Antibacterial Aerogels-Based Membranes by Customized Colloidal Functionalization of TEMPO-Oxidized Cellulose Nanofibers Incorporating CuO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CuO NPs and CNF Suspensions
2.2. Preparation of Nanocellulose/CuO Aerogels
2.3. Antimicrobial Analysis of the CNF/CuO Aerogels
2.3.1. Agar Disk Diffusion Method
2.3.2. Broth Dilution Method
3. Results and Discussion
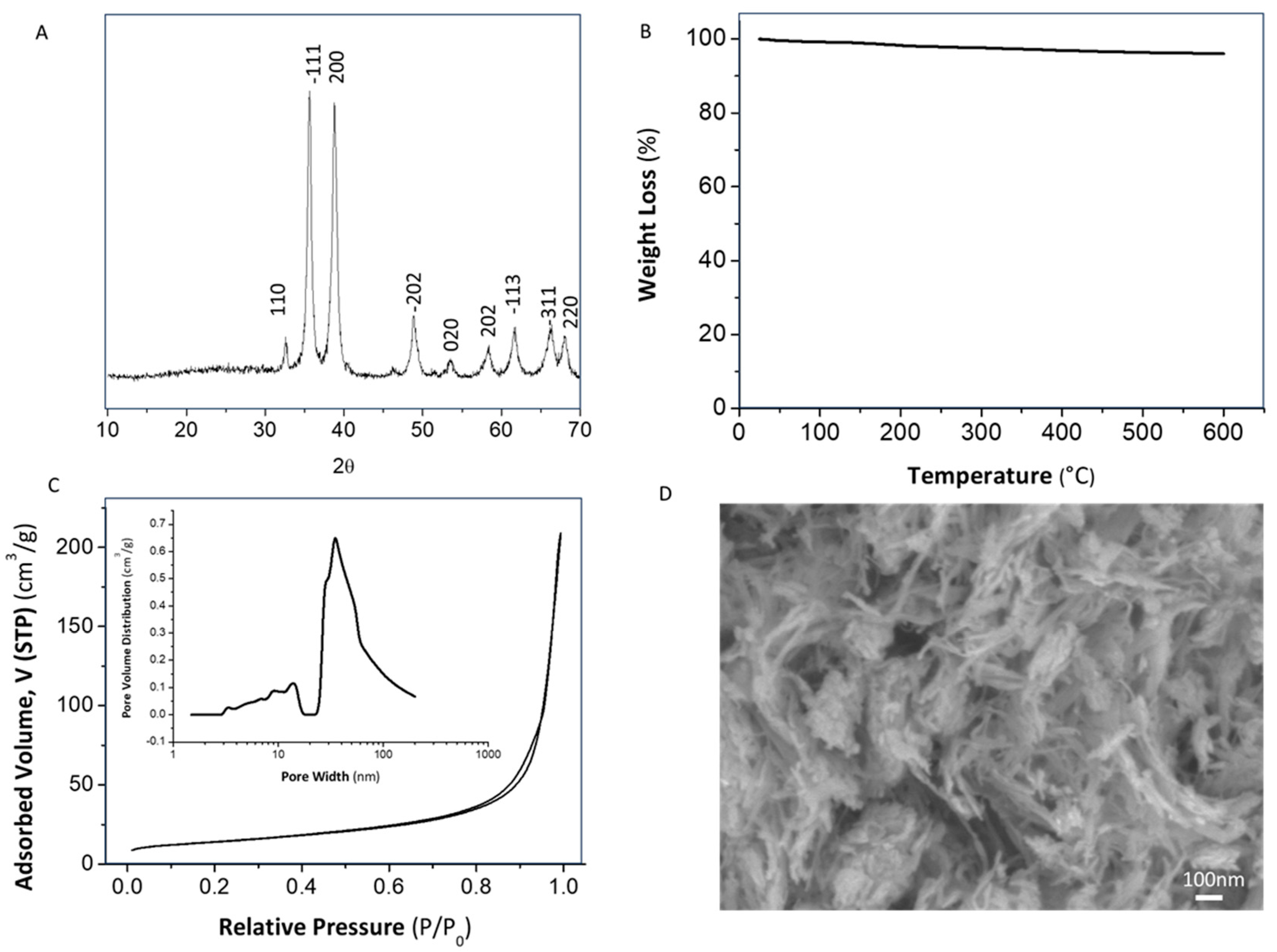
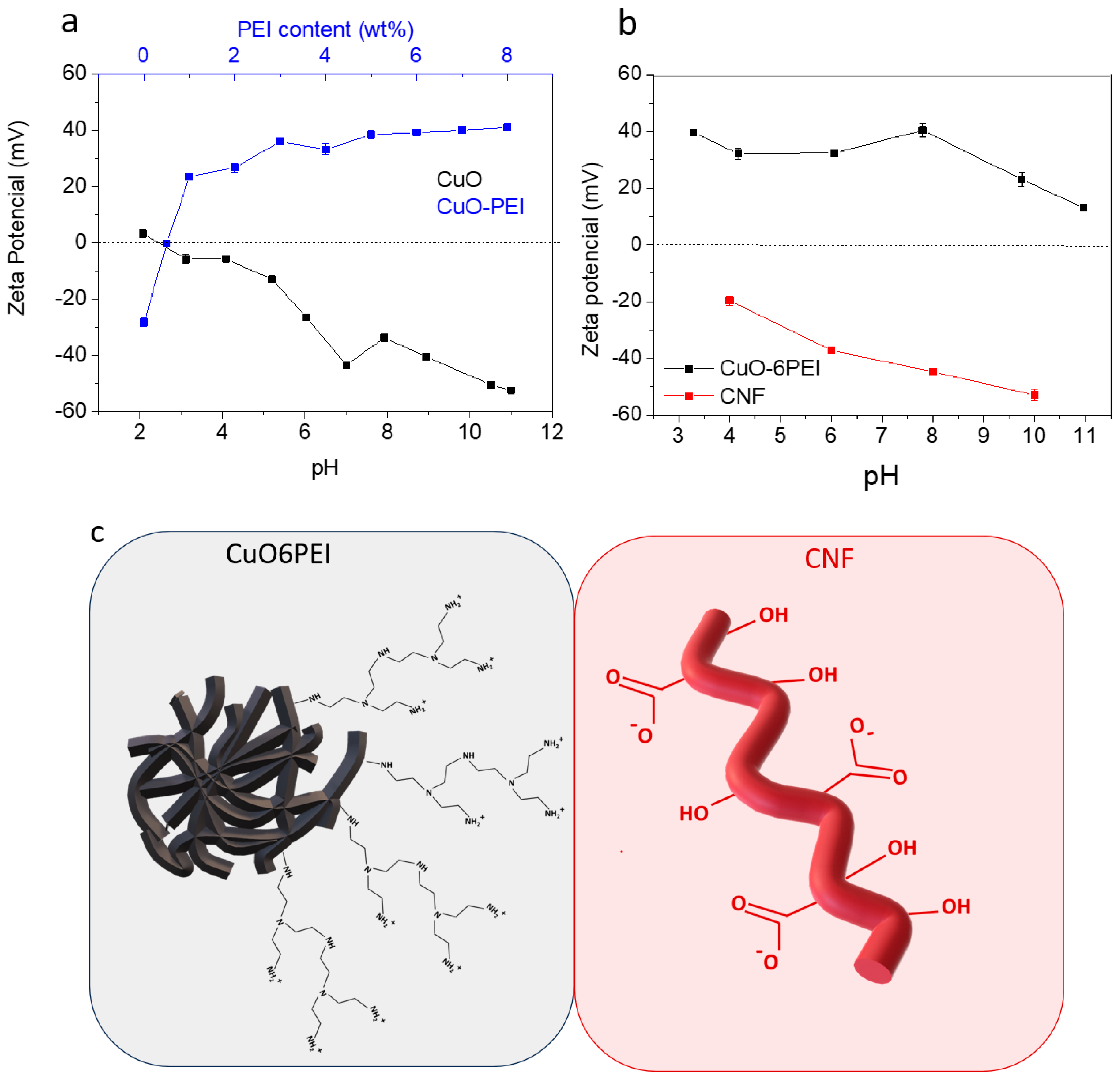
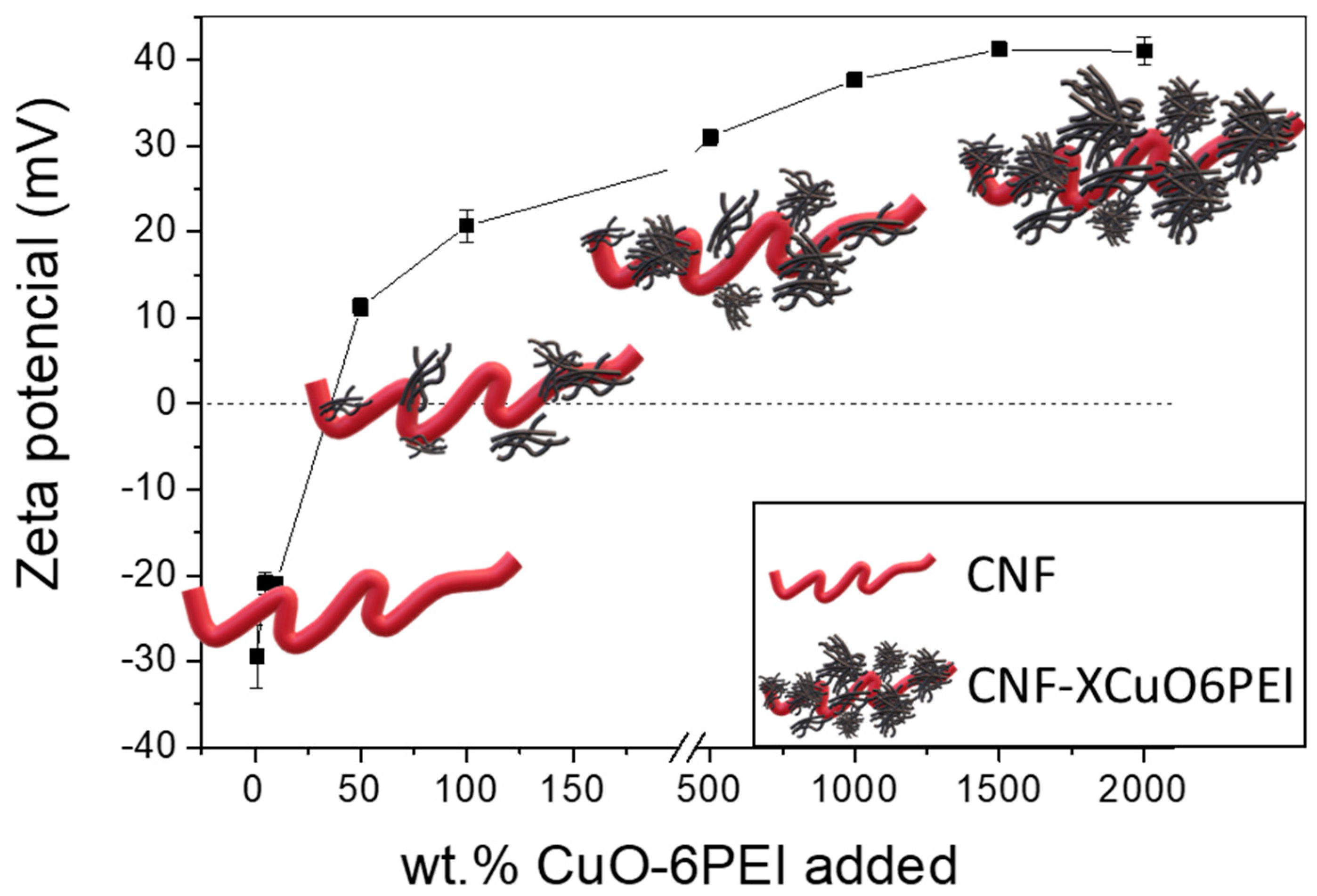
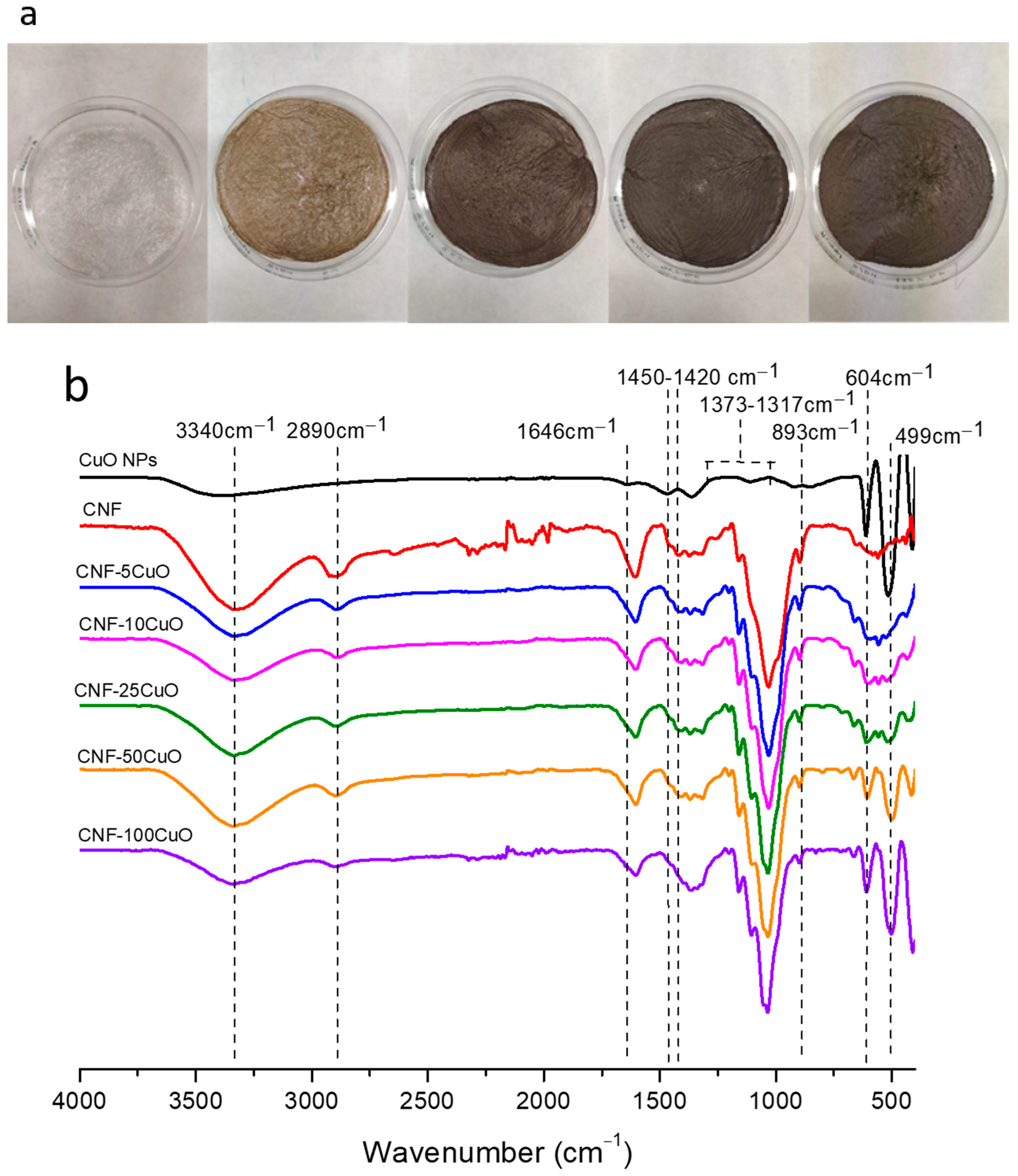
3.1. Synthesis and Characterization of the Involved Components
3.2. Blending Formulation and Aerogels Preparation
3.3. Antimicrobial Activity of Aerogels
4. Conclusions
- -
- It has been demonstrated that the application of a controlled amount of ultrasonic power in a concrete volume of sulfate precursor solution (W cm−2 mol−1) allowed the instantaneous formation of a pure CuO monoclinic nanophase. The direct obtaining of this species avoided the application of further thermal treatments that are conventionally required in the absence of sonochemistry.
- -
- In terms of processing of nanocomposite materials, it can be concluded that the design of CNF–CuO heterostructures by means of a colloidal route provides numerous possibilities that are unattainable using traditional methods.
- -
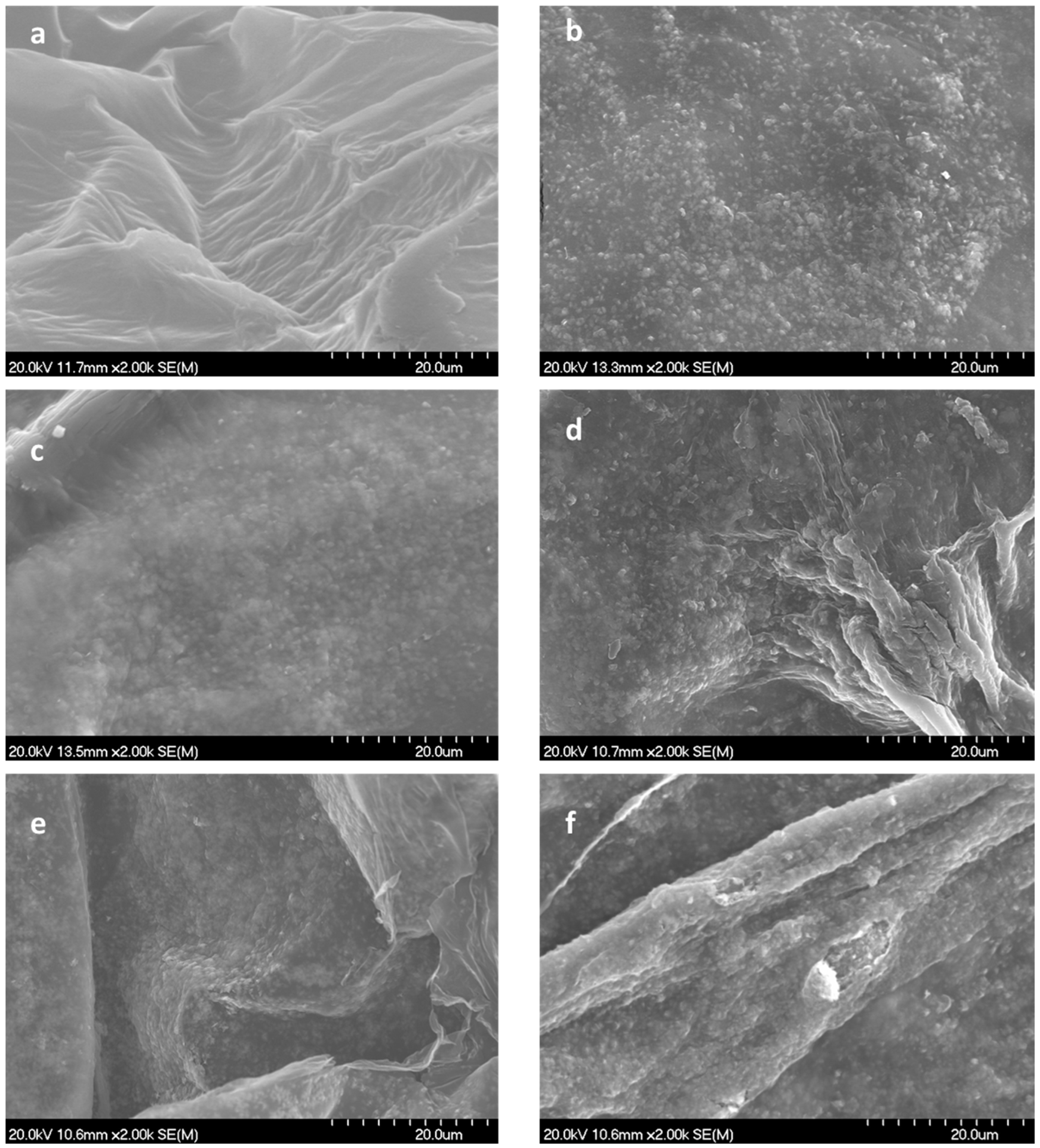
- The fundamental hypothesis of the present work has been confirmed. The TEMPO-Oxidized CNF showed idoneous surface charge to promote their electrostatic interaction with highly dispersed CuO nanostructures. It was observed that with increasing CuO concentration in the formulation, the CNF surface was completely covered, increasing the exposition of the inorganic phase towards the media and the corresponding improvement in the functional performance of the final aerogels.
- -
- All samples showed a positive response against Escherichia coli and Staphylococcus aureus. An increase of the antibacterial response of the aerogels, measured by agar disk diffusion test, was observed with the increase of CuO NPs incorporated, obtaining a width of the antimicrobial “halo” (nwhalo) from 0 to 0.6 and 0.35 for S. aureus and E. coli, respectively. Furthermore, the aerogels were also able to deactivate S. aureus and E. coli in less than 5 h when the antibacterial assays were analyzed by a broth dilution method.
- -
- It is therefore concluded that the use of CNF as a support for antimicrobial materials favors the adequate dispersion and support of the nanoparticles, and its aerogel conformation and specific cross-linked structure facilitate good contact and transfer towards different media, increasing the efficiency of the antimicrobial activity. Although this work involved the incorporation of CuO NPs into a specific nanocellulose-based support through cross-linking phenomena, the colloidal procedure, herein described, opens a door to ad hoc design of multifunctional membranes, filters, or flexible substrates, which requires the inclusion of other alternative compositions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric Antimicrobial Membranes Enabled by Nanomaterials for Water Treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Ren, G.; Wan, K.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.; Wei, G. Recent Advance in Biomass Membranes: Fabrication, Functional Regulation, and Antimicrobial Applications. Carbohydr. Polym. 2023, 305, 120537. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Paulose, T.A.P.; Philip, E.; Thomas, D.; Madhavan, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Pandey, A.; Sindhu, R. Updates on High Value Products from Cellulosic Biorefinery. Fuel 2022, 308, 122056. [Google Scholar] [CrossRef]
- Doh, H.; Lee, M.H.; Whiteside, W.S. Physicochemical Characteristics of Cellulose Nanocrystals Isolated from Seaweed Biomass. Food Hydrocoll. 2020, 102, 105542. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Ahmed, A.; Oun, S.S.; Rhim, J.-W. Multifunctional Nanocellulose/Metal and Metal Oxide Nanoparticle Hybrid Nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef]
- Cabanas-Polo, S.; Gonzalez, Z.; Sanchez-Herencia, A.J.; Ferrari, B. Influence of Ultrasound on the Instantaneous Synthesis of Tridimensional α-Ni(OH)2 Nanostructures and Derived NiO Nanoparticles. CrystEngComm 2015, 17, 6193–6206. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Shen, W.; Wu, Y.; Corriou, J.-P. Highly Sensitive, Flexible and Transparent TiO2/Nanocellulose Humidity Sensor for Respiration and Skin Monitoring. Ceram. Int. 2023, 49, 2204–2214. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Z.; Dutta, A.; Chen, X.; Gao, P.; Li, R.; Yan, J.; Niu, G.; Wang, Y.; Du, S.; et al. Superhydrophobic, Stretchable Kirigami Pencil-on-Paper Multifunctional Device Platform. Chem. Eng. J. 2023, 465, 142774. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Paul, J.; Ahankari, S.S. Nanocellulose-Based Aerogels for Water Purification: A Review. Carbohydr. Polym. 2023, 309, 120677. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparation and Properties of Carbohydrate-Based Composite Films Incorporated with CuO Nanoparticles. Carbohydr. Polym. 2017, 169, 264–271. [Google Scholar] [CrossRef]
- Heidari, H.; Teimuri, F.; Ahmadi, A.R. Nanocellulose-Based Aerogels Decorated with Ag, CuO and ZnO Nanoparticles: Synthesis, Characterization and the Antibacterial Activity. Polyhedron 2022, 213, 115629. [Google Scholar] [CrossRef]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of Novel Nanohybrids by Impregnation of CuO Nanoparticles into Bacterial Cellulose and Chitosan Nanofibers: Characterization, Antimicrobial and Release Properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Oun, A.A.; Rhim, J.-W. Preparation of Antimicrobial Hybrid Nano-Materials Using Regenerated Cellulose and Metallic Nanoparticles. Int. J. Biol. Macromol. 2018, 107, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal Oxides Nanoparticles via Sol-Gel Method: A Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Postnova, I.; Khlebnikov, O.; Silant’ev, V.; Shchipunov, Y. Dimensionally Stable Cellulosic Aerogels Functionalized by Titania. Pure Appl. Chem. 2018, 90, 1755–1771. [Google Scholar] [CrossRef]
- Antoniw, J.M.; Hallman, M.T.; Kiriakou, M.V.; Morse, T.; Cranston, E.D. Colloidal Stability Window for Carboxylated Cellulose Nanocrystals: Considerations for Handling, Characterization, and Formulation. Langmuir 2023, 39, 10321–10334. [Google Scholar] [CrossRef]
- Franks, G.V.; Tallon, C.; Studart, A.R.; Sesso, M.L.; Leo, S. Colloidal Processing: Enabling Complex Shaped Ceramics with Unique Multiscale Structures. J. Am. Ceram. Soc. 2017, 100, 458–490. [Google Scholar] [CrossRef]
- Carrasco, S.; Espinosa, E.; González, Z.; Cruz-Yusta, M.; Sánchez, L.; Rodríguez, A. Simple Route to Prepare Composite Nanocellulose Aerogels: A Case of Photocatalytic De-NOx Materials Application. ACS Sustain. Chem. Eng. 2023, 11, 2354–2363. [Google Scholar] [CrossRef]
- De Haro-Niza, J.; Rincón, E.; Gonzalez, Z.; Espinosa, E.; Rodríguez, A. Nanocellulose from Spanish Harvesting Residues to Improve the Sustainability and Functionality of Linerboard Recycling Processes. Nanomaterials 2022, 12, 4447. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Frígols, B.; Serrano-Aroca, A. Antimicrobial Characterization of Advanced Materials for Bioengineering Applications. J. Vis. Exp. 2018, e57710. [Google Scholar] [CrossRef]
- Rangel, W.M.; Boca Santa, R.A.A.; Riella, H.G. A Facile Method for Synthesis of Nanostructured Copper (II) Oxide by Coprecipitation. J. Mater. Res. Technol. 2020, 9, 994–1004. [Google Scholar] [CrossRef]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO Nanoparticles by Precipitation Method Using Different Precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Jagadeesan, M.S.; Movlaee, K.; Krishnakumar, T.; Leonardi, S.G.; Neri, G. One-Step Microwave-Assisted Synthesis and Characterization of Novel CuO Nanodisks for Non-Enzymatic Glucose Sensing. J. Electroanal. Chem. 2019, 835, 161–168. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific Surface Area and Pore-Size Distribution in Clays and Shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Yus, J.; Sanchez-Herencia, A.J.; Dewalque, J.; Manceriu, L.; Henrist, C.; Ferrari, B. A Colloidal Approach to Prepare Binder and Crack-Free TiO2 Multilayer Coatings from Particulate Suspensions: Application in DSSCs. J. Eur. Ceram. Soc. 2019, 39, 366–375. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.P.V.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta Potential beyond Materials Science: Applications to Bacterial Systems and to the Development of Novel Antimicrobials. Biochim. Biophys. Acta–Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- Kaffashsaie, E.; Yousefi, H.; Nishino, T.; Matsumoto, T.; Mashkour, M.; Madhoushi, M.; Kawaguchi, H. Direct Conversion of Raw Wood to TEMPO-Oxidized Cellulose Nanofibers. Carbohydr. Polym. 2021, 262, 117938. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules 2022, 12, 232. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective Toxicity of ZnO Nanoparticles toward Gram-Positive Bacteria and Cancer Cells by Apoptosis through Lipid Peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.; Chen, C.; Yu, H.; Yuan, H. Antibacterial Activity and Mechanism of Oxidized Bacterial Nanocellulose with Different Carboxyl Content. Macromol. Biosci. 2023, 23, 2200459. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dai, J.; Huang, L.; Si, Y.; Yu, J.; Ding, B. Biomimetic and Superelastic Silica Nanofibrous Aerogels with Rechargeable Bactericidal Function for Antifouling Water Disinfection. ACS Nano 2020, 14, 8975–8984. [Google Scholar] [CrossRef] [PubMed]
- Gnanamani, A.; Hariharan, P.; Paul-Satyaseela, M. Staphylococcus aureus: Overview of Bacteriology, Clinical Diseases, Epidemiology, Antibiotic Resistance and Therapeutic Approach. In Frontiers in Staphylococcus aureus; Enany, S., Crotty Alexander, L., Eds.; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Ananthanarayan, R.; Jayaram Paniker, C.K. Ananthanarayan and Paniker’s Textbook of Microbiology; Orient Blackswan: Hyderabad, India, 2017. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usala, E.; Espinosa, E.; El Arfaoui, W.; Morcillo-Martín, R.; Ferrari, B.; González, Z. Antibacterial Aerogels-Based Membranes by Customized Colloidal Functionalization of TEMPO-Oxidized Cellulose Nanofibers Incorporating CuO. Bioengineering 2023, 10, 1312. https://doi.org/10.3390/bioengineering10111312
Usala E, Espinosa E, El Arfaoui W, Morcillo-Martín R, Ferrari B, González Z. Antibacterial Aerogels-Based Membranes by Customized Colloidal Functionalization of TEMPO-Oxidized Cellulose Nanofibers Incorporating CuO. Bioengineering. 2023; 10(11):1312. https://doi.org/10.3390/bioengineering10111312
Chicago/Turabian StyleUsala, Elena, Eduardo Espinosa, Wasim El Arfaoui, Ramón Morcillo-Martín, Begoña Ferrari, and Zoilo González. 2023. "Antibacterial Aerogels-Based Membranes by Customized Colloidal Functionalization of TEMPO-Oxidized Cellulose Nanofibers Incorporating CuO" Bioengineering 10, no. 11: 1312. https://doi.org/10.3390/bioengineering10111312
APA StyleUsala, E., Espinosa, E., El Arfaoui, W., Morcillo-Martín, R., Ferrari, B., & González, Z. (2023). Antibacterial Aerogels-Based Membranes by Customized Colloidal Functionalization of TEMPO-Oxidized Cellulose Nanofibers Incorporating CuO. Bioengineering, 10(11), 1312. https://doi.org/10.3390/bioengineering10111312









