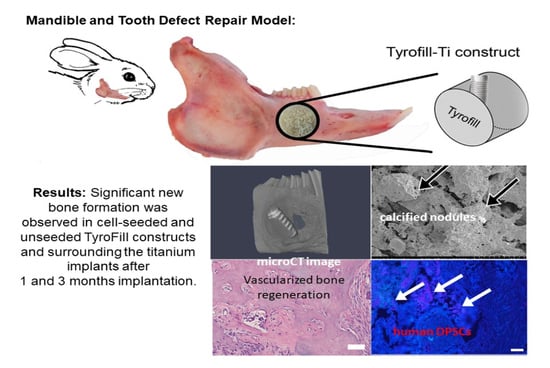
TyroFill–Titanium Implant Constructs for the Coordinated Repair of Rabbit Mandible and Tooth Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TyroFill (E1001(1k)-DCPD) Scaffolds
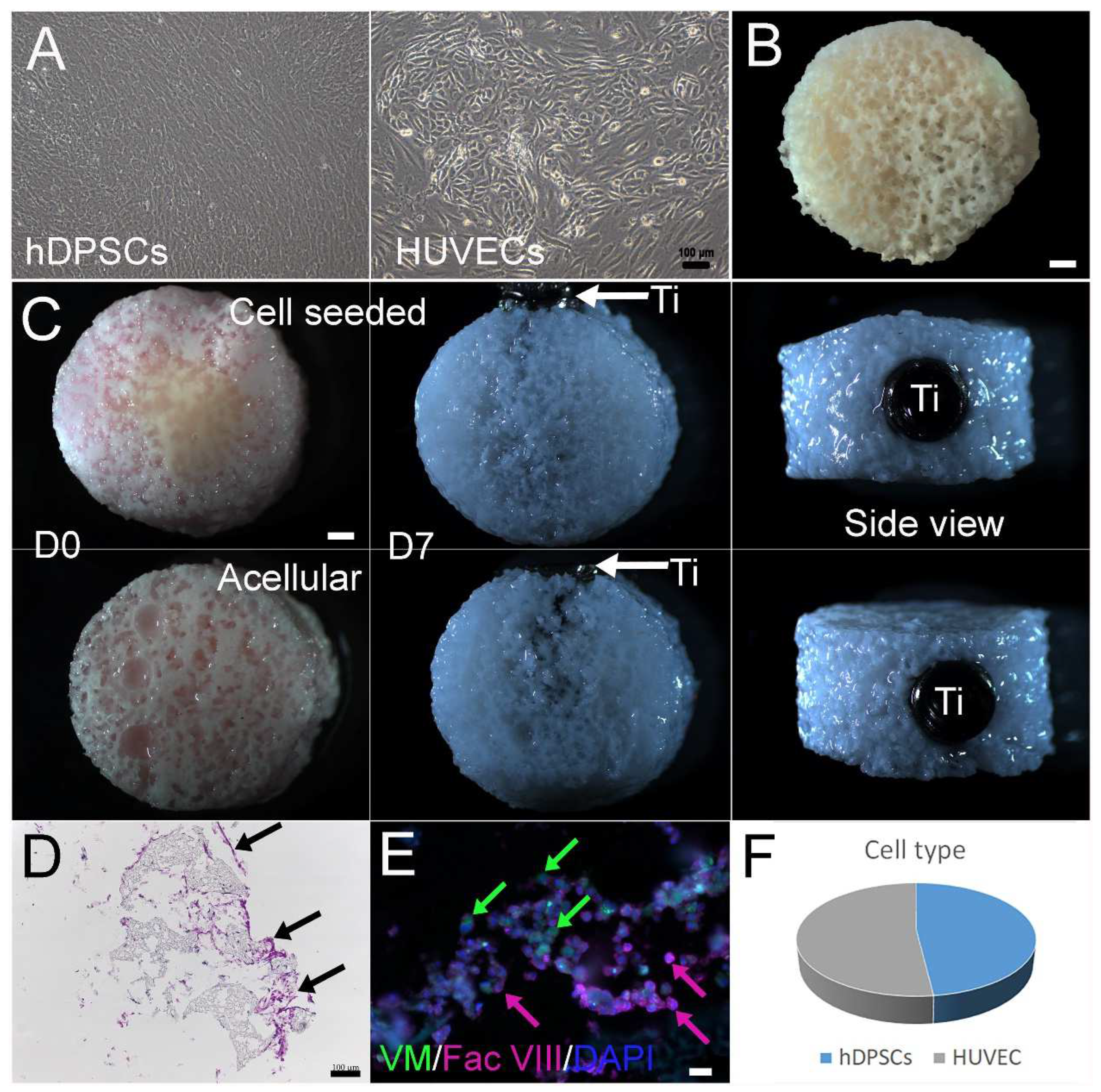
2.2. Cell Seeding
2.3. Rabbit Mandible Defect Repair Model
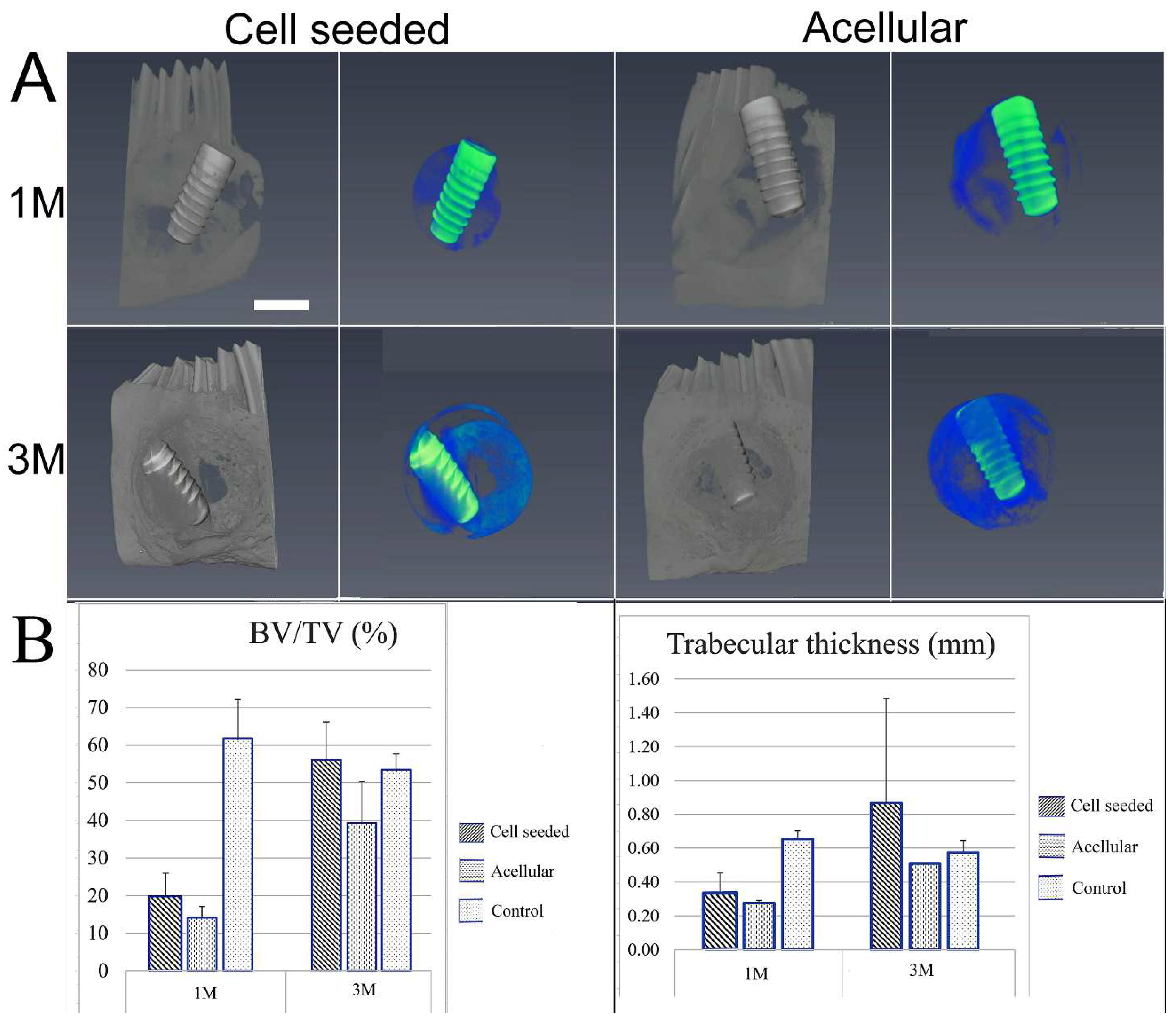
2.4. Evaluation of Bioengineered Mandibular Bone Implants
2.5. Surface Characterization of the Titanium Dental Implants
3. Results
3.1. Construction and In Vitro Culture of 3D Bone–Tooth Constructs
3.2. Post-Surgical Analyses
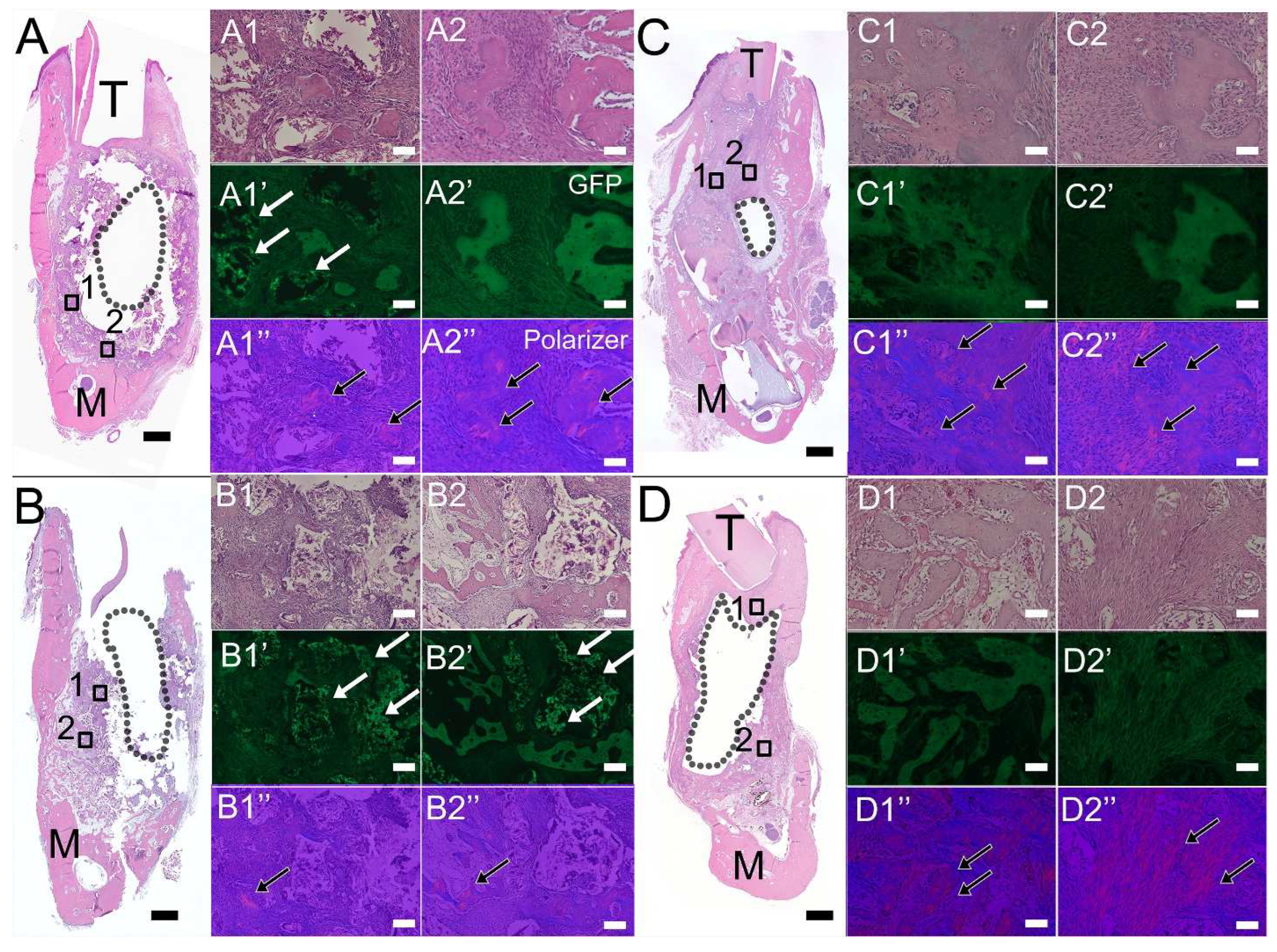
3.3. Histological Analyses of Bioengineered Constructs
3.4. Characterization of Harvested Dental Implant Surfaces
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Telischi, F.F. Traumatic facial nerve injuries: Review of diagnosis and treatment. J. Craniomaxillofac. Trauma 1995, 1, 30–41. [Google Scholar]
- Caballero, M.; Jones, D.C.; Shan, Z.; Soleimani, S.; van Aalst, J.A. Tissue Engineering Strategies to Improve Osteogenesis in the Juvenile Swine Alveolar Cleft Model. Tissue Eng. Part C Methods 2017, 23, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Molnar, B.; Jung, A.K.; Papp, Z.; Martin, A.; Orban, K.; Prohl, A.; Jung, O.; Barbeck, M.; Windisch, P. Comparative analysis of lateral maxillary sinus augmentation with a xenogeneic bone substitute material in combination with piezosurgical preparation and bony wall repositioning or rotary instrumentation and membrane coverage: A prospective randomized clinical and histological study. Clin. Oral. Investig. 2022, 26, 5261–5272. [Google Scholar] [CrossRef] [PubMed]
- Cevolani, L.; Bianchi, G.; Costantino, E.; Staals, E.; Lucarelli, E.; Spazzoli, B.; Frisoni, T.; Donati, D.M. Minimally invasive treatment of long bone non-unions with bone marrow concentrate, demineralized bone matrix and platelet-rich fibrin in 38 patients. J. Tissue Eng. Regen. Med. 2021, 15, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Pagni, G.; Park, C.H.; Braun, T.M.; Holman, L.A.; Yi, E.; Tarle, S.A.; Bartel, R.L.; Giannobile, W.V. Stem cell therapy for craniofacial bone regeneration: A randomized, controlled feasibility trial. Cell Transplant. 2013, 22, 767–777. [Google Scholar] [CrossRef]
- Sodek, J.; McKee, M.D. Molecular and cellular biology of alveolar bone. Periodontol. 2000 2000, 24, 99–126. [Google Scholar] [CrossRef]
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef]
- Song, W.; Bo, X.; Ma, X.; Hou, K.; Li, D.; Geng, W.; Zeng, J. Craniomaxillofacial derived bone marrow mesenchymal stem/stromal cells (BMSCs) for craniomaxillofacial bone tissue engineering: A literature review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e650–e659. [Google Scholar] [CrossRef]
- Karaplis, A.C. Embryonic Development of Bone and Regulation of Intramembranous and Endochondral Bone Formation. Princ. Bone Biol. 2002, 1, 33–58. [Google Scholar]
- Sullivan, W.G.; Szwajkun, P.R. Revascularization of cranial versus iliac crest bone grafts in the rat. Plast. Reconstr. Surg. 1991, 87, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Filho Cerruti, H.; Kerkis, I.; Kerkis, A.; Tatsui, N.H.; da Costa Neves, A.; Bueno, D.F.; da Silva, M.C. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: Clinical case reports. Artif. Organs 2007, 31, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dennis, J.E.; Solchaga, L.A.; Awadallah, A.S.; Goldberg, V.M.; Caplan, A.I. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001, 7, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 2006, 24, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; van Kuppevelt, T.H.; Daamen, W.F.; Bian, Z.; Jansen, J.A. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials 2006, 27, 5658–5668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; van Osch, G.J.; van den Dolder, J.; Jansen, J.A. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng. Part A 2008, 14, 285–294. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Kim, J.; Magno, M.H.; Alvarez, P.; Darr, A.; Kohn, J.; Hollinger, J.O. Osteogenic differentiation of pre-osteoblasts on biomimetic tyrosine-derived polycarbonate scaffolds. Biomacromolecules 2011, 12, 3520–3527. [Google Scholar] [CrossRef]
- Kim, J.; Magno, M.H.; Waters, H.; Doll, B.A.; McBride, S.; Alvarez, P.; Darr, A.; Vasanji, A.; Kohn, J.; Hollinger, J.O. Bone regeneration in a rabbit critical-sized calvarial model using tyrosine-derived polycarbonate scaffolds. Tissue Eng. Part A 2012, 18, 1132–1139. [Google Scholar] [CrossRef]
- Guda, T.; Darr, A.; Silliman, D.T.; Magno, M.H.; Wenke, J.C.; Kohn, J.; Brown Baer, P.R. Methods to analyze bone regenerative response to different rhBMP-2 doses in rabbit craniofacial defects. Tissue Eng. Part C Methods 2014, 20, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McBride, S.; Donovan, A.; Darr, A.; Magno, M.H.; Hollinger, J.O. Tyrosine-derived polycarbonate scaffolds for bone regeneration in a rabbit radius critical-size defect model. Biomed. Mater. 2015, 10, 035001. [Google Scholar] [CrossRef] [PubMed]
- Shariff, K.A.; Tsuru, K.; Ishikawa, K. Fabrication of dicalcium phosphate dihydrate-coated beta-TCP granules and evaluation of their osteoconductivity using experimental rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ahluwalia, I.P.; Literman, R.; Kaplan, D.L.; Yelick, P.C. Human dental pulp progenitor cell behavior on aqueous and hexafluoroisopropanol based silk scaffolds. J. Biomed. Mater. Res. A 2011, 97, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Saxena, S.; Fakhrzadeh, A.; Rudolph, S.; Young, S.; Kohn, J.; Yelick, P.C. Use of Human Dental Pulp and Endothelial Cell Seeded Tyrosine-Derived Polycarbonate Scaffolds for Robust in vivo Alveolar Jaw Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 796. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Magno, M.H.; Ortiz, O.; McBride, S.; Darr, A.; Kohn, J.; Hollinger, J.O. Next-generation resorbable polymer scaffolds with surface-precipitated calcium phosphate coatings. Regen. Biomater. 2015, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Chang, W.; Fakhrzadeh, A.; Murthy, N.S.; Zhang, W.; Kohn, J.; Yelick, P.C. Calcium phosphate enriched synthetic tyrosine-derived polycarbonate-dicalcium phosphate dihydrate polymer scaffolds for enhanced bone regeneration. Materialia 2020, 9, 100616. [Google Scholar] [CrossRef]
- Liu, R.; Lei, T.; Dusevich, V.; Yao, X.; Liu, Y.; Walker, M.P.; Wang, Y.; Ye, L. Surface characteristics and cell adhesion: A comparative study of four commercial dental implants. J. Prosthodont. 2013, 22, 641–651. [Google Scholar] [CrossRef]
- Popov, V.K.; Evseev, A.V.; Ivanov, A.L.; Roginski, V.V.; Volozhin, A.I.; Howdle, S.M. Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication. J. Mater. Sci. Mater. Med. 2004, 15, 123–128. [Google Scholar] [CrossRef]
- Antonov, E.N.; Bagratashvili, V.N.; Whitaker, M.J.; Barry, J.J.; Shakesheff, K.M.; Konovalov, A.N.; Popov, V.K.; Howdle, S.M. Three-Dimensional Bioactive and Biodegradable Scaffolds Fabricated by Surface-Selective Laser Sintering. Adv. Mater. 2004, 17, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Touyz, L.Z.; Afrashtehfar, K.I. Cryogenically salvaged teeth as a potential source for grafting dentoalveolar, periodontal or maxillofacial defects. Med. Hypotheses 2016, 92, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shi, H.; Liang, Y.; Lu, T.; Lin, Z.; Ye, J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped beta-tricalcium phosphate. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110481. [Google Scholar] [CrossRef] [PubMed]
- Putranti, N.A.R.; Kunimatsu, R.; Rikitake, K.; Hiraki, T.; Nakajima, K.; Abe, T.; Tsuka, Y.; Sakata, S.; Nakatani, A.; Nikawa, H.; et al. Combination of Carbonate Hydroxyapatite and Stem Cells from Human Deciduous Teeth Promotes Bone Regeneration by Enhancing BMP-2, VEGF and CD31 Expression in Immunodeficient Mice. Cells 2022, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.J.; Harley, B.A.C. Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 2021, 11, 17809–17827. [Google Scholar] [CrossRef] [PubMed]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; Garcia, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Hsiong, S.X.; Mooney, D.J. Regeneration of vascularized bone. Periodontol. 2000 2006, 41, 109–122. [Google Scholar] [CrossRef]
- Clarkin, C.E.; Gerstenfeld, L.C. VEGF and bone cell signalling: An essential vessel for communication? Cell Biochem. Funct. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Athanasopoulos, A.N.; Schneider, D.; Keiper, T.; Alt, V.; Pendurthi, U.R.; Liegibel, U.M.; Sommer, U.; Nawroth, P.P.; Kasperk, C.; Chavakis, T. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J. Biol. Chem. 2007, 282, 26746–26753. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Gilbert, S.R.; Clemens, T.L. Oxygen sensing and osteogenesis. Ann. N. Y Acad. Sci. 2007, 1117, 1–11. [Google Scholar] [CrossRef]
- Shaoki, A.; Xu, J.Y.; Sun, H.; Chen, X.S.; Ouyang, J.; Zhuang, X.M.; Deng, F.L. Osseointegration of three-dimensional designed titanium implants manufactured by selective laser melting. Biofabrication 2016, 8, 045014. [Google Scholar] [CrossRef]
- Barba, D.; Alabort, E.; Reed, R.C. Synthetic bone: Design by additive manufacturing. Acta Biomater. 2019, 97, 637–656. [Google Scholar] [CrossRef]
- Nimmawitt, P.; Aliyu, A.A.A.; Lohwongwatana, B.; Arunjaroensuk, S.; Puncreobutr, C.; Mattheos, N.; Pimkhaokham, A. Understanding the Stress Distribution on Anatomic Customized Root-Analog Dental Implant at Bone-Implant Interface for Different Bone Densities. Materials 2022, 15, 6379. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Kohn, J.; Yelick, P.C. TyroFill–Titanium Implant Constructs for the Coordinated Repair of Rabbit Mandible and Tooth Defects. Bioengineering 2023, 10, 1277. https://doi.org/10.3390/bioengineering10111277
Zhang W, Kohn J, Yelick PC. TyroFill–Titanium Implant Constructs for the Coordinated Repair of Rabbit Mandible and Tooth Defects. Bioengineering. 2023; 10(11):1277. https://doi.org/10.3390/bioengineering10111277
Chicago/Turabian StyleZhang, Weibo, Joachim Kohn, and Pamela C. Yelick. 2023. "TyroFill–Titanium Implant Constructs for the Coordinated Repair of Rabbit Mandible and Tooth Defects" Bioengineering 10, no. 11: 1277. https://doi.org/10.3390/bioengineering10111277
APA StyleZhang, W., Kohn, J., & Yelick, P. C. (2023). TyroFill–Titanium Implant Constructs for the Coordinated Repair of Rabbit Mandible and Tooth Defects. Bioengineering, 10(11), 1277. https://doi.org/10.3390/bioengineering10111277