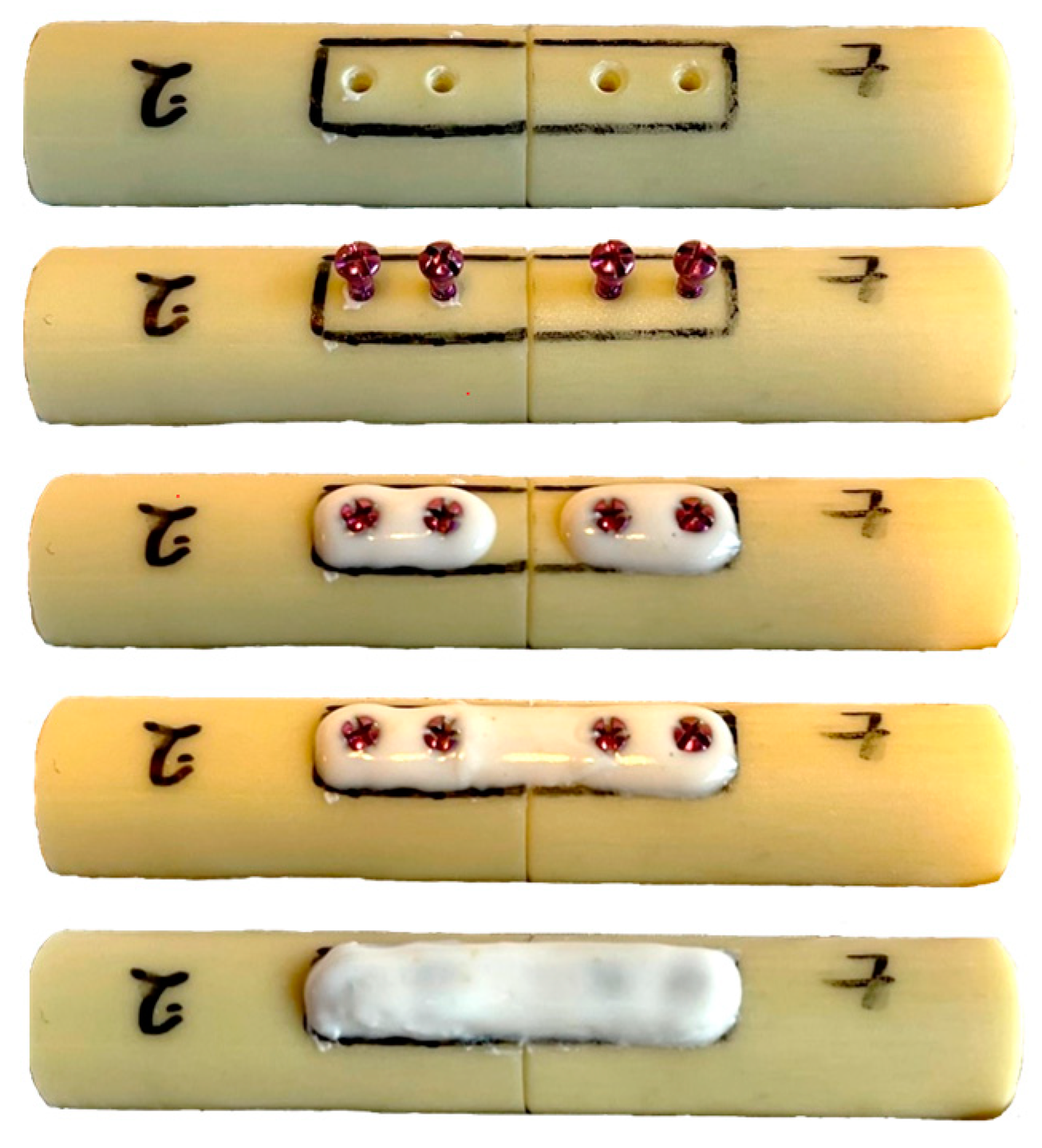
2. Materials and Methods
3. Results
3.1. Inter-Surgeon Variation
3.2. Intra-Surgeon Variation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.D.; Abolhassani, H.; Aboyans, V.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Harith, H.; Malekani, J.; Schmutz, B.; Schuetz, M.; Yarlagadda, P.K. A method for optimal fit of patient-specific fracture fixation plates. Proc. Inst. Mech. Eng. Part. L J. Mater. Des. Appl. 2016, 230, 282–290. [Google Scholar] [CrossRef]
- Hollensteiner, M.; Sandriesser, S.; Bliven, E.; von Rüden, C.; Augat, P. Biomechanics of Osteoporotic Fracture Fixation. Curr. Osteoporos. Rep. 2019, 17, 363–374. [Google Scholar] [CrossRef]
- Lampropoulou-Adamidou, K.; Karampinas, P.K.; Chronopoulos, E.; Vlamis, J.; Korres, D.S. Currents of plate osteosynthesis in osteoporotic bone. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 427–433. [Google Scholar] [CrossRef]
- Wähnert, D.; Frank, A.; Ueberberg, J.; Heilmann, L.F.; Sauzet, O.; Raschke, M.J.; Gehweiler, D. Development and first biomechanical validation of a score to predict bone implant interface stability based on clinical qCT scans. Sci. Rep. 2021, 11, 3273. [Google Scholar] [CrossRef] [PubMed]
- Lögters, T.T.; Lee, H.H.; Gehrmann, S.; Windolf, J.; Kaufmann, R.A. Proximal Phalanx Fracture Management. Hand 2018, 13, 376–383. [Google Scholar] [CrossRef]
- von Kieseritzky, J.; Alfort, H.; Granskog, V.; Hutchinson, D.; Stenlund, P.; Bogestål, Y.; Arner, M.; Håkansson, J.; Malkoch, M. DendroPrime as an adhesion barrier on fracture fixation plates: An experimental study in rabbits. J. Hand Surg. Eur. Vol. 2020, 45, 742–747. [Google Scholar] [CrossRef]
- Neumeister, M.W.; Winters, J.N.; Maduakolum, E. Phalangeal and Metacarpal Fractures of the Hand: Preventing Stiffness. Plast. Reconstr. Surg Glob. Open 2021, 9, e3871. [Google Scholar] [CrossRef]
- Hutchinson, D.J.; Granskog, V.; von Kieseritzky, J.; Alfort, H.; Stenlund, P.; Zhang, Y.; Arner, M.; Håkansson, J.; Malkoch, M. Highly Customizable Bone Fracture Fixation through the Marriage of Composites and Screws. Adv Funct Mater. 2021, 31, 2105187. [Google Scholar] [CrossRef]
- Schwarzenberg, P.; Rasmussen, T.C.; Hutchinson, D.J.; Mischler, D.; Horstmann, P.; Petersen, M.M.; Jacobsen, S.; Pastor, T.; Malkoch, M.; Wong, C.; et al. Biomechanical performance of a novel light—Curable bone fixation technique. Sci. Rep. 2023, 13, 9339. [Google Scholar] [CrossRef]
- Augat, P.; Hast, M.W.; Schemitsch, G.; Heyland, M.; Trepczynski, A.; Borgiani, E.; Russow, G.; Märdian, S.; Duda, G.N.; Hollensteiner, M.; et al. Biomechanical models: Key considerations in study design. OTA Int. Open Access J. Orthop. Trauma. 2021, 4, e099. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Silva, M.J.; Krieg, J.C. Biomechanical testing of fracture fixation constructs: Variability, validity, and clinical applicability. J. Am. Acad. Orthop. Surg. 2012, 20, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Brouwer de Koning, S.G.; de Winter, N.; Moosabeiki, V.; Mirzaali, M.J.; Berenschot, A.; Witbreuk, M.M.E.H.; Lagerburg, V. Design considerations for patient-specific bone fixation plates: A literature review. Med. Biol. Eng. Comput. 2023. [Google Scholar] [CrossRef]
- Park, S.M.; Park, S.; Park, J.; Choi, M.; Kim, L.; Noh, G. Design process of patient-specific osteosynthesis plates using topology optimization. J. Comput. Des. Eng. 2021, 8, 1257–1266. [Google Scholar] [CrossRef]
- Gutwald, R.; Jaeger, R.; Lambers, F.M. Customized mandibular reconstruction plates improve mechanical performance in a mandibular reconstruction model. Comput. Methods Biomech. Biomed. Engin 2017, 20, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.W.A.; Neumann, V.; Wenzel, L.; Gueorguiev, B.; Richards, R.G.; Gill, H.S.; Whitehouse, M.R.; Preatoni, E. Screw tightness and stripping rates vary between biomechanical researchers and practicing orthopaedic surgeons. J. Orthop. Surg. Res. 2021, 16, 185. [Google Scholar] [CrossRef]
- Fletcher, J.W.A.; Wenzel, L.; Neumann, V.; Richards, R.G.; Gueorguiev, B.; Gill, H.S.; Preatoni, E.; Whitehouse, M.R. Surgical performance when inserting non-locking screws: A systematic review. EFORT Open Rev. 2020, 5, 26–36. [Google Scholar] [CrossRef]
- Karam, M.D.; Westerlind, B.; Anderson, D.D.; arsh, J.L.; UI Orthopaedic Surgical Skills Training Committee. Development of an orthopaedic surgical skills curriculum for post-graduate year one resident learners—The University of Iowa experience. Iowa Orthop. J. 2013, 33, 178–184. [Google Scholar]
- Stirling, E.R.B.; Lewis, T.L.; Ferran, N.A. Surgical skills simulation in trauma and orthopaedic training. J. Orthop. Surg. Res. 2014, 9, 126. [Google Scholar] [CrossRef]
- Joeris, A.; Höglinger, M.; Meier, F.; Knöfler, F.; Scholz, S.; Brügger, U.; Denk, E.; Gutzwiller, F.; Prein, J.; Renner, N.; et al. The impact of the AO Foundation on fracture care: An evaluation of 60 years AO Foundation. Injury 2019, 50, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Egol, K.A.; Kubiak, E.N.; Fulkerson, E.; Kummer, F.J.; Koval, K.J. Biomechanics of locked plates and screws. J. Orthop. Trauma. 2004, 18, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.; MacDermid, J.; Robinson, S.; Szekeres, M.; Ferreira, L.; Lalone, E. Evaluation of individual finger forces during activities of daily living in healthy individuals and those with hand arthritis. J. Hand Ther. 2020, 33, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; van Munster, V.D.M.; Dijkstra, P.U.; Bos, R.R.M.; Vissink, A.; van Bakelen, N.B.; van Minnen, B. Reliability and accuracy of the torque applied to osteosynthesis screws by maxillofacial surgeons and residents. Sci. Rep. 2022, 12, 14411. [Google Scholar] [CrossRef]
- Acker, W.B.; Tai, B.L.; Belmont, B.; Shih, A.J.; Irwin, T.A.; Holmes, J.R. Two-Finger Tightness: What Is It? Measuring Torque and Reproducibility in a Simulated Model. J. Orthop. Trauma. 2016, 30, 273–277. [Google Scholar] [CrossRef]
- Fletcher, J.W.A.; Neumann, V.; Silva, J.; Burdon, A.; Mys, K.; Panagiotopoulou, V.C.; Gueorguiev, B.; Richards, R.G.; Whitehouse, M.R.; Preatoni, E.; et al. Augmented screwdrivers can increase the performance of orthopaedic surgeons compared with use of normal screwdrivers. Sci. Rep. 2022, 12, 20076. [Google Scholar] [CrossRef]
- Stoesz, M.J.; Gustafson, P.A.; Patel, B.V.; Jastifer, J.R.; Chess, J.L. Surgeon perception of cancellous screw fixation. J. Orthop. Trauma. 2014, 28, e1–e7. [Google Scholar] [CrossRef]
- Caiti, G.; Dobbe, J.G.G.; Bervoets, E.; Beerens, M.; Strackee, S.D.; Strijkers, G.J.; Streekstra, G.J. Biomechanical considerations in the design of patient-specific fixation plates for the distal radius. Med. Biol. Eng. Comput. 2019, 57, 1099–1107. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar]
- Bottlang, M.; Schemitsch, C.E.; Nauth, A.; Routt, M.J.; Egol, K.A.; Cook, G.E.M.; Schemitsch, E.H.M. Biomechanical concepts for fracture fixation. J. Orthop. Trauma. 2015, 29, S28–S33. [Google Scholar] [CrossRef]


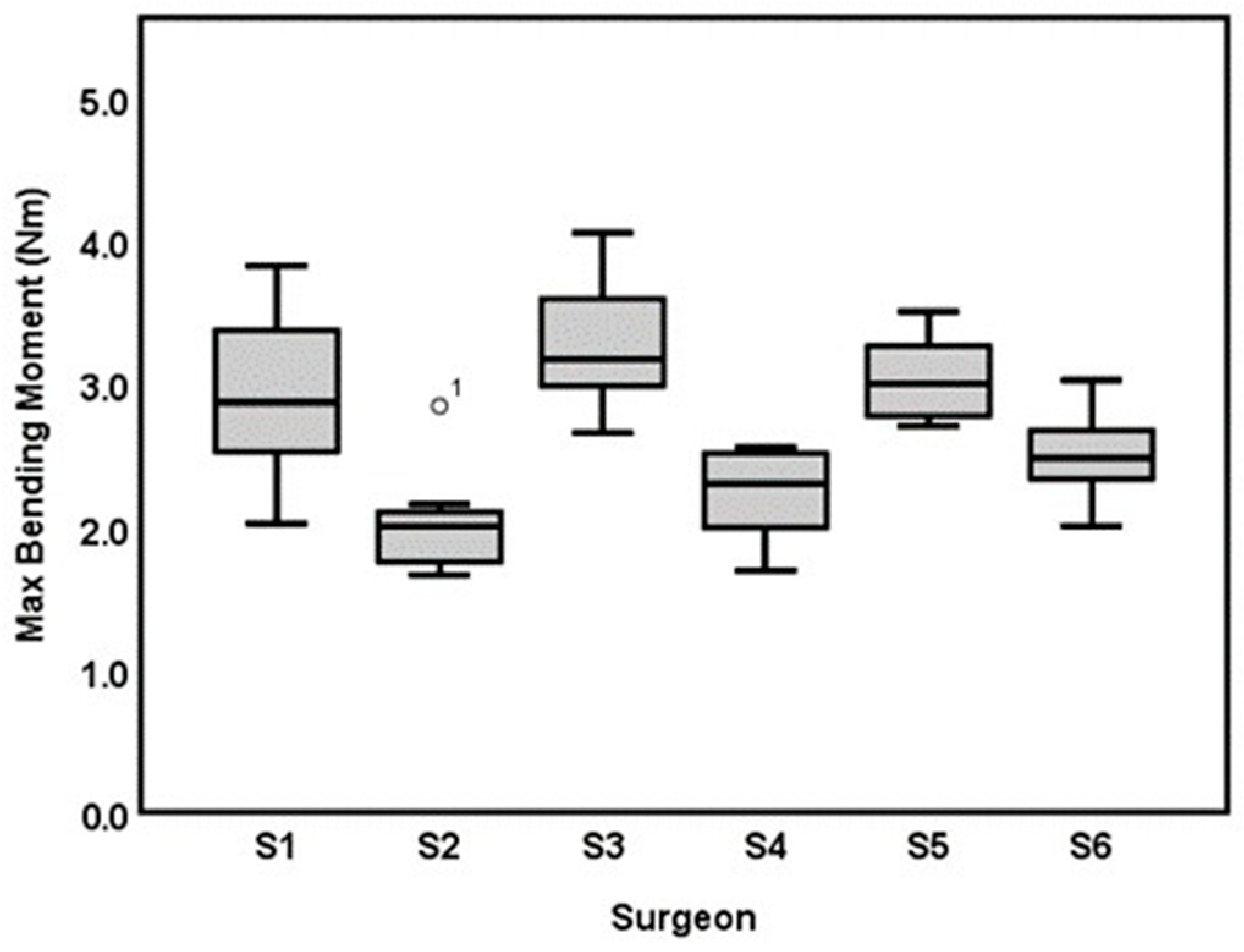
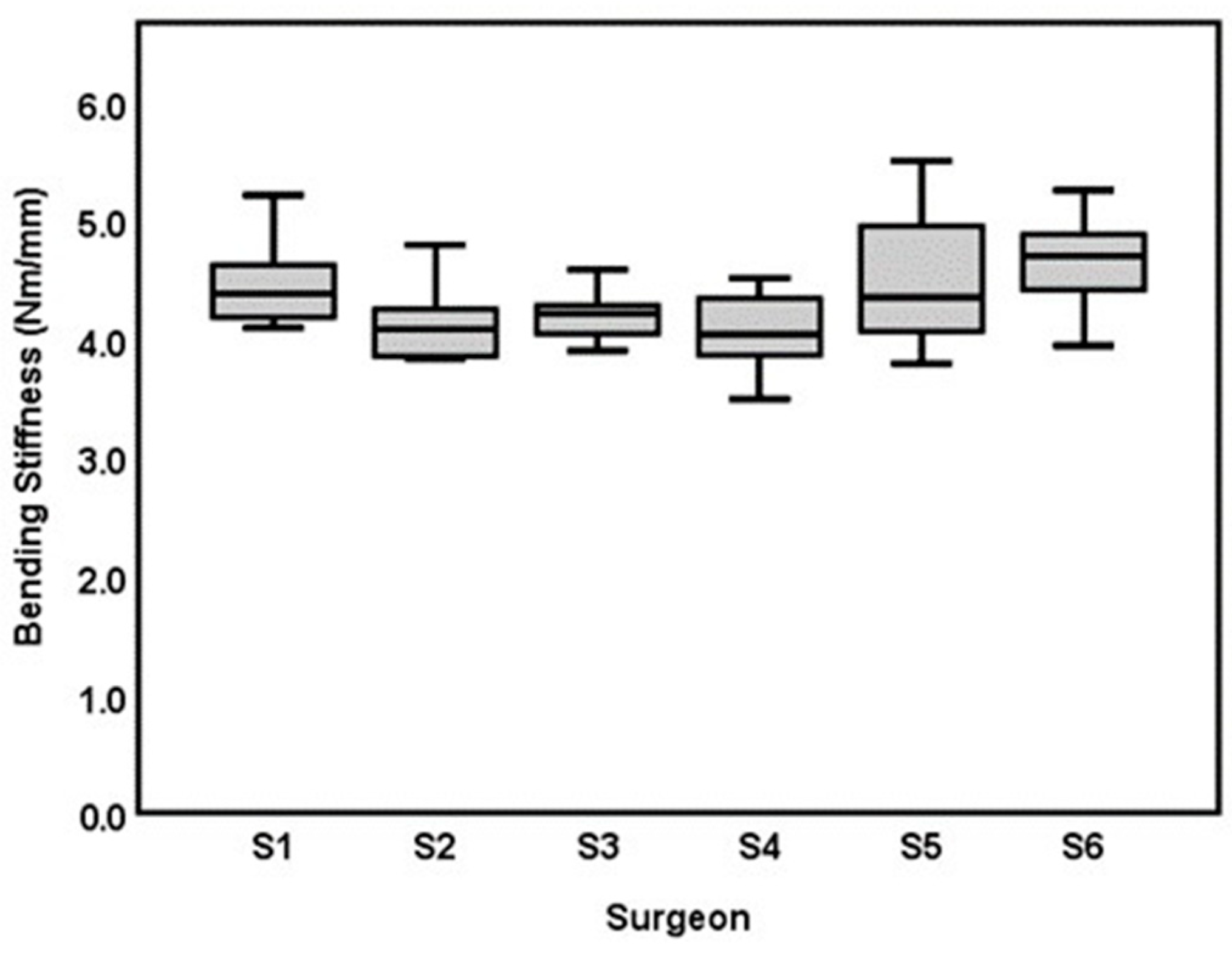
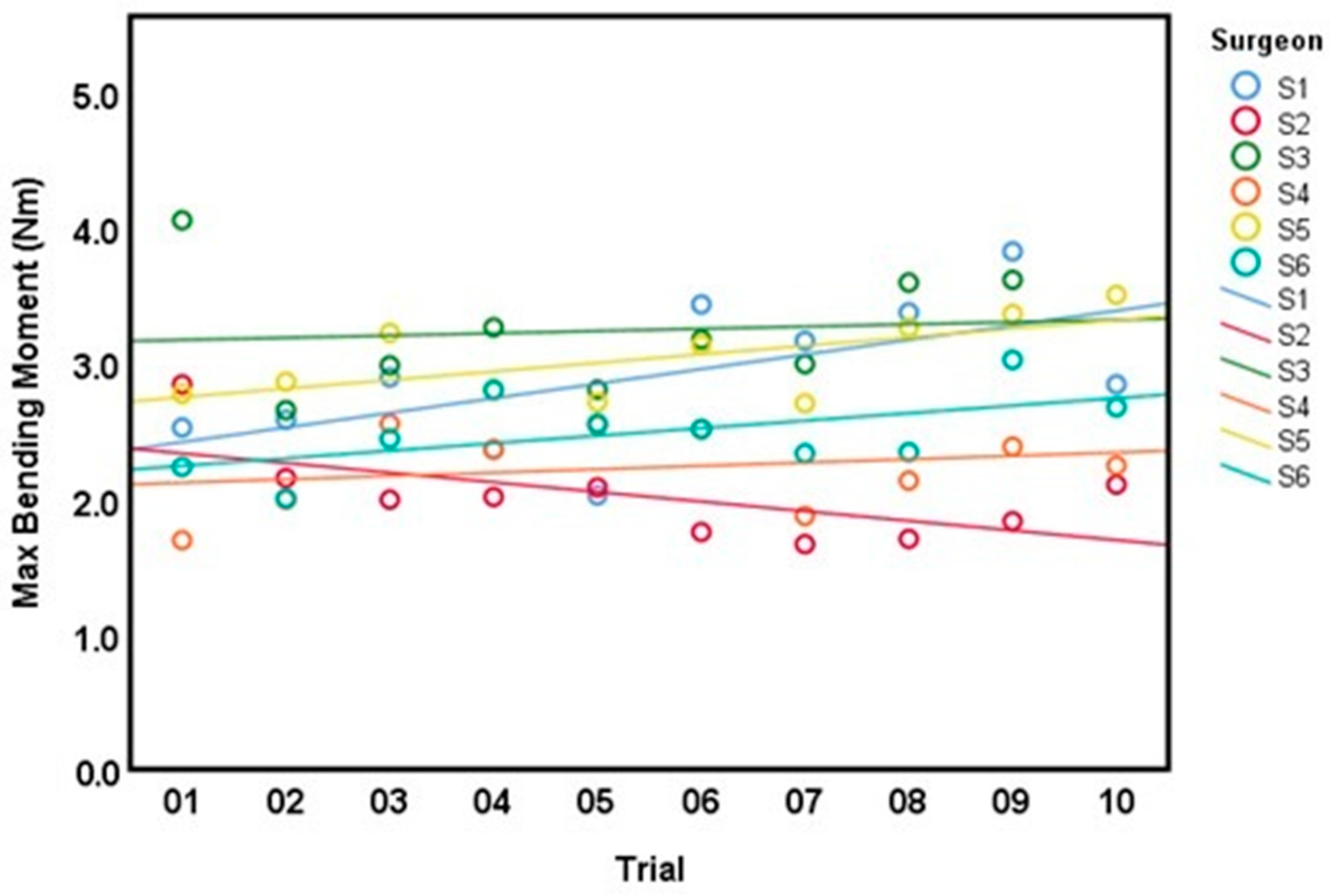
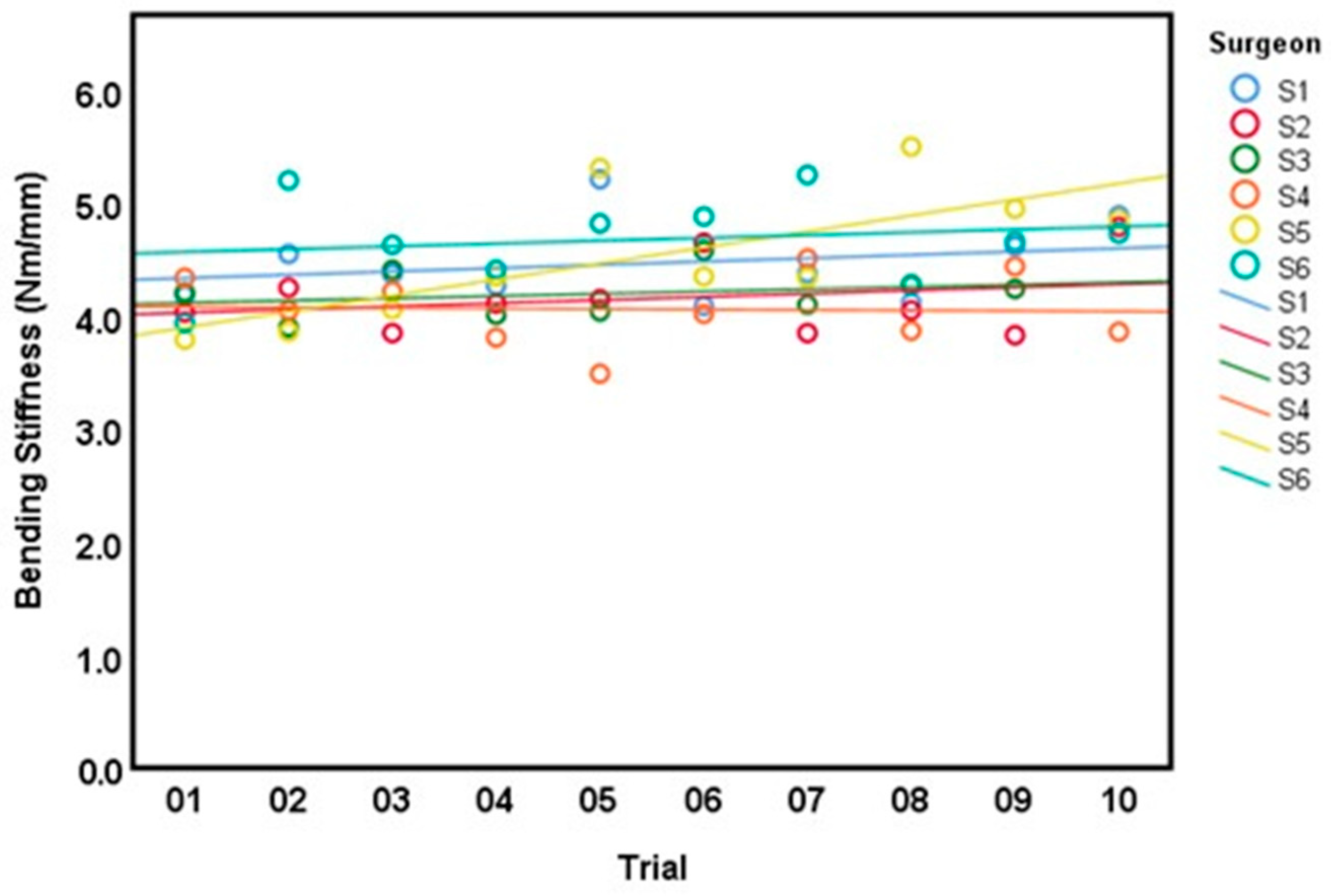
| Surgeon | Bending Moment (Nm)Mean ± SD (CV) | Bending Stiffness (Nm/mm) Mean ± SD (CV) | Cross Sectional Area (mm2) Mean ± SD | Time (min:s), Mean ± SD |
|---|---|---|---|---|
| Surgeon 1: Consultant hand surgeon | 2.90 ± 0.55 (19%) | 4.47 ± 0.36 (8%) | 12.17 ± 2.02 | 13:51 ± 00:27 |
| Surgeon2: Specialist hand surgeon | 2.01 ± 0.34 (18%) | 4.16 ± 0.33 (8%) | 9.24 ± 1.52 | 08:24 ± 00.25 |
| Surgeon 3: Specialist arthroplasty surgeon | 3.23 ± 0.45 (14%) | 4.20 ± 0.21 (5%) | 12.88 ± 1.36 | 11:22 ± 00.34 |
| Surgeon 4: 1st-year Resident | 2.23 ± 0.30 (13.5%) | 4.06 ± 0.32 (7.8%) | 9.14 ± 1.12 | 13:32 ± 01:05 |
| Surgeon 5: Intern (surgical novice) | 3.03 ± 0.30 (10%) | 4.54 ± 0.59 (13%) | 12.77 ± 1.18 | 16:29 ± 01:08 |
| Surgeon 6: 1st-year resident (AdhFix experienced) | 2.49 ± 0.29 (11.6%) | 4.68 ± 0.40 (8.5%) | 9.75 ± 0.84 | 12:24 ± 00:40 |
| Total trials (N = 59) | 2.64 ± 0.57 (21.6%) | 4.35 ± 0.44 (10.1%) | 10.96 ± 2.12 | 12:41 ± 02:37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colding-Rasmussen, T.; Schwarzenberg, P.; Horstmann, P.F.; Ottesen, C.B.S.; Garcia, J.S.J.; Hutchinson, D.J.; Malkoch, M.; Petersen, M.M.; Varga, P.; Tierp-Wong, C.N.E. Biomechanical Variability and Usability of a Novel Customizable Fracture Fixation Technique. Bioengineering 2023, 10, 1146. https://doi.org/10.3390/bioengineering10101146
Colding-Rasmussen T, Schwarzenberg P, Horstmann PF, Ottesen CBS, Garcia JSJ, Hutchinson DJ, Malkoch M, Petersen MM, Varga P, Tierp-Wong CNE. Biomechanical Variability and Usability of a Novel Customizable Fracture Fixation Technique. Bioengineering. 2023; 10(10):1146. https://doi.org/10.3390/bioengineering10101146
Chicago/Turabian StyleColding-Rasmussen, Thomas, Peter Schwarzenberg, Peter Frederik Horstmann, Casper Bent Smedegaard Ottesen, Jorge San Jacinto Garcia, Daniel John Hutchinson, Michael Malkoch, Michael Mørk Petersen, Peter Varga, and Christian Nai En Tierp-Wong. 2023. "Biomechanical Variability and Usability of a Novel Customizable Fracture Fixation Technique" Bioengineering 10, no. 10: 1146. https://doi.org/10.3390/bioengineering10101146