Recent Progress in Catalyst Development of the Hydrogenolysis of Biomass-Based Glycerol into Propanediols—A Review
Abstract
:1. Introduction
2. Catalysts for Selective Glycerol Hydrogenolysis to 1,2-PDO
2.1. Cu-Based Catalysts
2.1.1. Influence of the Properties of the Cu Metal Component
2.1.2. Effects of Support Properties
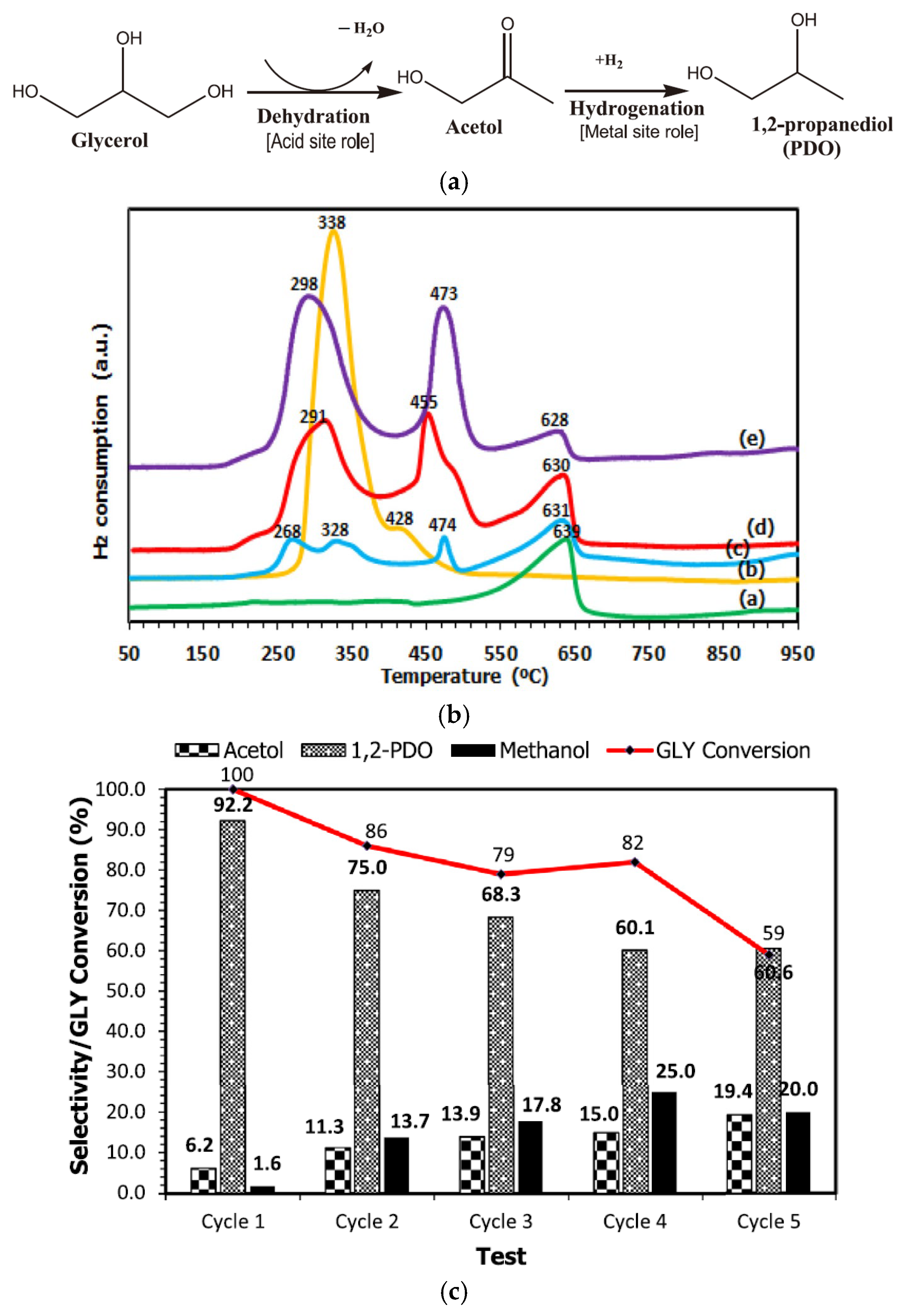
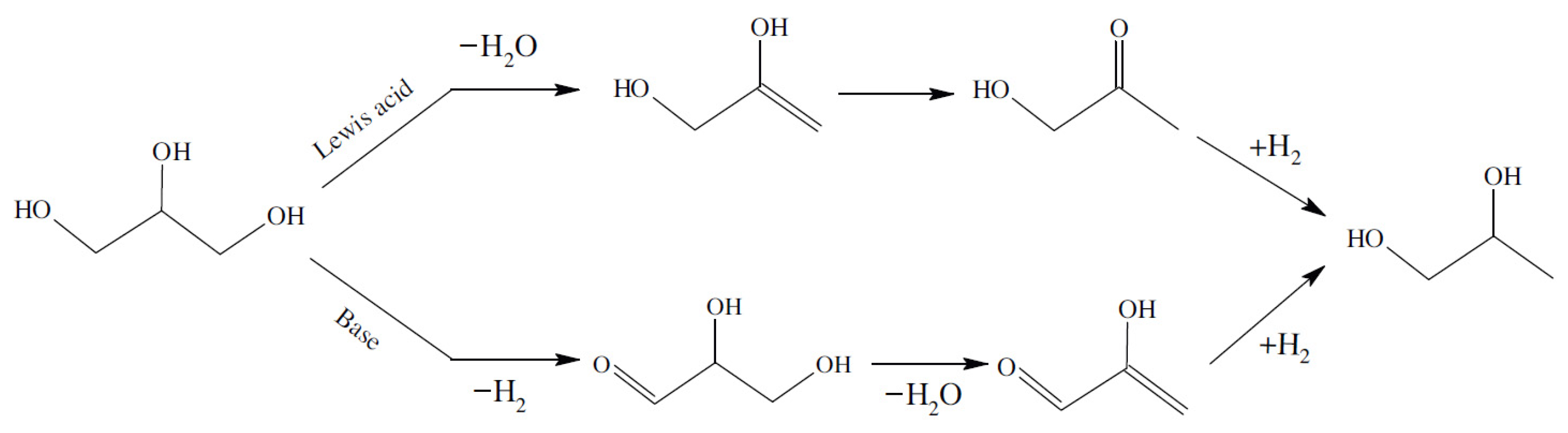
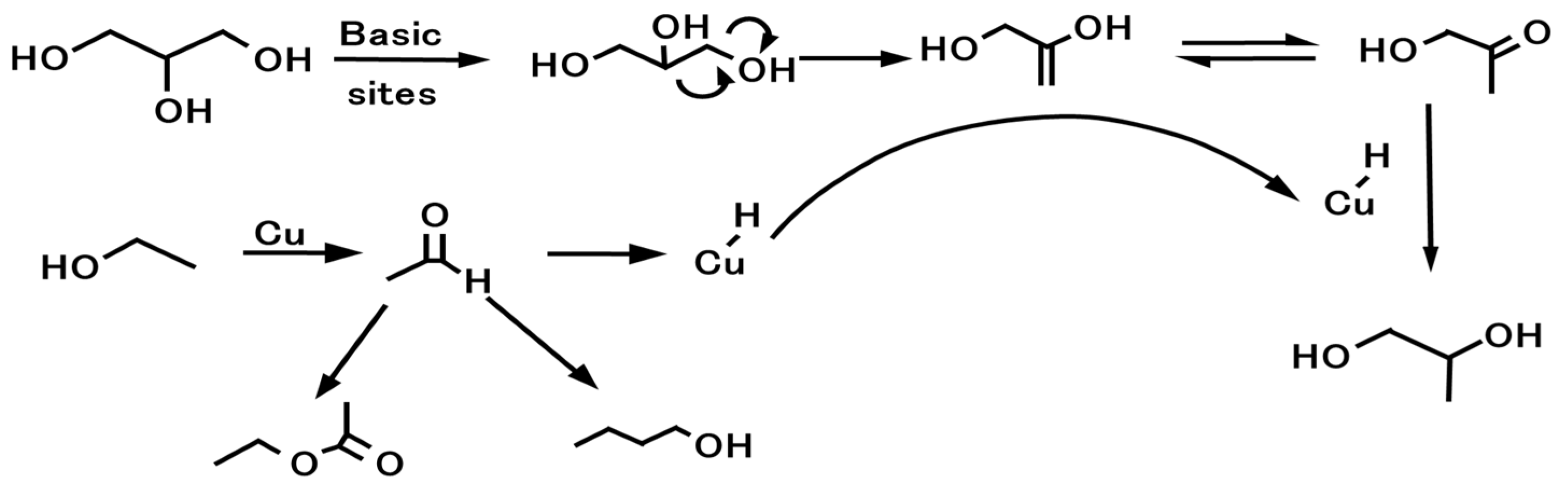
2.1.3. Cu Metal and Support Synergic Effect
2.1.4. Effects of Promoters
2.2. Pt-, Ru-, and Pd-Based Catalysts
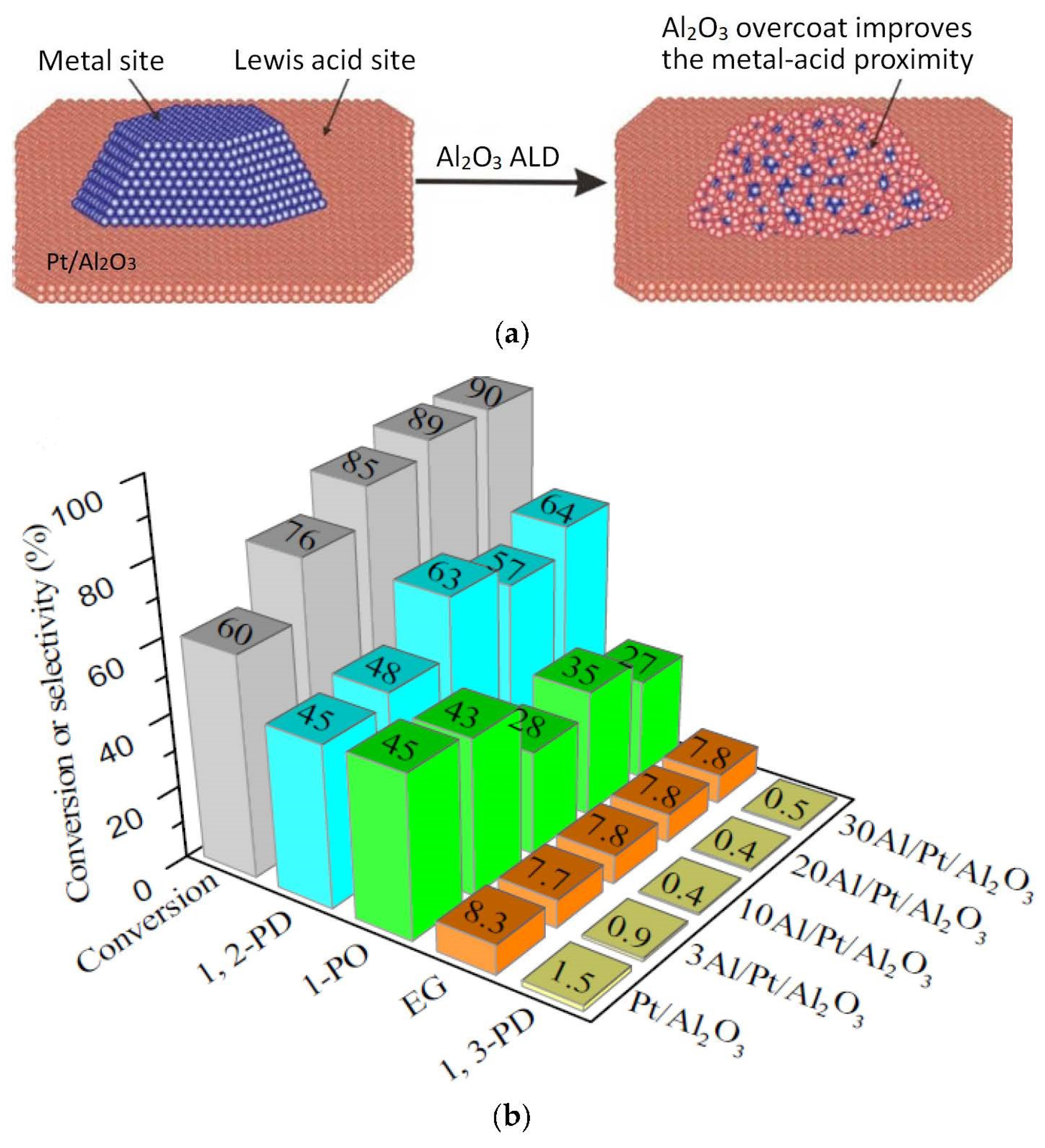
2.2.1. Pt-Based Catalysts
2.2.2. Ru-Based Catalysts
2.2.3. Pd-Based Catalysts
3. Selective Glycerol Hydrogenolysis to 1,3-PDO over Pt-Based and Ir-Based Catalysts
3.1. Pt-Based Catalysts
3.1.1. WOx Species and Supports
3.1.2. Doping and Modification of Pt Catalysts
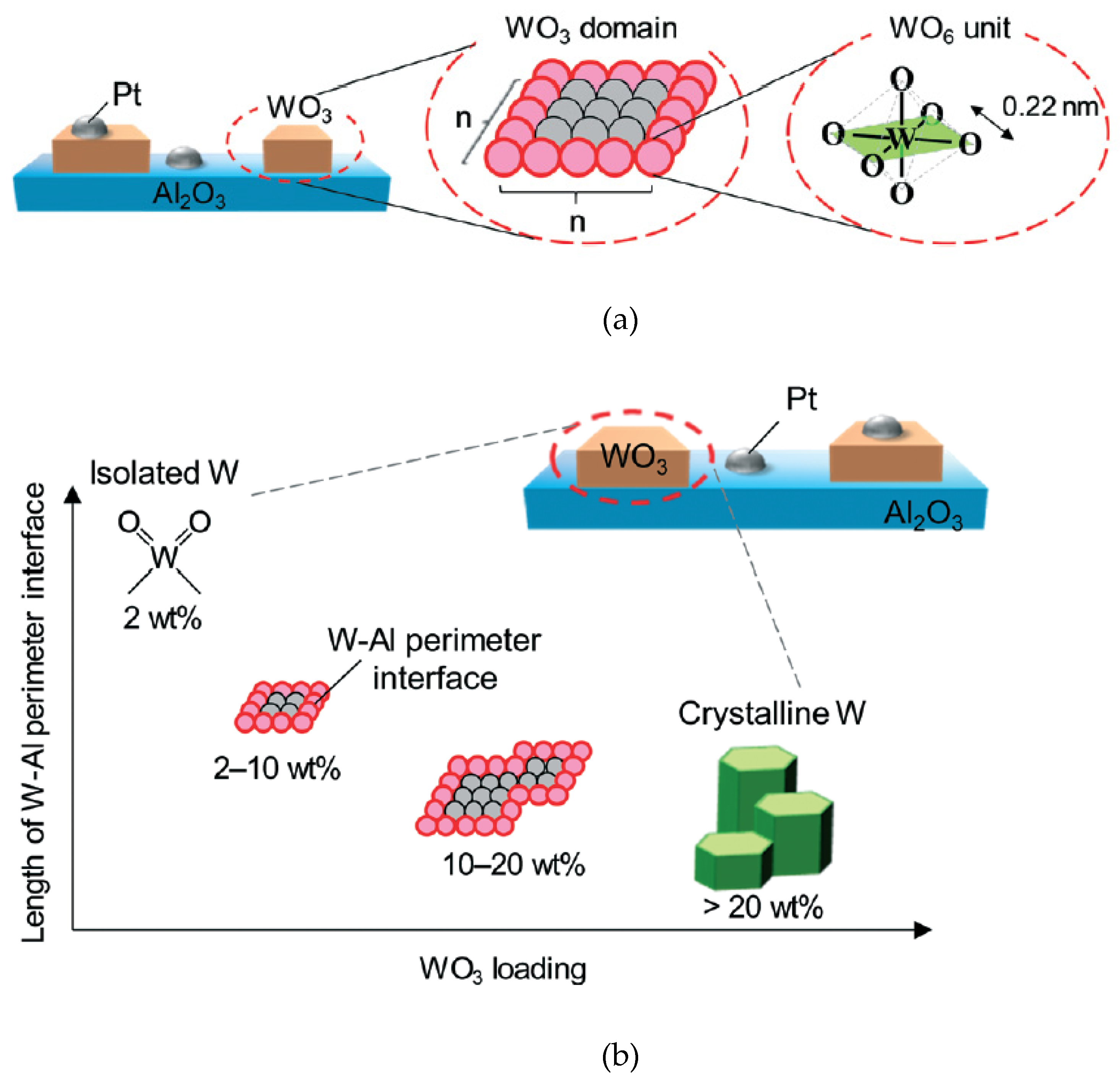
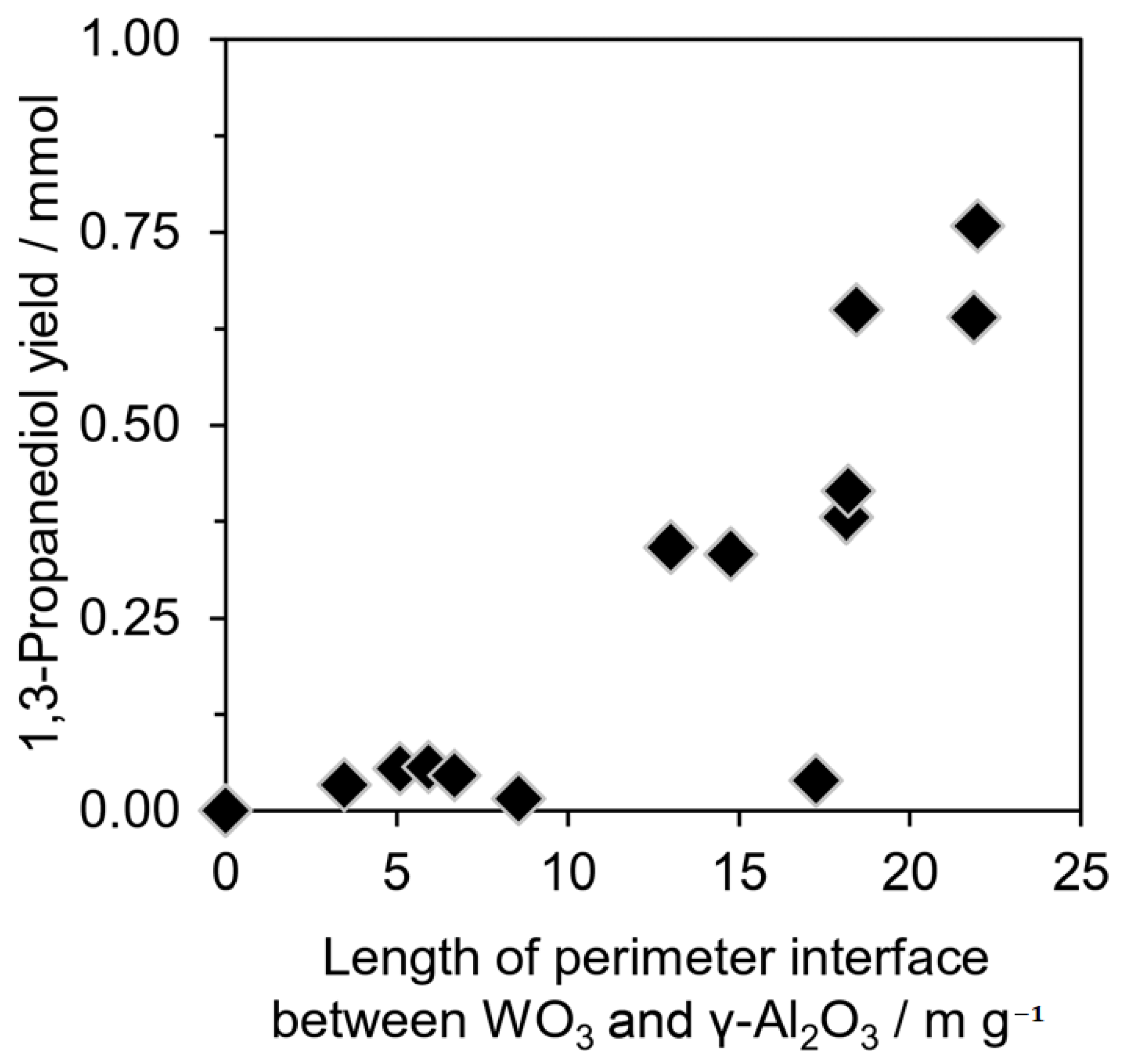
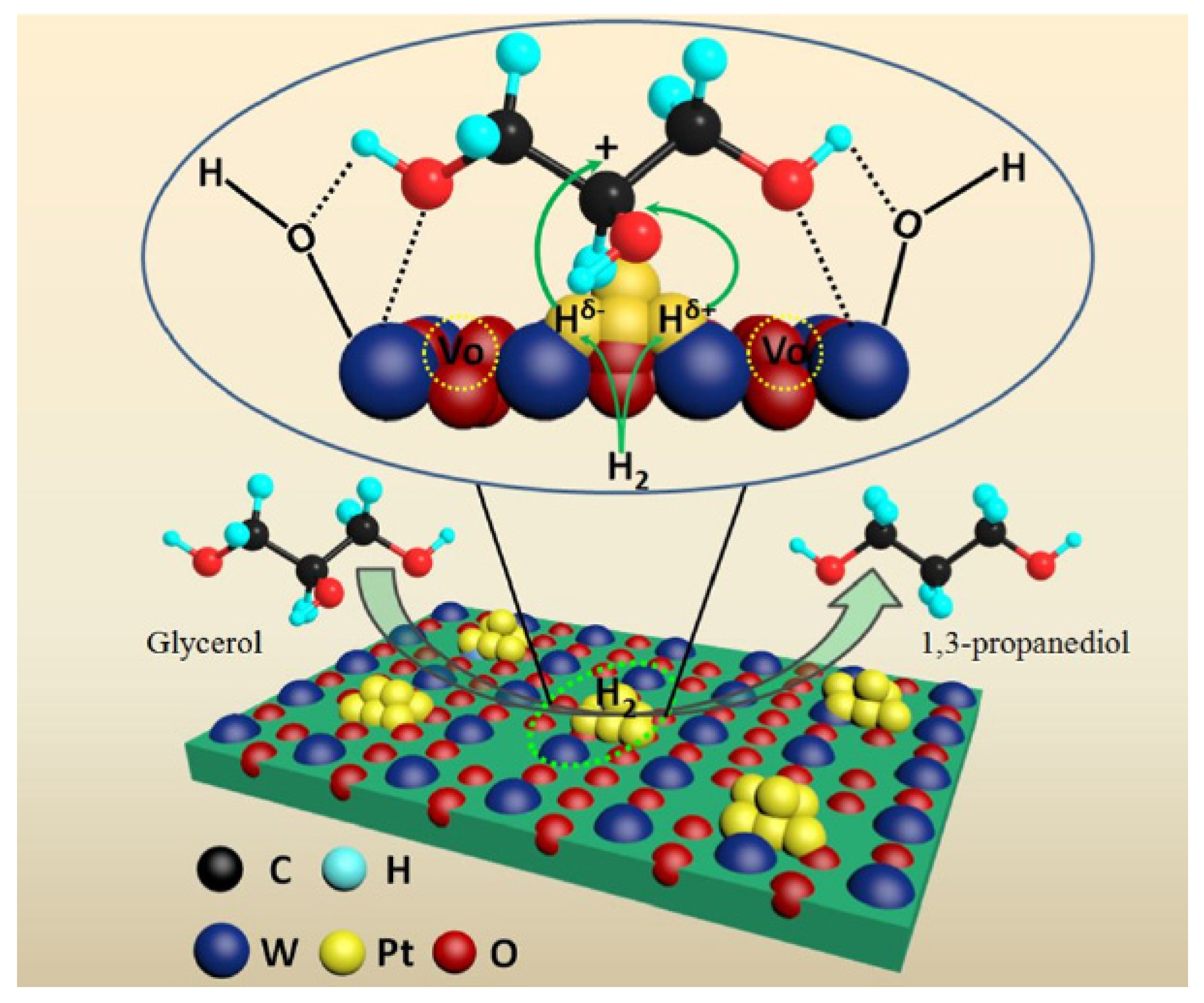
3.1.3. Effects of the Structures and Properties of WOx and Supports
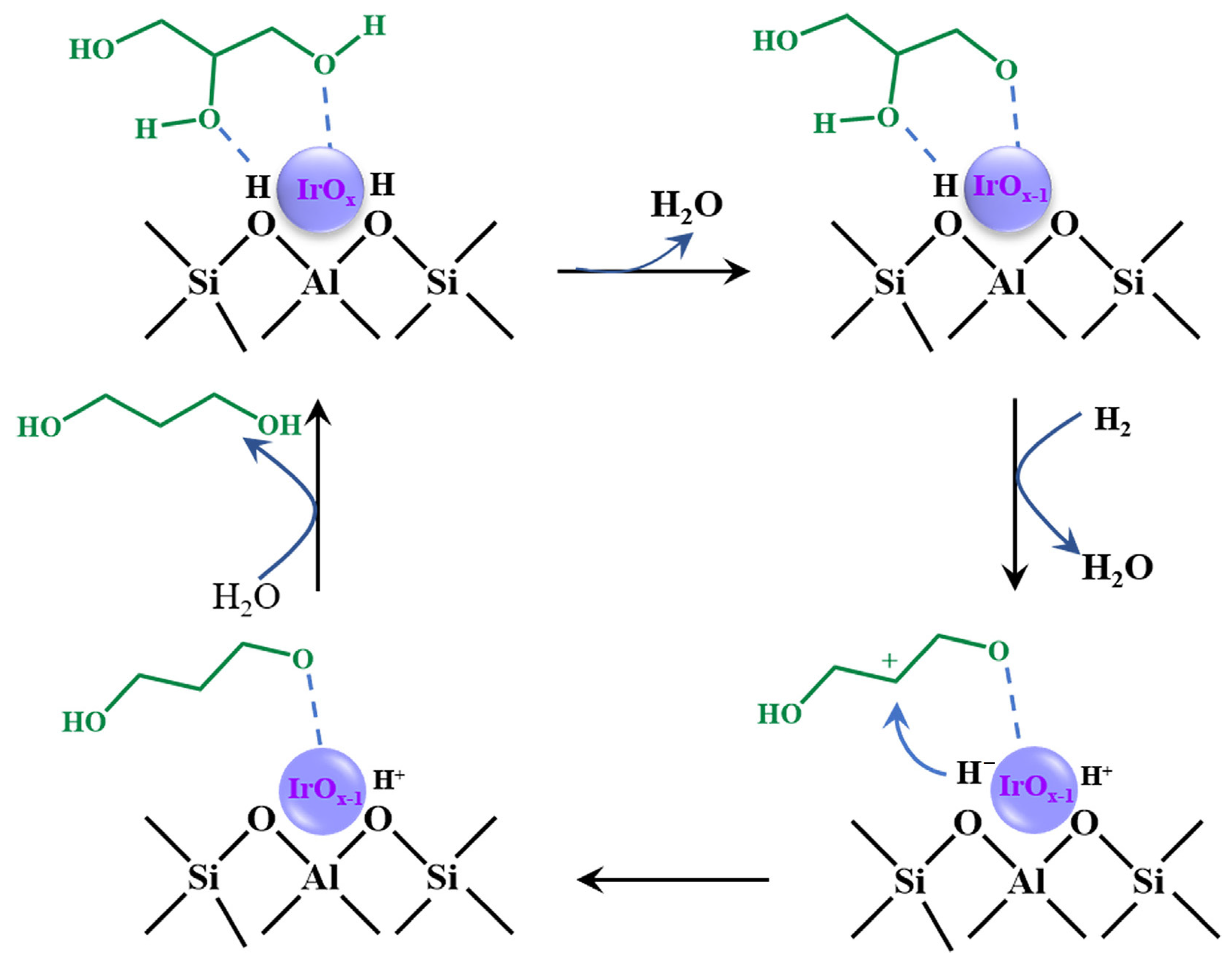
3.2. Ir-Based Catalysts
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleme, J.J.; Varbanov, P.S.; Ocoń, P.; Chin, H.H. Towards efficient and clean process integration: Utilisation of renewable resources and energy-saving technologies. Energies 2019, 12, 4092. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Biruntha, M.; Krishnan, R.Y.; Muthusamy, G.; Karmegam, N. Recent development patterns, utilization and prospective of biofuel production: Emerging nanotechnological intervention for environmental sustainability-A review. Fuel 2022, 314, 122757. [Google Scholar] [CrossRef]
- Arthur, J.; Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Tschaplinski, T. The path forward for biofuels and biomaterials. Science 2006, 5760, 484–489. [Google Scholar]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemes, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Ameen, M.; Ahmad, M.; Zafar, M.; Munir, M.; Mujtaba, M.M.; Sultana, S.; El-Khatib, S.E.; Soudagar, M.E.M.; Kalam, M.A. Prospects of catalysis for process sustainability of eco-green biodiesel synthesis via transesterification: A state-of-the-art review. Sustainability 2022, 14, 7032. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.R.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Mamtani, K.; Shahbaz, K.; Farid, M.M. Glycerolysis of free fatty acids: A review. Renew. Sustain. Energy Rev. 2021, 137, 110501. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2015, 116, 1432–1439. [Google Scholar] [CrossRef]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Al-Qahtani, N. Heterogeneous catalysts for conversion of biodiesel-waste glycerol into high-added-value chemicals. Catalysts 2022, 12, 767. [Google Scholar]
- Zakaria, Z.Y.; Jusoh, M.; Kader, S.S.; Idris, S.S. Challenges & opportunities on catalytic conversion of glycerol to value aAdded chemicals. Bull. Chem. React. Eng. 2021, 16, 525–547. [Google Scholar]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Ruy, A.D.D.S.; Alves, R.M.D.B.; Hewer, T.L.R.; Pontes, D.D.A.; Pontes, L.A.M. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal. Today 2021, 381, 243–253. [Google Scholar]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 183, 75–92. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, X.; Shen, Y. Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem. Rev. 2010, 11, 5253–5277. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Xu, C. Recent advancements in catalytic conversion of glycerol into propylene glycol: A review. Catal. Rev. 2016, 58, 309–336. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic materials for the hydrogenolysis of glycerol to 1,3-propanediol. J. Mater. Chem. A 2014, 2, 6688–6702. [Google Scholar] [CrossRef]
- Lee, C.S.; Aroua, M.K.; Daud, W.M.A.W.; Cognet, P.; Pérès-Lucchese, Y.; Fabre, P.L.; Reynes, O.; Latapie, L. A review: Conversion of bioglycerol into 1,3-propanediol via biological and chemical method. Renew. Sustain. Energy Rev. 2015, 42, 963–972. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Wang, A. Selective hydrogenolysis of glycerol to 1,3-propanediol over Pt-W based catalysts. Chin. J. Catal. 2020, 41, 1311–1319. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, H.; Zhu, X.; Lu, R.; Shi, L.; Lu, F. Effect of tungsten species on selective hydrogenolysis of glycerol to 1,3-Propanediol. ChemSusChem 2021, 14, 569–581. [Google Scholar] [CrossRef]
- Bhowmik, S.; Darbha, S. Advances in solid catalysts for selective hydrogenolysis of glycerol to 1,3-propanediol. Catal. Rev. 2021, 63, 639–703. [Google Scholar] [CrossRef]
- Mane, R.; Jeon, Y.; Rode, C. A review on non-noble metal catalysts for glycerol hydrodeoxygenation to 1,2-propanediol with and without external hydrogen. Green Chem. 2022, 24, 6751–6781. [Google Scholar] [CrossRef]
- Luadthong, C.; Kuboon, S.; Ratanatawanate, C.; Nualpaeng, W.; Viriya-Empikul, N.; Faungnawakij, K.; Pavasant, P. Cu-based spinels for catalytic hydrogenolysis of glycerol to 1,2-propanediol. Sci. Adv. Mater. 2017, 9, 34–41. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Effect of hydrogen donor on glycerol hydrodeoxygenation to 1,2-propanediol. Catal. Today 2020, 355, 727–736. [Google Scholar] [CrossRef]
- Mondal, S.; Janardhan, R.; Meena, M.L.; Biswas, P. Highly active Cu-Zn-Mg-Al-O catalyst derived from layered double hydroxides (LDHs) precursor for selective hydrogenolysis of glycerol to 1,2-propanediol. J. Environ. Chem. Eng. 2017, 5, 5695–5706. [Google Scholar] [CrossRef]
- Durán-Martín, D.; Granados, M.L.; Fierró, J.L.G.; Pinel, C. Deactivation of CuZn catalysts used in glycerol hydrogenolysis to obtain 1,2-propanediol. Top. Catal. 2017, 60, 1062–1071. [Google Scholar] [CrossRef]
- Skuhrovcová, L.; Kolena, J.; Tišler, Z.; Kocík, J. Cu–Zn–Al mixed oxides as catalysts for the hydrogenolysis of glycerol to 1,2-propanediol. React. Kinet. Mech. Cat. 2019, 127, 241–257. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Dmitriev, G.S.; Zanaveskin, K.L.; Egorova, T.B.; Khadzhiev, S.N. Selective hydrogenolysis of glycerol to 1,2-propylene glycol on ultrafine copper particles. Pet. Chem. 2017, 57, 1074–1080. [Google Scholar] [CrossRef]
- Manuale, D.L.; Santiago, L.V.; Torres, G.C.; Sepúlveda, J.H.; Yori, J.C. Hydrogenolysis of glycerol to 1,2-propanediol in a continuous flow trickle bed reactor. J. Chem. Technol. Biot. 2017, 93, 1050–1064. [Google Scholar] [CrossRef]
- Jorge, H.S.F.; Manuale, D.; Santiago, L.; Carrara, N.; Torres, G.; Vera, C.; Gonçalves, M.; Carvalho, W.A.; Mandelli, D. Selective glycerol hydrogenolysis to propylene glycol in a continuous flow trickle bed reactor using copper chromite and Cu/Al2O3 catalysts. Quím. Nova 2017, 40, 371–377. [Google Scholar]
- Lanxi, Z.; Huiguo, W.; Ning, L.; Guohua, L.; Xin, X. Research on hydrogenolysis of glycerol to 1,2-propylene glycol by using supported Raney Cu/Al2O3. China Pet. Process. Petrochem. Technol. 2019, 21, 27–34. [Google Scholar]
- Khadzhiev, V.I.; Dmitriev, G.S.; Mel’Chakov, I.S.; Shorina, T.E.; Maksimov, A.L. Kinetics of hydrogenolysis of glycerol into 1,2-propylene glycol on a copper catalyst. Kinet. Catal. 2019, 60, 802–807. [Google Scholar] [CrossRef]
- Malaya, N.; Zhongshun, Y.; Hengfu, S.; Chunbao, X. Selective glycerol hydrogenolysis and crude glycerol (a by-product or waste stream from the biodiesel industry) to 1,2-propanediol over B2O3 promoted Cu/Al2O3 catalysts. Catalysts 2017, 7, 196. [Google Scholar]
- Azri, N.; Ramli, I.; Nda-Umar, U.I.; Shamsuddin, M.R.; Taufiq-Yap, Y.H. Copper-dolomite as effective catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Taiwan Inst. Chem. Eng. 2020, 112, 34–51. [Google Scholar] [CrossRef]
- Pandhare, N.N.; Pudi, S.M.; Biswas, P.; Sinha, S. Selective hydrogenolysis of glycerol to 1,2-propanediol over highly active and stable Cu/MgO catalyst in vapor phase. Org. Process. Res. Dev. 2016, 20, 1059–1067. [Google Scholar] [CrossRef]
- Mishra, N.K.; Kumar, P.; Srivastava, V.C.; Tangar, U.L. Synthesis of Cu-based catalysts for hydrogenolysis of glycerol to 1,2-propanediol with in-situ generated hydrogen. J. Environ. Chem. Eng. 2021, 9, 105263. [Google Scholar] [CrossRef]
- Luciani, G.; Ruoppolo, G.; Landi, G.; Gargiulo, V.; Alfè, M.; Benedetto, A.D. Glycerol hydrogenolysis to 1,2-propanediol over novel Cu/ZrO2 catalysts. Catalysts 2022, 12, 72. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Rempel, G.L.; Ng, F.T.T. Glycerol hydrogenolysis to produce 1,2-propanediol in absence of molecular hydrogen using a Pd promoted Cu/MgO/Al2O3 catalyst. Catalysts 2021, 11, 1299. [Google Scholar] [CrossRef]
- Liu, Y.; Mai, C.T.Q.; Ng, F.T.T. Glycerol hydrogenolysis with in situ hydrogen produced via methanol steam reforming: The promoting effect of Pd on a Cu/ZnO/Al2O3 catalyst. Catalysts 2021, 11, 110. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, Y.K.; Chang, J.S.; Chae, H.J.; Hwang, D.W. Vapor-phase hydrogenolysis of glycerol to 1,2-propanediol using a chromium-free Ni-Cu-SiO2 nanocomposite catalyst. Catal. Commun. 2016, 84, 5–10. [Google Scholar] [CrossRef]
- Du, H.; Chen, S.; Wang, H.; Lu, J. Acidic alumina overcoating on platinum nanoparticles: Close metal–acid proximity enhances bifunctionality for glycerol hydrogenolysis. Chin. J. Catal. 2017, 38, 1237–1244. [Google Scholar] [CrossRef]
- Kang, Y.; Bu, X.; Wang, G.; Wang, X.; Li, Q.; Feng, Y. A highly active Cu–Pt/SiO2 bimetal for the glycerol hydrogenolysis to 1,2-propanediol. Catal. Lett. 2016, 146, 1408–1414. [Google Scholar] [CrossRef]
- Villa, A.; Manzoli, M.; Vindigni, F.; Chinchilla, L.E.; Botton, G.A.; Prati, L. Diols production from glycerol over Pt-based catalysts: On the role played by the acid sites of the support. Catal. Lett. 2017, 147, 2523–2533. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, G.; Wei, M. PtIn alloy catalysts toward selective glycerol hydrogenolysis to 1,2-propanediol. Ind. Eng. Chem. Res. 2020, 59, 12999–13006. [Google Scholar] [CrossRef]
- Gogoi, P.; Chilukuri, S.; Thirumalaiswamy, R. An active K-OMS-2 supported catalyst for glycerol hydrogenolysis. Chem. Select 2021, 6, 8700–8708. [Google Scholar] [CrossRef]
- He, B.; Li, C.; Xiao, Z.; Wang, B.; Liang, C. Glycerol hydrogenolysis over ruthenium supported on lanthanum modified ZrO2 catalysts. Reac. Kinet. Mech. Cat. 2017, 122, 101–115. [Google Scholar] [CrossRef]
- Bellè, A.; Kusada, K.; Kitagawa, H.; Perosa, A.; Castoldi, L.; Polidoro, D.; Selva, M. Carbon-supported WOx–Ru-based catalysts for the selective hydrogenolysis of glycerol to 1,2-propanediol. Catal. Sci. Technol. 2022, 12, 259–272. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; Xiong, W.; Ding, H.; He, B. Hydrogenolysis of glycerol to 1,2-propanediol and ethylene glycol over Ru-Co/ZrO2 catalysts. Catalysts 2016, 6, 51. [Google Scholar] [CrossRef]
- Magdy, S.; Anne, W.; Valea, K.W.; Anna, B.; Hristiana, V.; Bodo, F.; Jakob, A. Superior CNT-supported bimetallic RuCu catalyst for the highly selective hydrogenolysis of glycerol to 1,2-propanediol. Catal. Sci. Technol. 2021, 11, 6649–6653. [Google Scholar]
- Cai, F.; Jin, F.; Hao, J.; Xiao, G. Selective hydrogenolysis of glycerol to 1,2-propanediol on Nb-modified PdZrAl catalysts. Catal. Commun. 2019, 131, 105801. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; He, X.; Cao, Y.; Wang, C. Sulfate-functionalized metal–organic frameworks supporting Pd nanoparticles for the hydrogenolysis of glycerol to 1,2-propanediol. New J. Chem. 2021, 45, 21263–21269. [Google Scholar] [CrossRef]
- Ardila, A.N.; Sánchez-Castillo, M.A.; Zepeda, T.A.; Villa, A.L.; Fuentes, G.A. Glycerol hydrodeoxygenation to 1,2-propanediol catalyzed by CuPd/TiO2-Na. Appl. Catal. B Environ. 2017, 219, 658–671. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Zhang, B.; Zhang, C.; Lin, W.; Cheng, H.; Zhao, F. Efficient conversion of glycerol to 1,2-propenadiol over ZnPd/ZnO-3Al catalyst: The significant influences of calcination temperature. Catal. Today 2017, 302, 210–216. [Google Scholar] [CrossRef]
- Dam, J.; Hanefeld, U. Renewable chemicals: Dehydroxylation of glycerol and polyols. ChemSusChem 2011, 4, 1017–1034. [Google Scholar] [PubMed]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Perspective on catalyst development for glycerol reduction to C3 chemicals with molecular hydrogen. Res. Chem. Intermed. 2018, 44, 3879–3903. [Google Scholar]
- Zhang, D.; Zhang, Q.; Zhou, Z.; Li, Z.; Meng, K.; Fang, T.; You, Z.; Zhang, G.; Yin, B.; Shen, J. Hydrogenolysis of glycerol to 1,3-propanediol: Are spatial and electronic configuration of “Metal-Solid Acid” interface key for active and durable catalysts? ChemCatChem 2022, 14, 1867–3880. [Google Scholar] [CrossRef]
- Samudrala, S.P.; Kandasamy, S.; Bhattacharya, S. Turning biodiesel waste glycerol into 1,3-Propanediol: Catalytic performance of sulphuric acid-activated montmorillonite supported platinum catalysts in glycerol hydrogenolysis. Sci. Rep. 2018, 8, 7484. [Google Scholar]
- Kurosaka, T.; Maruyama, H.; Naribayashi, I.; Sasaki, Y. Production of 1,3-propanediol by hydrogenolysis of glycerol catalyzed by Pt/WO3/ZrO2. Catal. Commun. 2008, 9, 1360–1363. [Google Scholar]
- Qin, L.Z.; Song, M.J.; Chen, C.L. Aqueous-phase deoxygenation of glycerol to 1,3-propanediol over Pt/WO3/ZrO2 catalysts in a fixed-bed reactor. Green Chem. 2010, 12, 1466–1472. [Google Scholar]
- Mizugaki, T.; Yamakawa, T.; Arundhathi, R.; Mitsudome, T.; Kaneda, K. Selective hydrogenolysis of glycerol to 1,3-propanediol catalyzed by Pt nanoparticles-AlOx/WO3. Chem. Lett. 2012, 41, 1720–1722. [Google Scholar]
- Nakagawa, Y.; Shinmi, Y.; Koso, S.; Tomishige, K. Direct hydrogenolysis of glycerol into 1,3-propanediol over rhenium-modified iridium catalyst. J. Catal. 2010, 272, 191–194. [Google Scholar] [CrossRef]
- Yasushi, A.; Yasunori, S.; Shuichi, K.; Takeshi, K.; Yoshinao, N.; Keiichi, T. Reaction mechanism of the glycerol hydrogenolysis to 1,3-propanediol over Ir–ReOx/SiO2 catalyst. Appl. Catal. B Environ. 2011, 105, 117–127. [Google Scholar]
- Shinmi, Y.; Koso, S.; Kubota, T.; Nakagawa, Y.; Tomishige, K. Modification of Rh/SiO2 catalyst for the hydrogenolysis of glycerol in water. Appl. Catal. B Environ. 2010, 94, 318–326. [Google Scholar]
- Ma, L.; Li, Y.; He, D.H. Influence of catalyst pretreatment on catalytic properties and performances of Ru-Re/SiO2 in glycerol hydrogenolysis to propanediols. Catal. Today 2010, 149, 148–156. [Google Scholar]
- Ma, L.; Li, Y.; He, D.H. Glycerol hydrogenolysis to propanediols over Ru-Re/SiO2: Acidity of catalyst and role of Re. Chin. J. Catal. 2011, 32, 872–876. [Google Scholar]
- Wang, J.; Zhao, X.; Lei, N.; Li, L.; Zhang, L.; Xu, S.; Miao, S.; Pan, X.; Wang, A.; Zhang, T. Hydrogenolysis of glycerol to 1,3-propanediol under low hydrogen pressure over WOx-supported single/pseudo-single atom Pt catalyst. ChemSusChem 2016, 9, 784–790. [Google Scholar]
- Fan, Y.; Cheng, S.; Wang, H.; Ye, D.; Xie, S.; Pei, Y.; Hu, H.; Hua, W.; Li, Z.; Qiao, M. Nanoparticulate Pt on mesoporous SBA-15 doped with extremely low amount of W as a highly selective catalyst for glycerol hydrogenolysis to 1,3-propanediol. Green Chem. 2017, 19, 2174–2183. [Google Scholar]
- Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. Glycerol hydrogenolysis to 1,3-propanediol on tungstate/zirconia-supported platinum: Hydrogen spillover facilitated by Pt(1 1 1) formation. ChemCatChem 2016, 8, 3663–3671. [Google Scholar] [CrossRef]
- Tong, Q.; Gao, Q.; Xu, B.; Yu, L.; Fan, Y. Pt/WO3/ZrO2-catalyzed selective hydrogenolysis of glycerol to produce 1,3-propanediol. Chin. J. Org. Chem. 2017, 37, 753–758. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C. Stabilized hydrogenolysis of glycerol to 1,3-propanediol over Mg modified Pt/WOx –ZrO2 catalysts. React. Kinet. Mech. Cat. 2019, 128, 461–477. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, C.L. Hydrogenolysis of glycerol to 1,3-propanediol over Li2B4O7-modified tungsten-zirconium composite oxides supported platinum catalyst. Reac. Kinet. Mech. Cat. 2018, 124, 683–699. [Google Scholar]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Ma, X. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B Environ. 2018, 242, 410–421. [Google Scholar] [CrossRef]
- Edake, M.; Dalil, M.; Jaber, M.; Mahboub, D.i.; Dubois, J.L.; Patience, G.S. Catalytic glycerol hydrogenolysis to 1,3-propanediol in a gas-solid fluidized bed. RSC Adv. 2017, 7, 3853–3860. [Google Scholar] [CrossRef]
- Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. Effective hydrogenolysis of glycerol to 1,3-propanediol over metal-acid concerted Pt/WOx/Al2O3 catalysts. ChemCatChem 2019, 11, 3903–3912. [Google Scholar]
- Zhao, B.; Liang, Y.; Liu, L.; He, Q.; Dong, J. Facilitating PtWOx species interaction for efficient glycerol hydrogenolysis to 1,3-propanediol. ChemCatChem 2021, 13, 3695–3705. [Google Scholar] [CrossRef]
- Shi, G.; Cao, Z.; Xu, J.; Jin, K.; Bao, Y.; Xu, S. Effect of WOx doping into Pt/SiO2 catalysts for glycerol hydrogenolysis to 1,3-propanediol in liquid phase. Catal. Lett. 2018, 148, 2304–2314. [Google Scholar]
- Feng, S.; Zhao, B.; Liang, Y.; Liu, L.; Dong, J. Improving selectivity to 1,3-propanediol for glycerol hydrogenolysis using W and Al-incorporated SBA-15 as support for Pt nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 2661–2671. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, B.; Liu, L.; Dong, J. Platinum supported on WO3-doped aluminosilicate: A highly efficient catalyst for selective hydrogenolysis of glycerol to 1,3-propanediol. Ind. Eng. Chem. Res. 2017, 56, 11065–11074. [Google Scholar] [CrossRef]
- Yang, Z.; Lan, J.; Xin, Z.X.; Hai, X.S.; Yan, P.; Hua, Q.M.; Hua, L.Z.; Long, X.H. W-doped hierarchically porous silica nanosphere supported platinum for catalytic glycerol hydrogenolysis to 1,3-propanediol. Acta Chim. Sin. 2022, 80, 903–912. [Google Scholar]
- Cheng, S.J.; Fan, Y.Q.; Zhang, X.X.; Zeng, Y.; Xie, S.H.; Pei, Y.; Zeng, G.F.; Qiao, M.H.; Zong, B.N. Tungsten-doped siliceous mesocellular foams-supported platinum catalyst for glycerol hydrogenolysis to 1,3-propanediol. Appl. Catal. B Environ. 2021, 297, 120428. [Google Scholar] [CrossRef]
- Shi, G.; Xu, J.; Song, Z.; Cao, Z.; Jin, K.; Xu, S.; Yan, X. Selective hydrogenolysis of glycerol to 1,3-propanediol over Pt-WOx/SAPO-34 catalysts. Mol. Catal. 2018, 456, 22–30. [Google Scholar] [CrossRef]
- Priya, S.S.; Bhanuchander, P.; Kumar, V.P.; Dumbre, K.; Periasamy, S.R.; Bhargava, S.K.; Kantam, M.L. Platinum supported on H-mordenite: A highly efficient catalyst for selective hydrogenolysis of glycerol to 1,3-propanediol. ACS Sustain. Chem. Eng. 2016, 4, 1212–1222. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, Y.; Liu, L.; He, Q.; Dong, J. Discovering positively charged Pt for enhanced hydrogenolysis of glycerol to 1,3-propanediol. Green Chem. 2020, 22, 8254–8259. [Google Scholar] [CrossRef]
- Zeng, Y.; Jiang, L.; Zhang, X. Efect of titania polymorphs on the structure and catalytic performance of the Pt-WOx/TiO2 catalyst in glycerol hydrogenolysis to 1,3-propanediol. ACS Sustain. Chem. Eng. 2022, 10, 9532–9545. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Jia, H.Y.; Guo, Y.; Wang, Y.G.; Liu, X.H.; Xia, Q.N.; Li, X.; Wang, Y.Q. The promotional effect of sulfates on TiO2 supported Pt-WOx catalyst for hydrogenolysis of glycerol. ChemCatChem 2021, 13, 3953–3959. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, X.; Ren, Y.; Wang, J.; Lei, N.; Wang, A.; Zhang, T. Pt/Nb-WOx for the chemoselective hydrogenolysis of glycerol to 1,3-propanediol: Nb dopant pacifying the over-reduction of WOx supports. Chin. J. Catal. 2018, 39, 1027–1037. [Google Scholar] [CrossRef]
- Xi, Z.; Jia, W.; Zhu, Z. WO3–ZrO2–TiO2 composite oxide supported Pt as an efficient catalyst for continuous hydrogenolysis of glycerol. Catal. Lett. 2021, 151, 124–137. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Yang, M.; Lei, N.; Li, L.; Hou, B.; Miao, S.; Pan, X.; Wang, A.; Zhang, T. Selective hydrogenolysis of glycerol to 1,3-propanediol: Manipulating the frustrated lewis pairs by introducing gold to Pt/WOx. ChemSusChem 2017, 10, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, F.; Lei, N.; Yang, M.; Liu, F.; Zhili, M.Z.; Sun, Y.; Zhao, X.; Wang, A. Understanding the promotional effect of Au on Pt/WO3 in hydrogenolysis of glycerol to 1,3-propanediol. Chin. J. Catal. 2018, 39, 1366–1372. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Guan, W.; Wang, A.; Zhang, T. Promoting the effect of Au on the selective hydrogenolysis of glycerol to 1,3-propanediol over the Pt/WOx/Al2O3 catalyst. ACS Sustain. Chem. Eng. 2021, 9, 5705–5715. [Google Scholar] [CrossRef]
- Wen, Y.; Shen, W.; Li, Y.; Fang, Y. Promoting effect of Ru in the Pt-Ru/WOx/Al2O3 catalyst for the selective hydrogenolysis of glycerol to 1,3-propanediol. React. Kinet. Mech. Cat. 2020, 132, 219–233. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, S.Y.; Shen, W.; Fang, Y.J. Study on activity and stability of Pt-Ru/WOx/Al2O3 catalyst in the glycerol selective hydrogenolysis to 1,3-propanediol. Chem. Eng. Commun. 2023, 210, 1291–1304. [Google Scholar] [CrossRef]
- Aihara, T.; Miura, H.; Shishido, T. Effect of perimeter interface length between 2D WO3 monolayer domain and γ-Al2O3 on selective hydrogenolysis of glycerol to 1,3-propanediol. Catal. Sci. Technol. 2019, 9, 5359–5367. [Google Scholar] [CrossRef]
- Xu, W.; Niu, P.; Guo, H.; Jia, L.; Li, D. Hydrogenolysis of glycerol to 1,3-propanediol over a Al2O3-supported platinum tungsten catalyst with two-dimensional open structure. React. Kinet. Mech. Cat. 2021, 133, 173–189. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, B.; Liang, Y.; Liu, L.; Dong, J. Promoting role of oxygen deficiency on a WO3-supported Pt catalyst for glycerol hydrogenolysis to 1,3-propanediol. Ind. Eng. Chem. Res. 2020, 59, 7389–7397. [Google Scholar] [CrossRef]
- Yang, M.; Wu, K.; Sun, S.; Ren, Y. Regulating oxygen defects via atomically dispersed alumina on Pt/WOx catalyst for enhanced hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B Environ. 2022, 307, 121207. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Wang, X.F.; Yao, D.W.; Wang, Y.; Huang, S.Y.; Li, W.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Insight into the nature of Brnsted acidity of Pt-(WOx)n-H model catalysts in glycerol hydrogenolysis. J. Catal. 2020, 388, 154–163. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H. Selective hydrogenolysis of glycerol to 1,3-propanediol over supported platinum-tungsten oxide catalysts. Acta Phys. Chim. Sin. 2022, 38, 2207014. [Google Scholar] [CrossRef]
- Dolsiririttigul, N.; Numpilai, T.; Wattanakit, C.; Seubsai, A.; Faungnawakij, K.; Cheng, C.K.; Nijpanich, S.; Chanlek, N.; Witoon, T. Structure-activity relationships of Pt-WOx/Al2O3 prepared with different W contents and pretreatment conditions for glycerol conversion to 1,3-propanediol. Top. Catalysis 2023, 66, 205–222. [Google Scholar] [CrossRef]
- Tomishige, K.; Nakagawa, Y.; Tamura, M. Selective hydrogenolysis and hydrogenation using metal catalysts directly modified with metal oxide species. Green Chem. 2017, 19, 2876–2924. [Google Scholar] [CrossRef]
- Liu, L.J.; Kawakami, S.; Nakagawa, Y.; Tamura, M. Highly active iridium–rhenium catalyst condensed on silica support for hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B Environ. 2019, 256, 117775. [Google Scholar] [CrossRef]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Selective hydrogenolysis of glycerol to 1,3-propanediol over rhenium-oxide-modified iridium nanoparticles coating rutile titania support. ACS Catal. 2019, 9, 10913–10930. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ning, X.; Amada, Y.; Tomishige, K. Solid acid co-catalyst for the hydrogenolysis of glycerol to 1,3-propanediol over Ir-ReOx/SiO2. Appl. Catal. A Gen. 2012, 433–434, 128–134. [Google Scholar] [CrossRef]
- Sarun, C.; Wongsaphat, M.; Pooripong, S.; Thongthai, W.; Metta, C.; Kajornsak, F.; Anusorn, S. Hydrogenolysis of glycerol to 1,3-propanediol over H-ZSM-5-supported iridium and rhenium oxide catalysts. Catal. Today 2022, 397–399, 356–364. [Google Scholar]
- Wan, X.; Zhang, Q.; Zhu, M.; Zhao, Y.; Liu, Y.; Zhou, C.; Yang, Y.; Cao, Y. Interface synergy between IrOx and H-ZSM-5 in selective C–O hydrogenolysis of glycerol toward 1,3-propanediol. J. Catal. 2019, 375, 339–350. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, X.; Cao, Y.; Zhu, Y.A.; Zhou, J.; Zhou, X. Mechanistic insights into acid-affected hydrogenolysis of glycerol to 1,3-propanediol over an Ir–Re/SiO2 catalyst. Chem. Commun. 2022, 52, 2694–2697. [Google Scholar] [CrossRef]


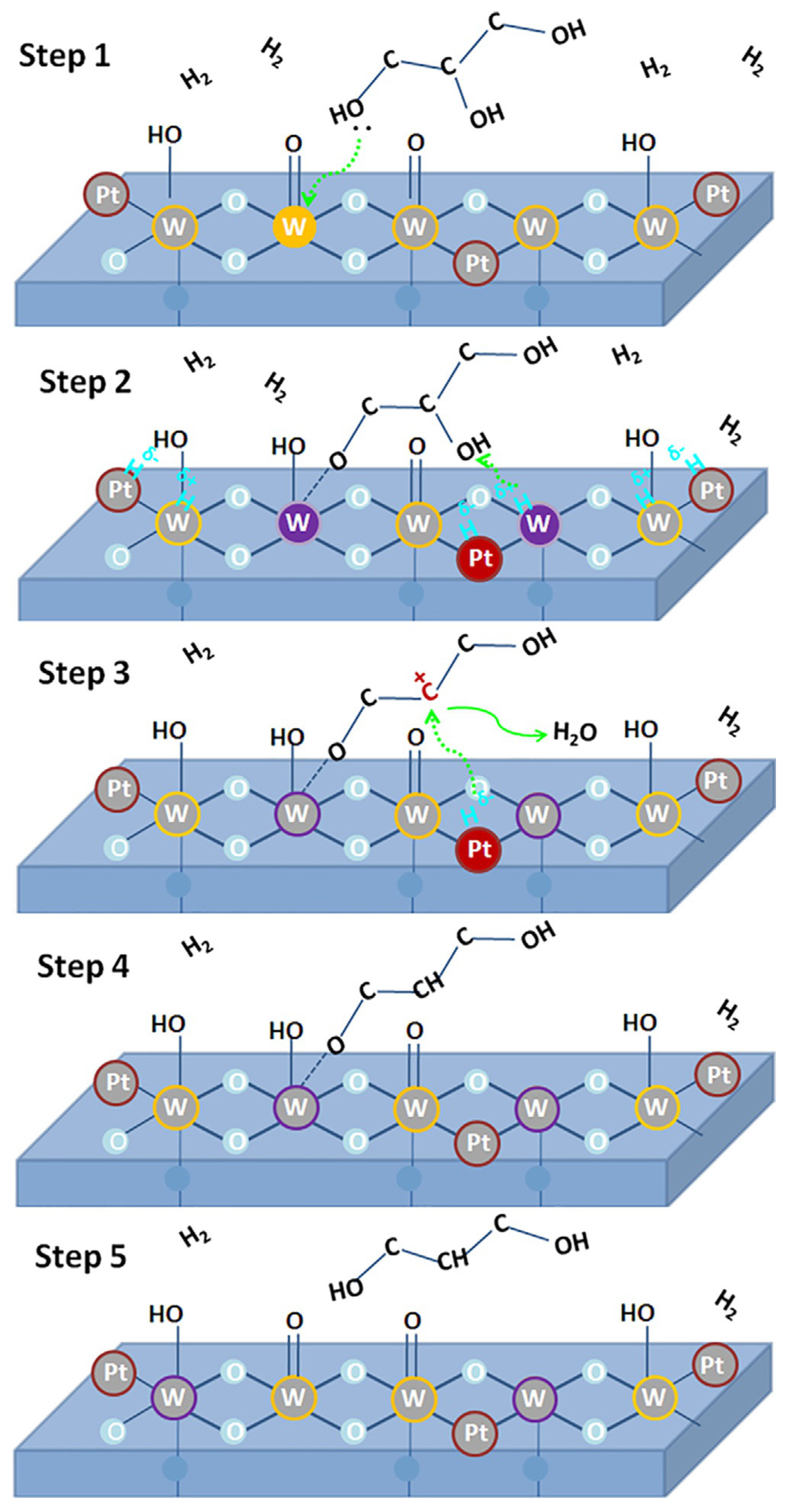
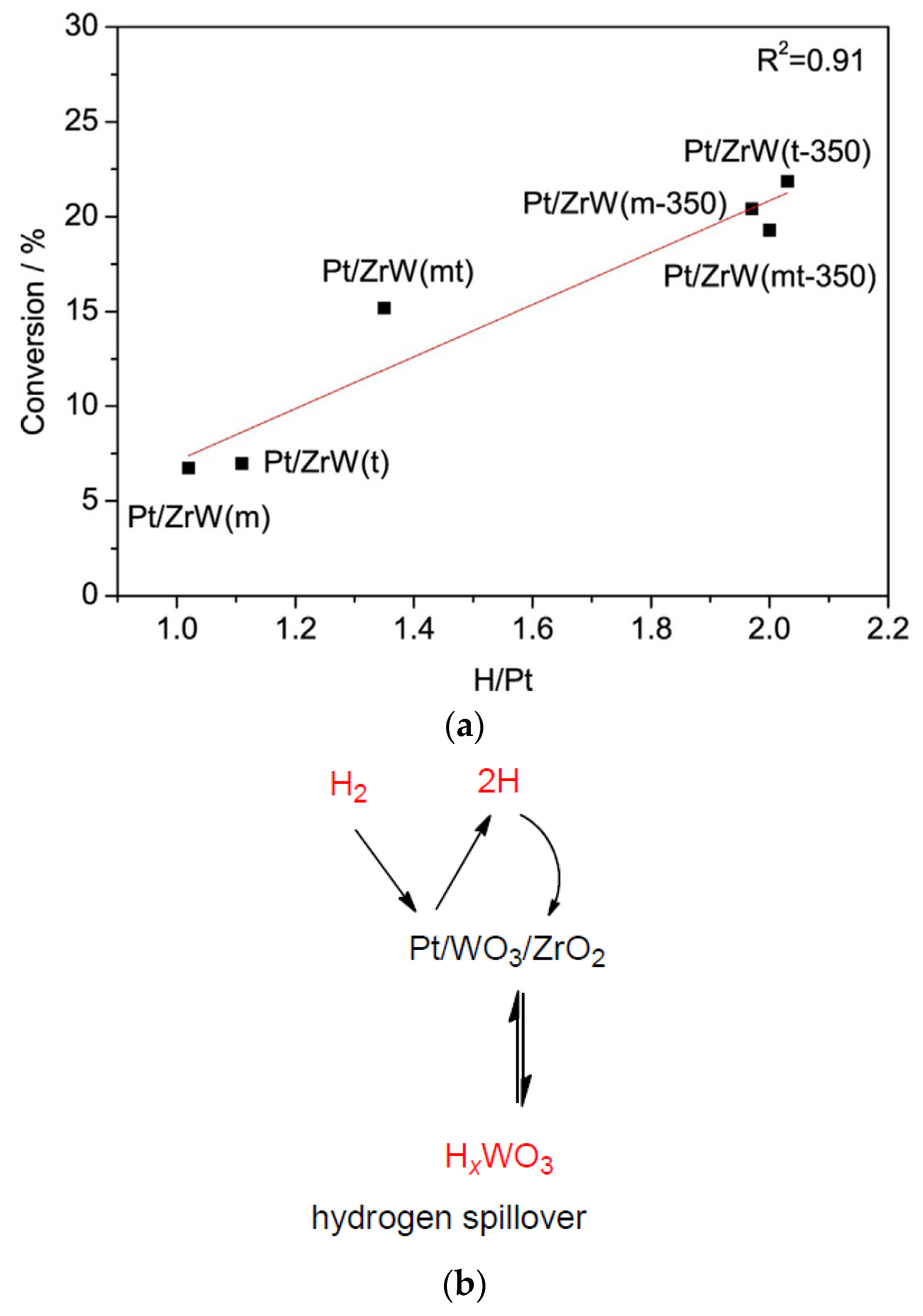
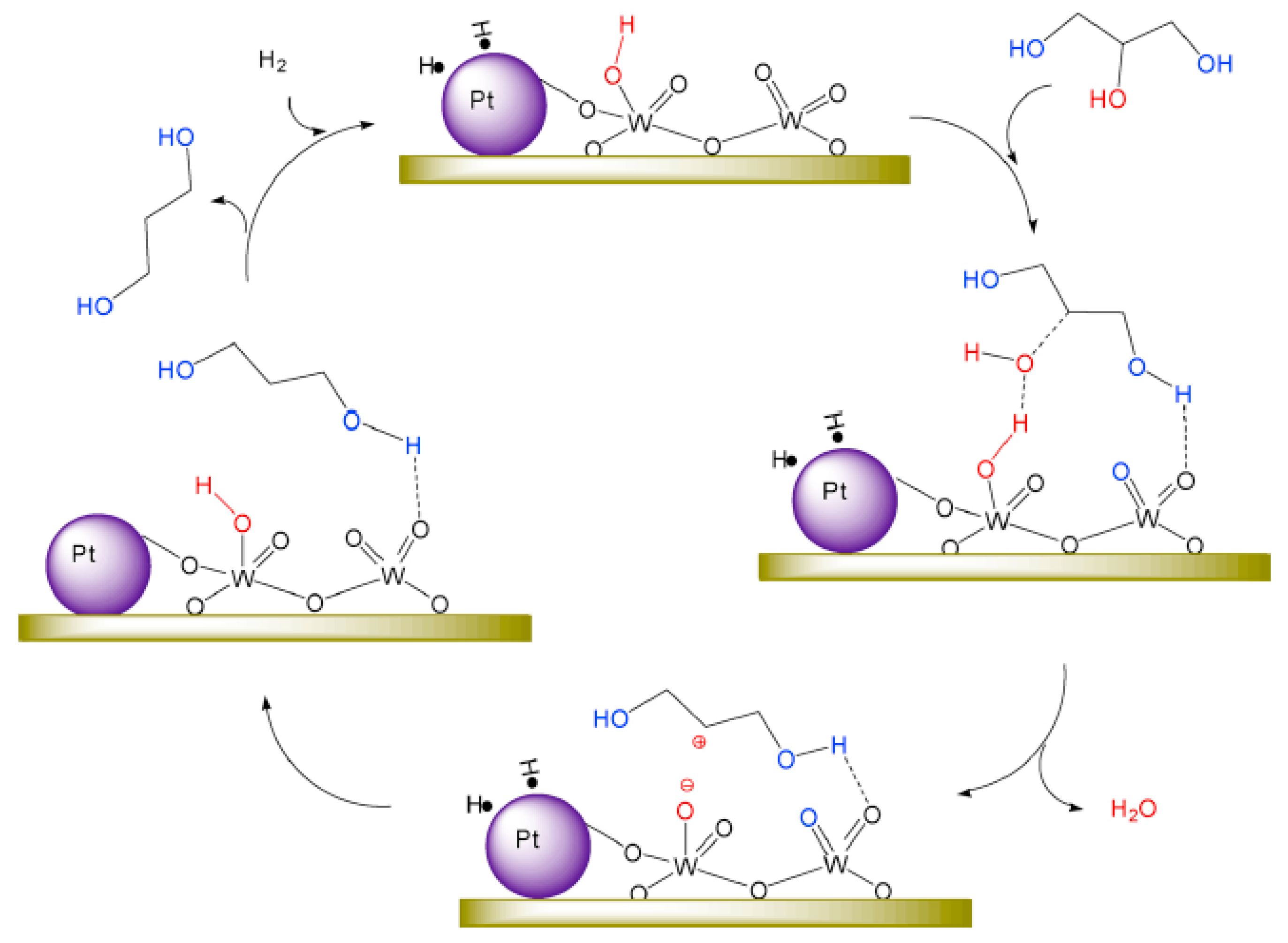
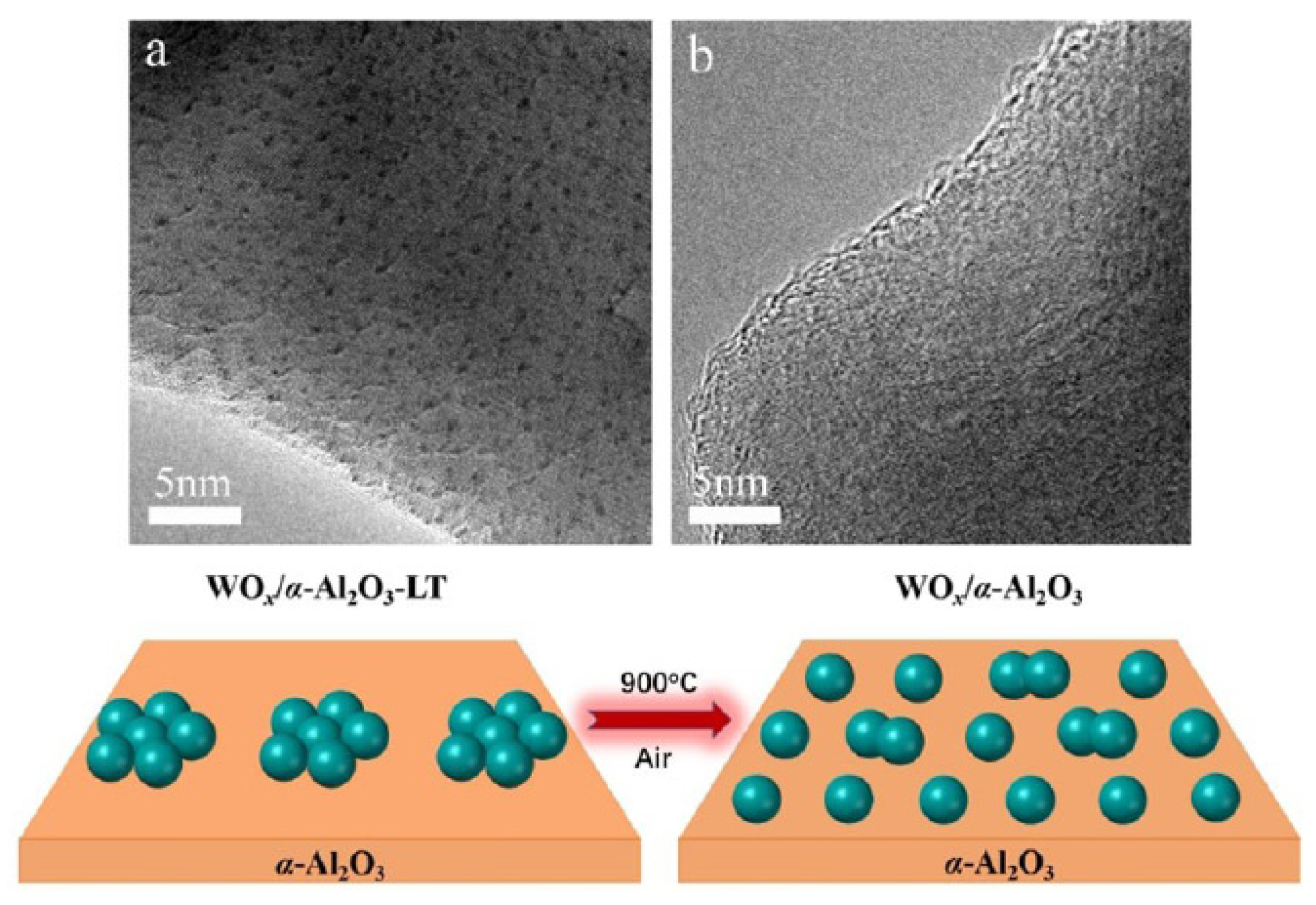
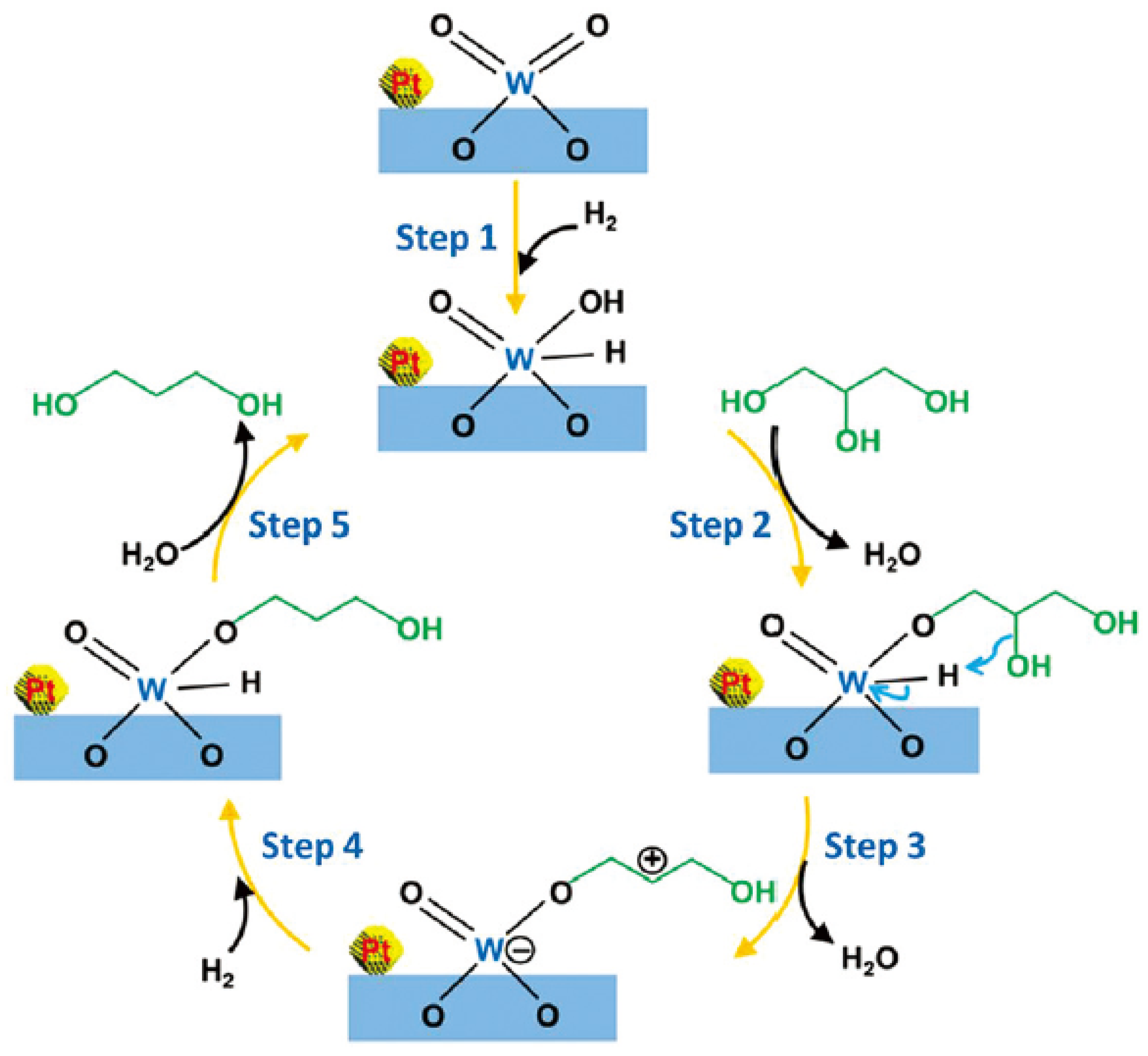

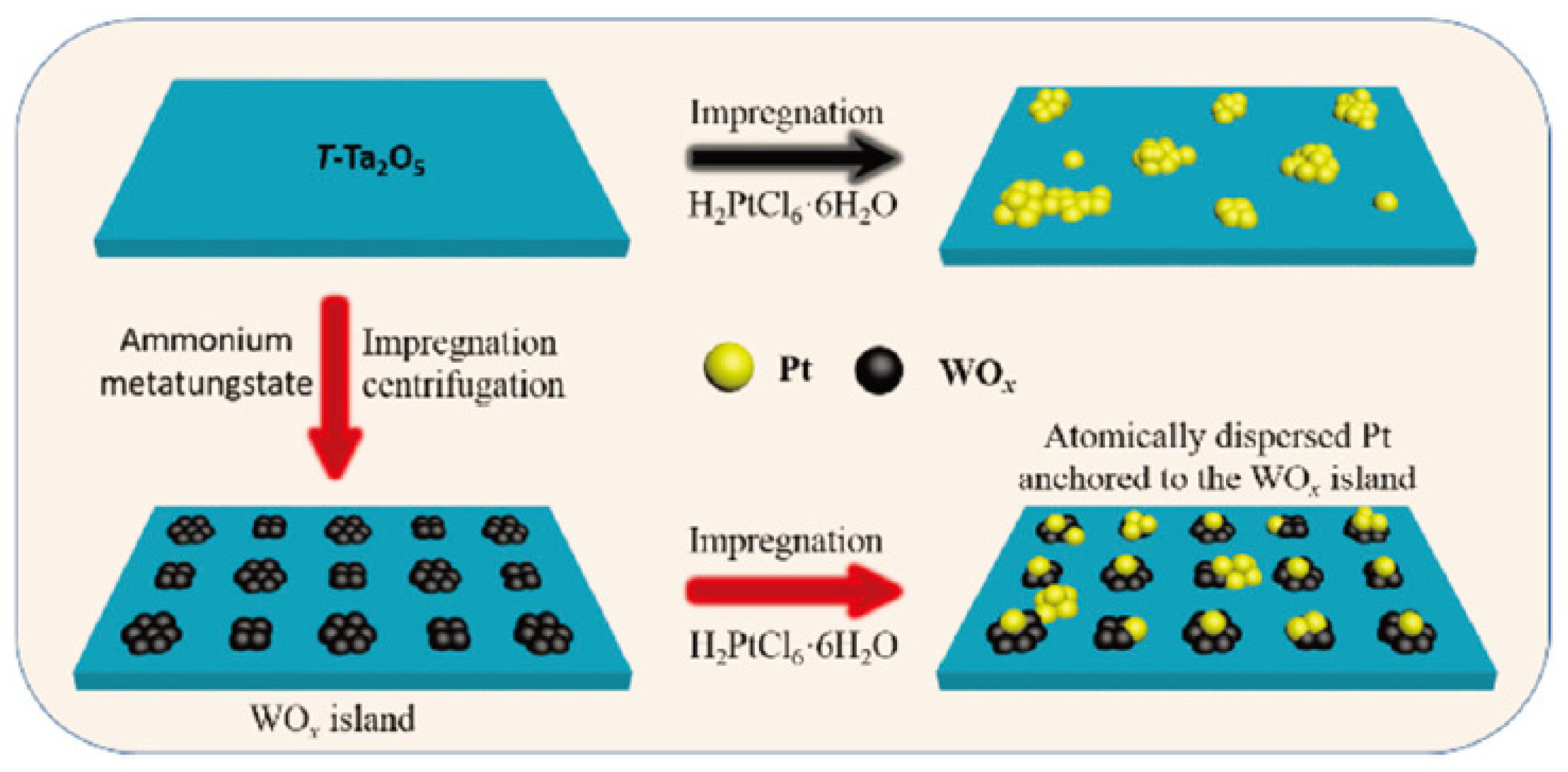
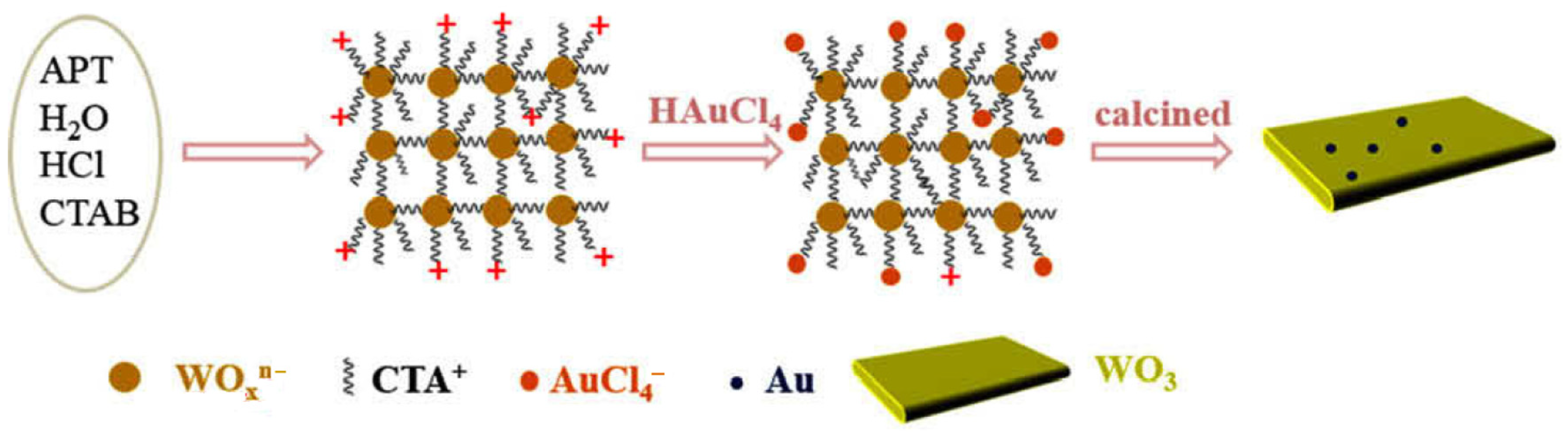

| Catalyst | Reaction Conditions | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Press. (bar) | T (h) | Conv. (%) | 1,2-PDO | Acetol | EG | |
| RANEY Cu | 240 | 30 | - | 86 | 66 | - | - |
| CuO/ZnO | 180 | 80 | 90 | 20 | 100 | ||
| Cu:ZnO | 200 | 42 | 12 | 17 | 29 | - | 3 |
| Cu–ZnO (1:1) | 200 | 60 | 6 | 2.6 TOF | 94 | - | 6 |
| CuO/ZnO–OG a | 200 | 70 | 7 | 46 | 90 | 1 | 1 |
| CuO/ZnO–CP b | 17 | 87 | 1 | 0 | |||
| Cu1.1/ZnO–HKUST-1 m | 230 | 20 | 2 | >99 | 95.6 | 4.4 | - |
| 20% Cu/ZnO/PAA n 20-NC (not calcined) | 200 | 30 | 24 | 70 | 87 | - | 12 |
| 20% Cu/ZnO/PAA520-NC | 200 | 30 | 24 | 70 | 87 | - | 13 |
| Cu/Al2O3, HY, HZSM, Hβ | 200 | 36 | 10 | 35 | 93.9 | - | - |
| Cu–STA/Al2O3 | 240 | 60 | - | 90 | 90 | - | 4 |
| Cu/Al2O3 | 200 | 50 | 24 | 59 | 79 | 7 | 1 |
| Cu–Al | 220 | 40 | - | 60 | 90 | 5 | 3 |
| Cu–Al CAP(Na2CO3) | 220 | 52 | 5 | 62 | 88 | 1 | 10 |
| Cu/fly ash | 220 | 52 | 5 | 37 | 84 | 4 | 3 |
| CuAl2O4 | 220 | 50 | 12 | 90 | 83 | - | - |
| Cu/boehmite | 200 | 40 | - | 77 | 93 | - | 0.2 |
| Cu/Al2O3 | 180–300 | 1 | 100 | 88 | 9 | - | |
| Cu/Al2O3 | 200 | 50 | 24 | 76 | 96 | 1 | 1 |
| Cu/Al2O3 | 205 | 50 | 23 | 89 | 94 | - | - |
| 15% Cu/Al2O3 | 200 | 1 | 180 g cat min mol−1 | 100 | 60 | 30 | - |
| 60% Cu/Al2O3 | 250 | ~50 | SLV o 0.55 (m3m−2 h−1) | 90 | 91.5 | - | 1.5 |
| Cu/SiO2 PG c | 200 | 90 | 12 | 73 | 94 | Trace | 2 |
| Cu/SiO2 IM d | 26 | 95 | 0.3 | 2 | |||
| Cu/SiO2-Na | 180 | 90 | 12 | 33 | 98.7 | Trace | 0.7 |
| Cu/SBA-15 | 250 | 83 | 6-7 | 96 | 92 | 3 | 3 |
| Cu/SiO2 IW e | 245 | 240 (218) | - | 13 | 76 | - | - |
| Cu/SiO2 IE f | 255 | 100 | 87 | 5 | 4 | ||
| Cu/SiO2 Hom-DP g | 200 | 90 | 12 | 55 | 96 | - | 2 |
| Het-DP h | 65 | 93 | - | 7 | |||
| 3CuB/SiO2 | 200 | 50 | 150 | 100 | 98 | 0.5 | 0.5 |
| 10% Cu/SBA-15(G) i | 210 | 40 | 1.5 | 59 | 98.7 | - | ~1 |
| 10% Cu/SBA-15(IM) | 210 | 40 | 1.5 | 8 | 99.2 | - | 0.8 |
| CuO-15 MgO-CP; | 180 | 30 | 20 | 72 | 98 | - | 1.3 |
| CuO-15 MgO-CP + NaOH | 82 | 96 | - | 0.3 | |||
| Cu/MgO | 200 | 40 | 8 | 49.3 | 92 | - | 6 |
| Cu/Mg–Al mixed-oxide | 210 | 90 | 24 | >95 | 85 | - | - |
| 10% Cu/MgO | 220 | 7.5 | 1.2h−1 | ||||
| (WHSV) p | 100 | 95.5 (y) | 1.6 | - | 105 | ||
| Cu0.4/Mg5.6Al2O8.6-CP (75% aq. gly) | 180 | 30 | 20 | 80 | 98.2 | 0.0 | 1 |
| Cu–Zn10HTr j | 200 | 20 | 8 | 38.5 | 61.6 | - | - |
| 5% CuO/Mg9Al2.7–Ga2.3O2 | 220 | 40 mL min−1 | 23 | 95 | 97 | - | - |
| Cu-Delaminated hectorites | 200 | 40 | 8 | 61.4 | 93 | - | - |
| 20% Cu/Dolomite | 180 | 20 | 6 | 100 | 92 | 6.2 | - |
| 20% Cu/Dolomite | 200 | 40 | 10 | 78.5 | 79 | 19 | - |
| 7% Cu/CGran k (750) | 220 | 50 | - | 22 | 94.7 | 3 | - |
| 15% Cu(M)–Zr(C) l | 240 | 20 | 24 | 80 | 95 | - | - |
| Catalyst | Size of Cu Crystallites (nm) | Glycerol Conversion (120 min) | Selectivity to 1,2-Propanediol (120 min) |
|---|---|---|---|
| HTc350 | 15.7 | 63.1 | 0.96 |
| HTc700 | 29.0 | 38.0 | 0.97 |
| Hydrox | 38.5 | 29.6 | 0.88 |
| OXAL | 17.0 | 27.6 | 0.93 |
| Combus | 42.4 | 20.0 | 0.92 |
| Mixox | 74.0 | 3.2 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Liu, H.; He, D. Recent Progress in Catalyst Development of the Hydrogenolysis of Biomass-Based Glycerol into Propanediols—A Review. Bioengineering 2023, 10, 1264. https://doi.org/10.3390/bioengineering10111264
Ma L, Liu H, He D. Recent Progress in Catalyst Development of the Hydrogenolysis of Biomass-Based Glycerol into Propanediols—A Review. Bioengineering. 2023; 10(11):1264. https://doi.org/10.3390/bioengineering10111264
Chicago/Turabian StyleMa, Lan, Huimin Liu, and Dehua He. 2023. "Recent Progress in Catalyst Development of the Hydrogenolysis of Biomass-Based Glycerol into Propanediols—A Review" Bioengineering 10, no. 11: 1264. https://doi.org/10.3390/bioengineering10111264
APA StyleMa, L., Liu, H., & He, D. (2023). Recent Progress in Catalyst Development of the Hydrogenolysis of Biomass-Based Glycerol into Propanediols—A Review. Bioengineering, 10(11), 1264. https://doi.org/10.3390/bioengineering10111264









