3D-Printed Medical-Grade Polycaprolactone (mPCL) Scaffold for the Surgical Treatment of Vaginal Prolapse and Abdominal Hernias
Abstract
:1. Introduction
2. Materials and Methods
2.1. mPCL Scaffold Preparation
2.2. Preparation of Platelet-Rich Plasma (PRP) and Loading of mPCL Mesh Scaffolds
2.3. Surgical Procedure
2.4. Mechanical Testing of Tissue–Mesh Complexes
2.5. Histology and Immunohistochemistry
2.5.1. Histology
Picrosirius Red staining
2.5.2. Immunohistochemistry
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results
3.1. Surgical Procedure & SEM
3.2. Mechanical Testing
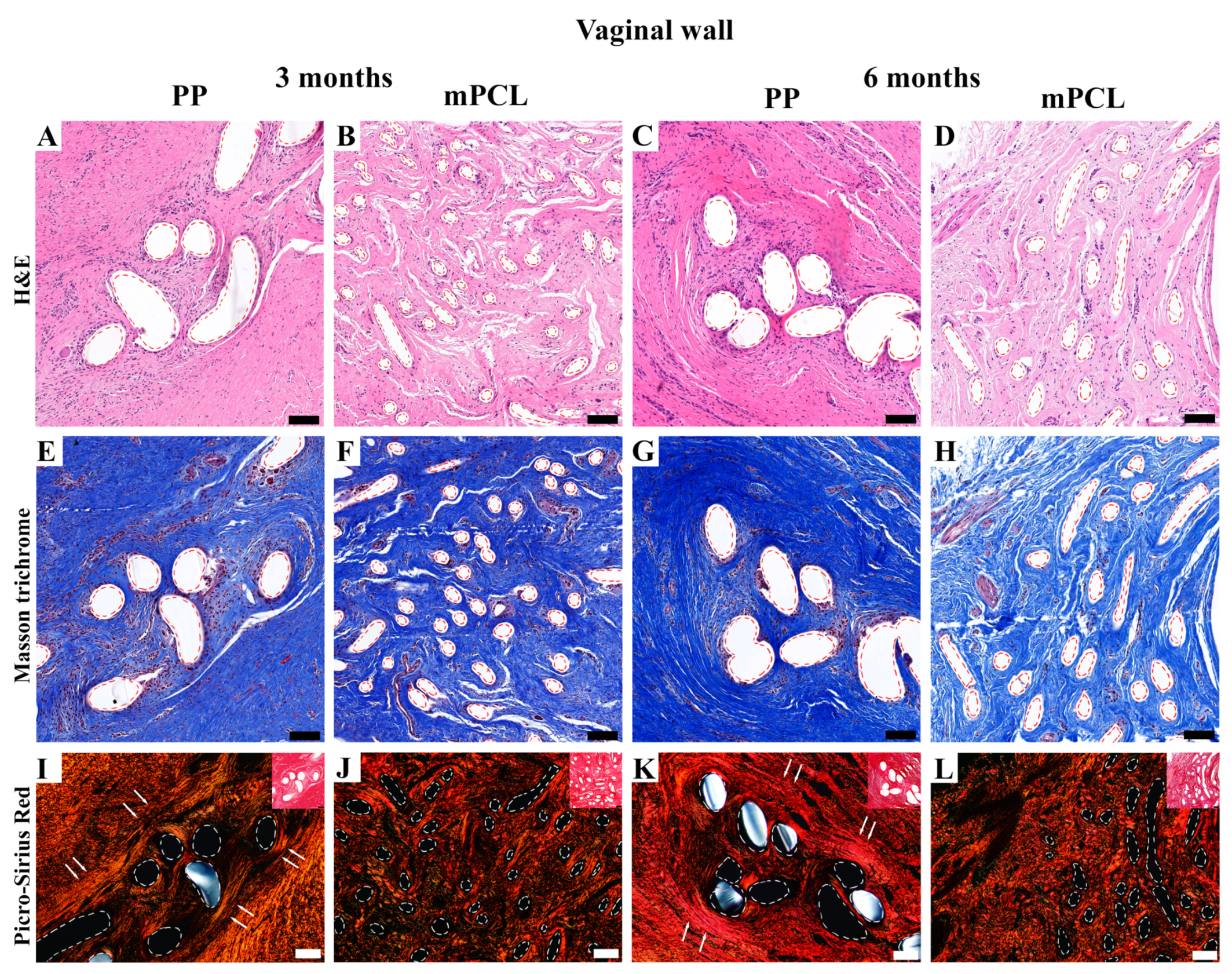
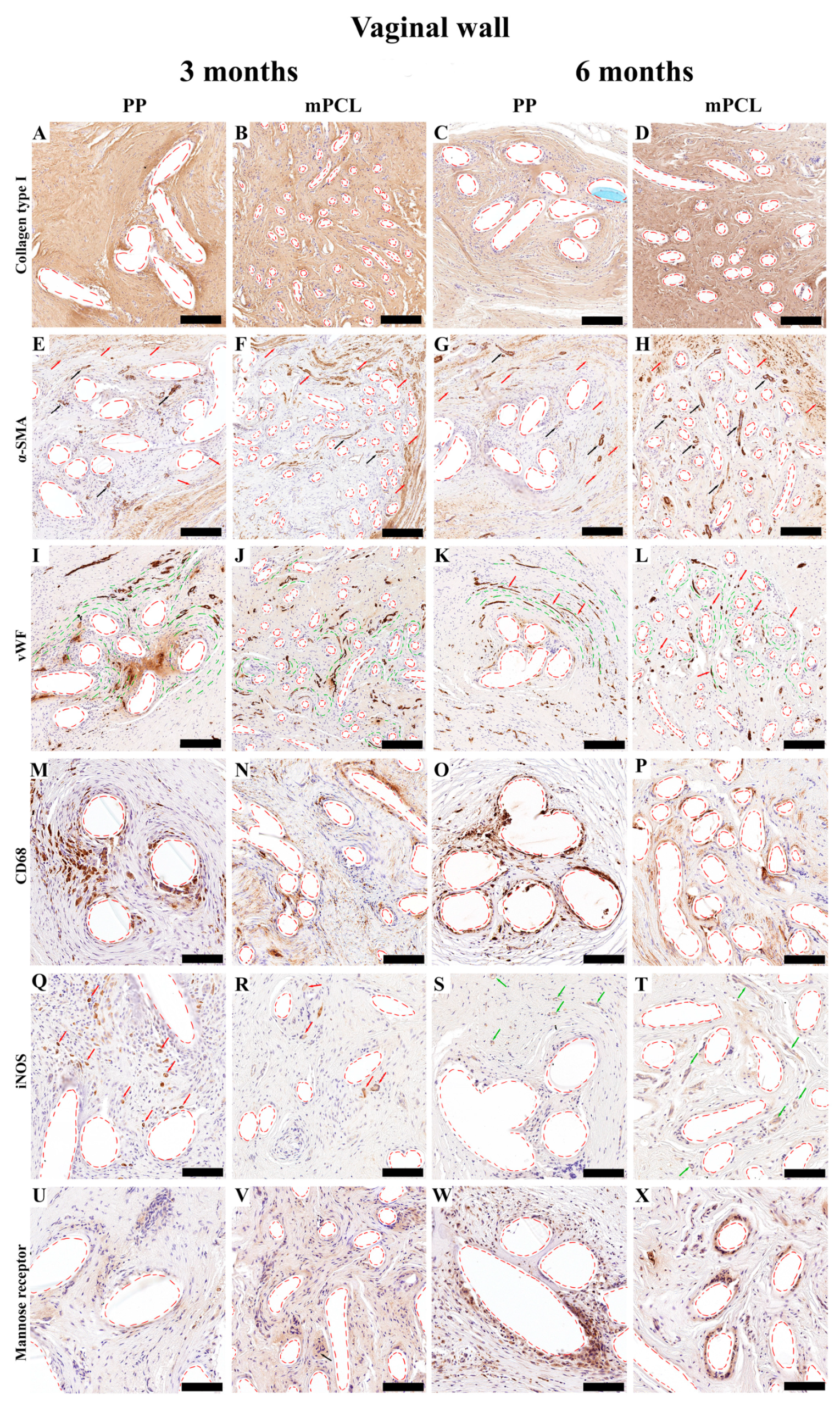
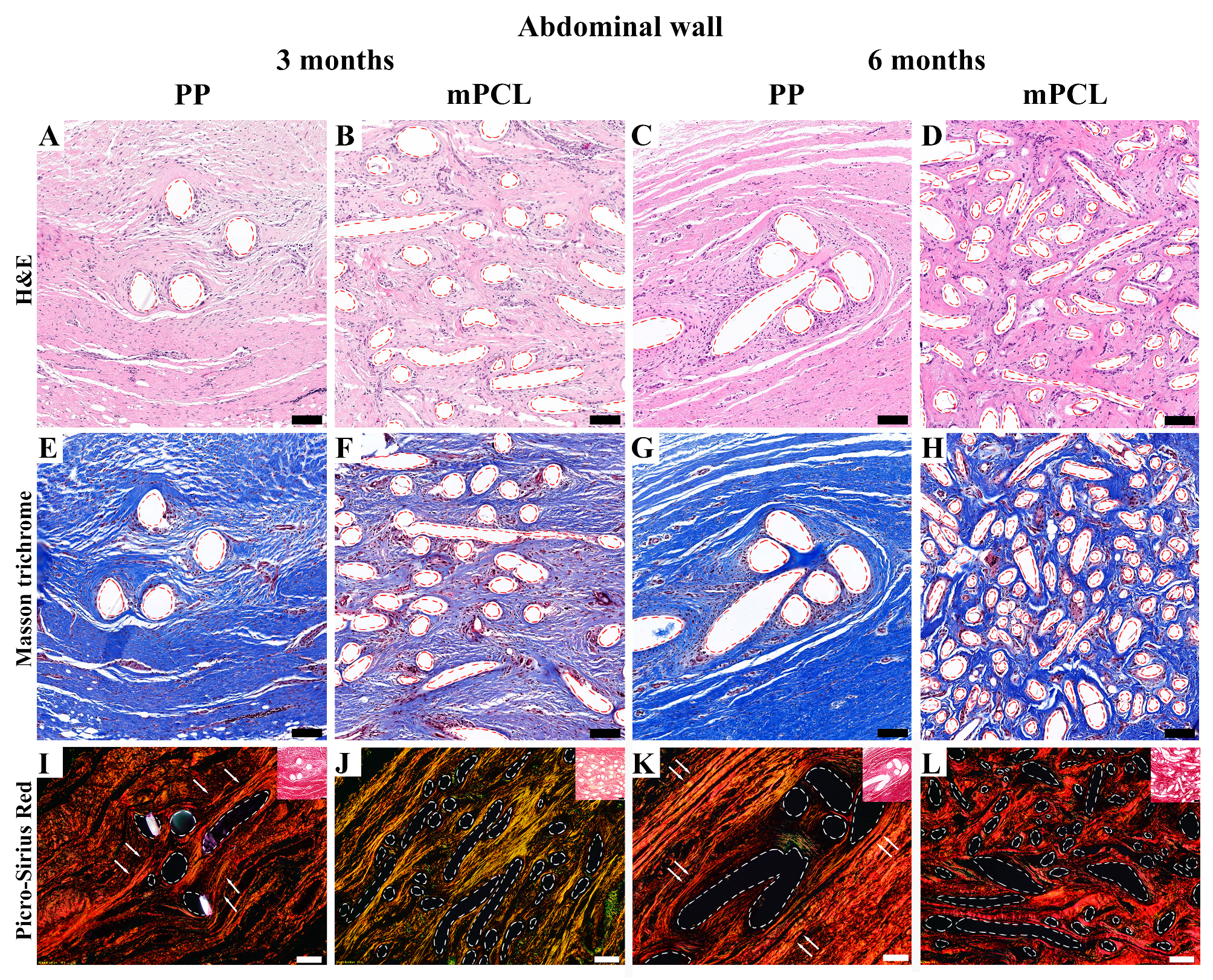
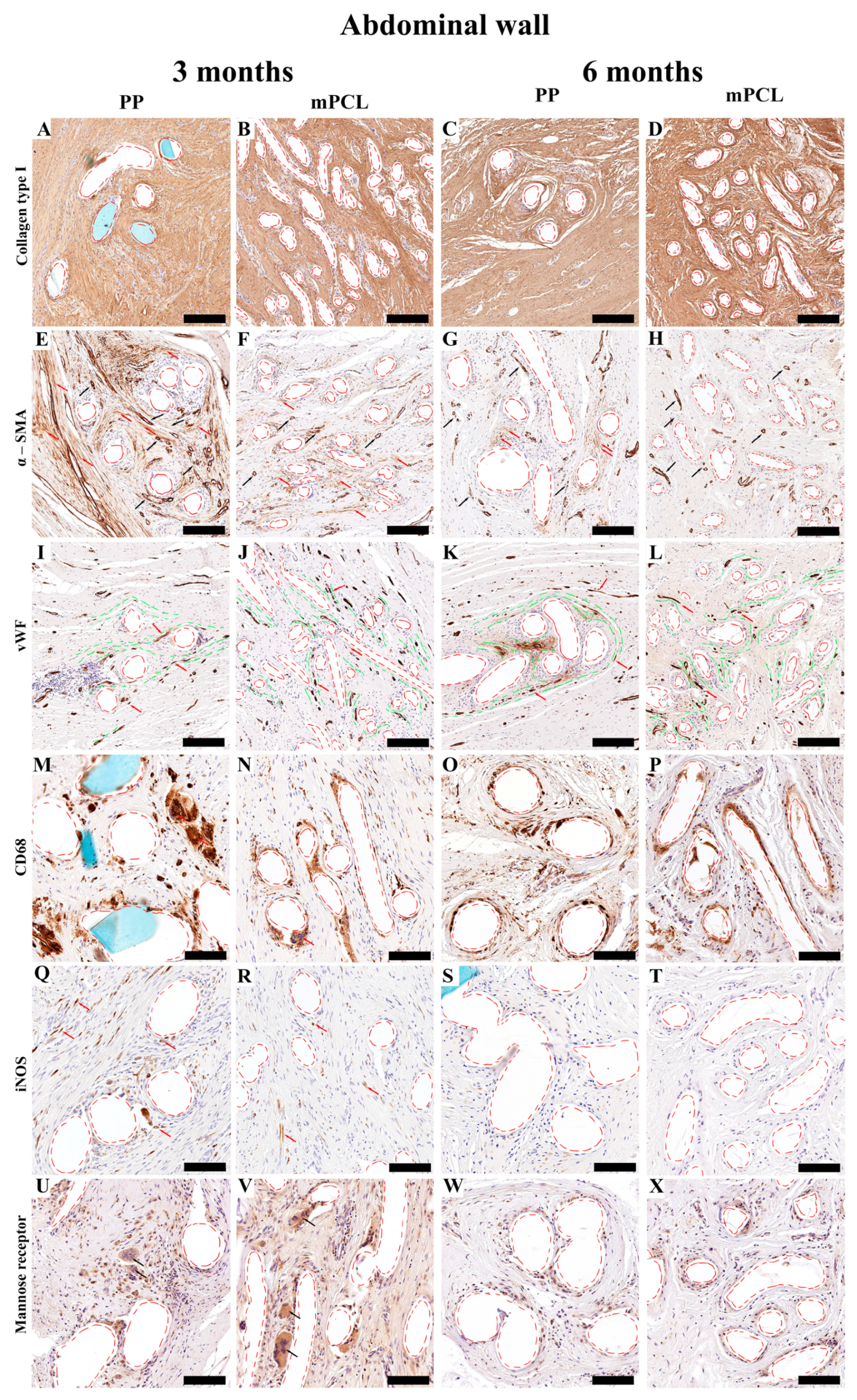
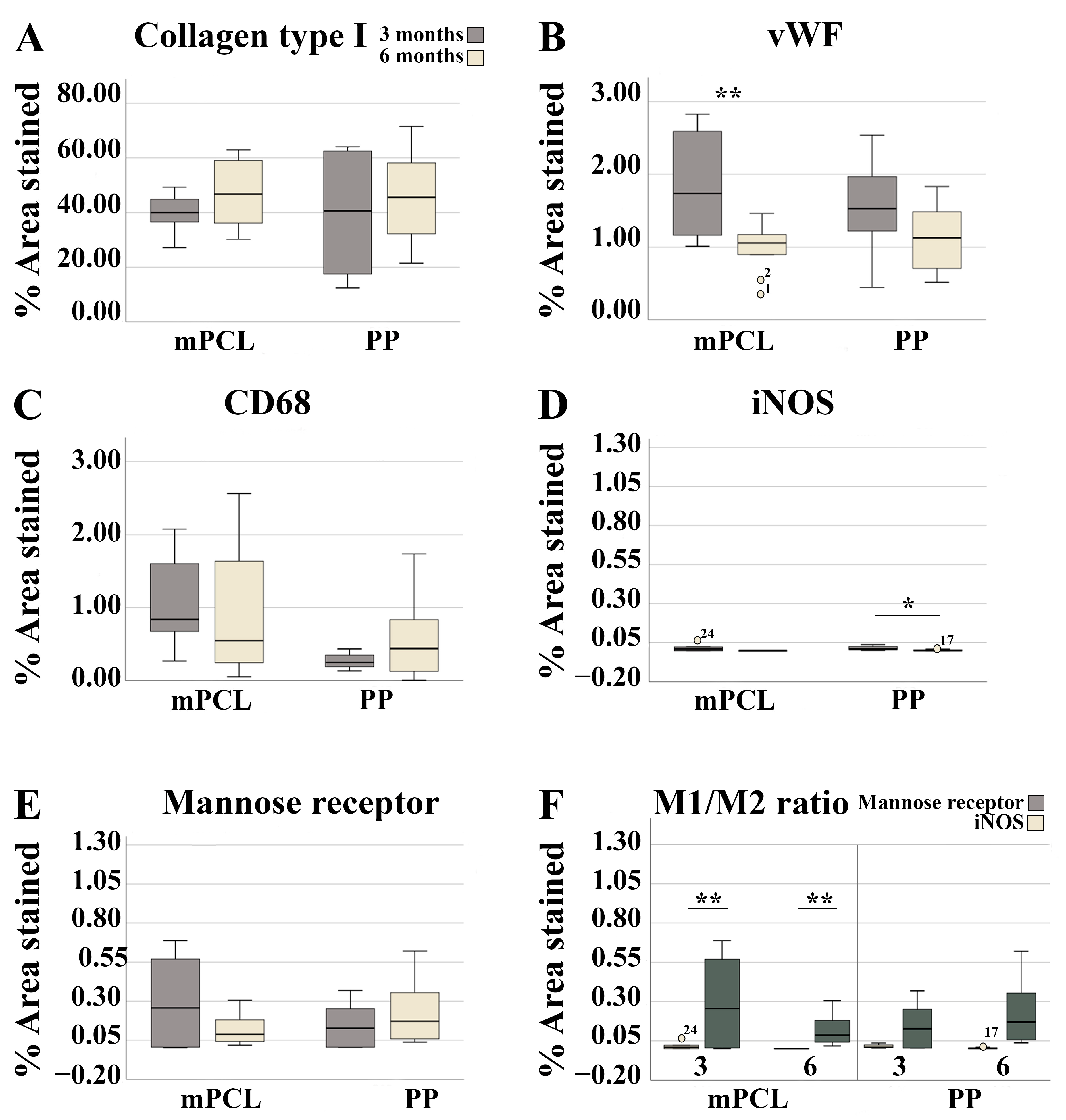
3.3. Histological, Immunohistochemical and Histomorphometric Analysis
3.3.1. Vaginal Tissue Site
3.3.2. Abdominal Tissue Site
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klosterhalfen, B.; Klinge, U. Retrieval Study at 623 Human Mesh Explants Made of Polypropylene-Impact of Mesh Class and Indication for Mesh Removal on Tissue Reaction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Brochhausen, C.; Schmitt, V.H.; Mamilos, A.; Schmitt, C.; Planck, C.N.E.; Rajab, T.K.; Hierlemann, H.; Kirkpatrick, C.J. Expression of CD68 Positive Macrophages in the Use of Different Barrier Materials to Prevent Peritoneal Adhesions—An Animal Study. J. Mater. Sci. Mater. Med. 2017, 28, 15. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; Lake, S.P. Mechanical Properties of the Abdominal Wall and Biomaterials Utilized for Hernia Repair. J. Mech. Behav. Biomed. Mater. 2017, 74, 411–427. [Google Scholar] [CrossRef]
- Finch, D.A.; Misra, V.A.; Hajibandeh, S. Open Darn Repair vs. Open Mesh Repair of Inguinal Hernia: A Systematic Review and Meta-Analysis of Randomised and Non-Randomised Studies. Hernia 2019, 23, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.N.; Badlani, G.H. Mesh Complications in Female Pelvic Floor Reconstructive Surgery and Their Management: A Systematic Review. Indian J. Urol. 2012, 28, 129–153. [Google Scholar] [CrossRef]
- Rovner, E.; de Tayrac, R.; Kirschner-Hermanns, R.; Veit-Rubin, N.; Anding, R. Is Polypropylene Mesh Material Fundamentally Safe for Use as a Reconstructive Material in Vaginal Surgery: ICI-RS 2019? Neurourol. Urodyn. 2020, 39, S132–S139. [Google Scholar] [CrossRef]
- Hilger, W.S.; Walter, A.; Zobitz, M.E.; Leslie, K.O.; Magtibay, P.; Cornella, J. Histological and Biomechanical Evaluation of Implanted Graft Materials in a Rabbit Vaginal and Abdominal Model. Am. J. Obstet. Gynecol. 2006, 195, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Kalaba, S.; Gerhard, E.; Winder, J.S.; Pauli, E.M.; Haluck, R.S.; Yang, J. Design Strategies and Applications of Biomaterials and Devices for Hernia Repair. Bioact. Mater. 2016, 1, 2–17. [Google Scholar] [CrossRef]
- Tayrac, R.; Alves, A.; Thérin, M. Collagen-Coated vs. Noncoated Low-Weight Polypropylene Meshes in a Sheep Model for Vaginal Surgery. A Pilot Study. Int. Urogynecol. J. 2007, 18, 513–520. [Google Scholar] [CrossRef]
- Sehreinemacher, M.H.F.; Emans, P.J.; Gijbels, M.J.J.; Greve, J.W.M.; Beets, G.L.; Bouvy, N.D. Degradation of Mesh Coatings and Intraperitoneal Adhesion Formation in an Experimental Model. Br. J. Surg. 2009, 96, 305–313. [Google Scholar] [CrossRef]
- Zinther, N.B.; Wara, P.; Friis-Andersen, H. Intraperitoneal Onlay Mesh: An Experimental Study of Adhesion Formation in a Sheep Model. Hernia 2010, 14, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Nolfi, A.L.; Brown, B.N.; Liang, R.; Palcsey, S.L.; Bonidie, M.J.; Abramowitch, S.D.; Moalli, P.A. Host Response to Synthetic Mesh in Women with Mesh Complications. Am. J. Obstet. Gynecol. 2016, 215, 206.e1–206.e8. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.D.; Dimarco, D.S.; Zobitz, M.E.; Elliott, D.S. Time Dependent Variations in Biomechanical Properties of Cadaveric Fascia, Porcine Dermis, Porcine Small Intestine Submucosa, Polypropylene Mesh and Autologous Fascia in the Rabbit Model: Implications for Sling Surgery. J. Urol. 2004, 171, 1970–1973. [Google Scholar] [CrossRef]
- Kobashi, K.C.; Govier, F.E. Management of Vaginal Erosion of Polypropylene Mesh Slings. J. Urol. 2003, 169, 2242–2243. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Rodríguez, M.; Gomez-Gil, V.; García-Honduvilla, N.; Buján, J.; Bellón, J.M. Early Tissue Incorporation and Collagen Deposition in Lightweight Polypropylene Meshes: Bioassay in an Experimental Model of Ventral Hernia. Surgery 2008, 144, 427–435. [Google Scholar] [CrossRef]
- Pierce, L.M.; Rao, A.; Baumann, S.S.; Glassberg, J.E.; Kuehl, T.J.; Muir, T.W. Long-Term Histologic Response to Synthetic and Biologic Graft Materials Implanted in the Vagina and Abdomen of a Rabbit Model. Am. J. Obstet. Gynecol. 2009, 200, 546.e1–546.e8. [Google Scholar] [CrossRef]
- Manodoro, S.; Endo, M.; Uvin, P.; Albersen, M.; Vláčil, J.; Engels, A.; Schmidt, B.; De Ridder, D.; Feola, A.; Deprest, J. Graft-Related Complications and Biaxial Tensiometry Following Experimental Vaginal Implantation of Flat Mesh of Variable Dimensions. BJOG An Int. J. Obstet. Gynaecol. 2013, 120, 244–250. [Google Scholar] [CrossRef]
- McGahan, L.; Scott, A. Herniorrhaphy for Inguinal and Femoral Hernia: Review of Clinical Evidence and Guidelines; 2014; Australian Safety & Efficacy Register of New Interventional Procedures–Surgical. Kent Town, Australia. Available online: https://www2.health.vic.gov.au/about/publications/researchandreports/herniorrhaphy-for-inguinal-and-femoral-hernias (accessed on 24 September 2023).
- Maher, C.; Feiner, B.; Baessler, K.; Christmann-Schmid, C.; Haya, N.; Marjoribanks, J. Transvaginal Mesh or Grafts Compared with Native Tissue Repair for Vaginal Prolapse. Cochrane Database Syst. Rev. 2016, 2016, CD012079. [Google Scholar] [CrossRef]
- Zargar, N.; Carr, A. The Regulatory Ancestral Network of Surgical Meshes. PLoS ONE 2018, 13, e0197883. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, J.W.; Park, J.K.; Song, E.H.; Park, S.A.; Kim, Y.S.; Shin, Y.S.; Kim, C.H. Tissue-Engineered Artificial Oesophagus Patch Using Three-Dimensionally Printed Polycaprolactone with Mesenchymal Stem Cells: A Preliminary Report. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 712–717. [Google Scholar] [CrossRef]
- Castrisos, G.; Gonzalez Matheus, I.; Sparks, D.; Lowe, M.; Ward, N.; Sehu, M.; Wille, M.-L.; Phua, Y.; Medeiros Savi, F.; Hutmacher, D.; et al. Regenerative Matching Axial Vascularisation of Absorbable 3D-Printed Scaffold for Large Bone Defects: A First in Human Series. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Savi, F.M.; Dlaska, C.E.; Saifzadeh, S.; Brierly, G.; Ren, E.; Cipitria, A.; Reichert, J.C.; Wille, M.L.; Schuetz, M.A.; et al. Convergence of Scaffold-Guided Bone Regeneration Principles and Microvascular Tissue Transfer Surgery. Sci. Adv. 2023, 9, eadd6071. [Google Scholar] [CrossRef]
- Chhaya, M.P.; Balmayor, E.R.; Hutmacher, D.W.; Schantz, J.-T. Transformation of Breast Reconstruction via Additive Biomanufacturing. Sci. Rep. 2016, 6, 28030. [Google Scholar] [CrossRef] [PubMed]
- Koleske, J.V. Blends Containing Poly(ɛ-Caprolactone) and Related Polymers. In Polymer Blends; Academic Press: New York, NY, USA, 1978; pp. 369–389. [Google Scholar]
- Seen, S.; Young, S.; Lang, S.S.; Lim, T.-C.; Amrith, S.; Sundar, G. Orbital Implants in Orbital Fracture Reconstruction: A Ten-Year Series. Craniomaxillofac. Trauma Reconstr. 2021, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, G.C.; Buettmann, E.G.; MacEwan, M.R.; Tang, M.E.; Frisella, M.M.; Matthews, B.D.; Deeken, C.R. Development of Novel Electrospun Absorbable Polycaprolactone (PCL) Scaffolds for Hernia Repair Applications. Surg. Endosc. 2012, 26, 2717–2728. [Google Scholar] [CrossRef]
- Plencner, M.; East, B.; Tonar, Z.; Otáhal, M.; Prosecká, E.; Rampichová, M.; Krejčí, T.; Litvinec, A.; Buzgo, M.; Míčková, A.; et al. Abdominal Closure Reinforcement by Using Polypropylene Mesh Functionalized with Poly-ε-Caprolactone Nanofibers and Growth Factors for Prevention of Incisional Hernia Formation. Int. J. Nanomed. 2014, 9, 3263–3277. [Google Scholar] [CrossRef]
- Zhao, W.; Ju, Y.M.; Christ, G.; Atala, A.; Yoo, J.J.; Lee, S.J. Diaphragmatic Muscle Reconstruction with an Aligned Electrospun Poly(ε-Caprolactone)/Collagen Hybrid Scaffold. Biomaterials 2013, 34, 8235–8240. [Google Scholar] [CrossRef]
- Plencner, M.; Prosecká, E.; Rampichová, M.; East, B.; Buzgo, M.; Vysloužilová, L.; Hoch, J.; Amler, E. Significant Improvement of Biocompatibility of Polypropylene Mesh for Incisional Hernia Repair by Using Poly-ε-Caprolactone Nanofibers Functionalized with Thrombocyte-Rich Solution. Int. J. Nanomed. 2015, 10, 2635–2646. [Google Scholar] [CrossRef]
- East, B.; Plencner, M.; Otahal, M.; Amler, E.; de Beaux, A.C. Dynamic Creep Properties of a Novel Nanofiber Hernia Mesh in Abdominal Wall Repair. Hernia 2019, 23, 1009–1015. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Direct Writing by Way of Melt Electrospinning. Adv. Mater. 2011, 23, 5651–5657. [Google Scholar] [CrossRef]
- Bas, O.; D’Angella, D.; Baldwin, J.G.; Castro, N.J.; Wunner, F.M.; Saidy, N.T.; Kollmannsberger, S.; Reali, A.; Rank, E.; De-Juan-Pardo, E.M.; et al. An Integrated Design, Material, and Fabrication Platform for Engineering Biomechanically and Biologically Functional Soft Tissues. ACS Appl. Mater. Interfaces 2017, 9, 29430–29437. [Google Scholar] [CrossRef]
- Konerding, M.A.; Chantereau, P.; Delventhal, V.; Holste, J.L.; Ackermann, M. Biomechanical and Histological Evaluation of Abdominal Wall Compliance with Intraperitoneal Onlay Mesh Implants in Rabbits: A Comparison of Six Different State-of-the-Art Meshes. Med. Eng. Phys. 2012, 34, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Savi, F.; Mieszczanek, P.; Revert, S.; Wille, M.L.; Bray, L.J. A New Automated Histomorphometric MATLAB Algorithm for Immunohistochemistry Analysis Using Whole Slide Imaging. Tissue Eng. Part C Methods 2020, 26, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Bas, O.; Catelas, I.; De-Juan-Pardo, E.M.; Hutmacher, D.W. The Quest for Mechanically and Biologically Functional Soft Biomaterials via Soft Network Composites. Adv. Drug Deliv. Rev. 2018, 132, 214–234. [Google Scholar] [CrossRef] [PubMed]
- Hympánová, L.; Rynkevic, R.; Román, S.; Mori da Cunha, M.G.M.C.; Mazza, E.; Zündel, M.; Urbánková, I.; Gallego, M.R.; Vange, J.; Callewaert, G.; et al. Assessment of Electrospun and Ultra-Lightweight Polypropylene Meshes in the Sheep Model for Vaginal Surgery. Eur. Urol. Focus 2018, 6, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Bellón, J.M.; Buján, J.; Contreras, L.; Hernando, A. Integration of Biomaterials Implanted into Abdominal Wall: Process of Scar Formation and Macrophage Response. Biomaterials 1995, 16, 381–387. [Google Scholar] [CrossRef]
- Diedrich, C.M.; Guler, Z.; Hympanova, L.; Vodegel, E.; Zündel, M.; Mazza, E.; Deprest, J.; Roovers, J.P. Evaluation of the Short-Term Host Response and Biomechanics of an Absorbable Poly-4-hydroxybutyrate Scaffold in a Sheep Model Following Vaginal Implantation. BJOG An Int. J. Obstet. Gynaecol. 2022, 129, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.; Rubod, C.; Brieu, M.; Dedet, B.; De Landsheere, L.; Delmas, V.; Cosson, M. Vagina, Abdominal Skin, and Aponeurosis: Do They Have Similar Biomechanical Properties? Int. Urogynecol. J. 2011, 22, 23–27. [Google Scholar] [CrossRef]
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Zhu, L.-M. Mesh Implants: An Overview of Crucial Mesh Parameters. World J. Gastrointest. Surg. 2015, 7, 226. [Google Scholar] [CrossRef]
- Elango, S.; Perumalsamy, S.; Ramachandran, K.; Vadodaria, K. Mesh Materials and Hernia Repair. BioMedicine 2017, 7, 16. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, Z.; Yang, Y.; Chi, J.; Qiao, X.; Han, B.; Liu, W. Study of a New Biodegradable Hernia Patch to Repair Abdominal Wall Defect in Rats. Carbohydr. Polym. 2017, 172, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.; Barron, L. Macrophages: Master Regulators of Inflammation and Fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory Effects of Biomaterial Modification Strategies on Macrophage Polarization and Bone Regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Corliss, B.A.; Azimi, M.S.; Munson, J.M.; Peirce, S.M.; Murfee, W.L. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation 2016, 23, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The Role of Macrophage Phenotype in Vascularization of Tissue Engineering Scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Dondossola, E.; Friedl, P. Host Responses to Implants Revealed by Intravital Microscopy. Nat. Rev. Mater. 2021, 7, 6–22. [Google Scholar] [CrossRef]
- Est, S.; Roen, M.; Chi, T.; Simien, A.; Castile, R.M.; Thompson, D.M.; Blatnik, J.A.; Deeken, C.R.; Lake, S.P. Multi-Directional Mechanical Analysis of Synthetic Scaffolds for Hernia Repair. J. Mech. Behav. Biomed. Mater. 2017, 71, 43–53. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Chhaya, M.P.; Wunner, F.M.; Jeon, J.E.; Klein, T.J.; Hutmacher, D.W. Enhancing Structural Integrity of Hydrogels by Using Highly Organised Melt Electrospun Fibre Constructs. Eur. Polym. J. 2015, 72, 451–463. [Google Scholar] [CrossRef]
- Richards, I. Prolapses in Sheep. The University of Ohio. 2021. Available online: https://u.osu.edu/sheep/2021/12/14/prolapses-in-sheep/ (accessed on 24 September 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo Serafini, M.; Mowat, A.; Mustafa, S.; Saifzadeh, S.; Shabab, T.; Bas, O.; O’Rourke, N.; W. Hutmacher, D.; Medeiros Savi, F. 3D-Printed Medical-Grade Polycaprolactone (mPCL) Scaffold for the Surgical Treatment of Vaginal Prolapse and Abdominal Hernias. Bioengineering 2023, 10, 1242. https://doi.org/10.3390/bioengineering10111242
Russo Serafini M, Mowat A, Mustafa S, Saifzadeh S, Shabab T, Bas O, O’Rourke N, W. Hutmacher D, Medeiros Savi F. 3D-Printed Medical-Grade Polycaprolactone (mPCL) Scaffold for the Surgical Treatment of Vaginal Prolapse and Abdominal Hernias. Bioengineering. 2023; 10(11):1242. https://doi.org/10.3390/bioengineering10111242
Chicago/Turabian StyleRusso Serafini, Mairim, Alexandra Mowat, Susanah Mustafa, Siamak Saifzadeh, Tara Shabab, Onur Bas, Nicholas O’Rourke, Dietmar W. Hutmacher, and Flavia Medeiros Savi. 2023. "3D-Printed Medical-Grade Polycaprolactone (mPCL) Scaffold for the Surgical Treatment of Vaginal Prolapse and Abdominal Hernias" Bioengineering 10, no. 11: 1242. https://doi.org/10.3390/bioengineering10111242
APA StyleRusso Serafini, M., Mowat, A., Mustafa, S., Saifzadeh, S., Shabab, T., Bas, O., O’Rourke, N., W. Hutmacher, D., & Medeiros Savi, F. (2023). 3D-Printed Medical-Grade Polycaprolactone (mPCL) Scaffold for the Surgical Treatment of Vaginal Prolapse and Abdominal Hernias. Bioengineering, 10(11), 1242. https://doi.org/10.3390/bioengineering10111242









