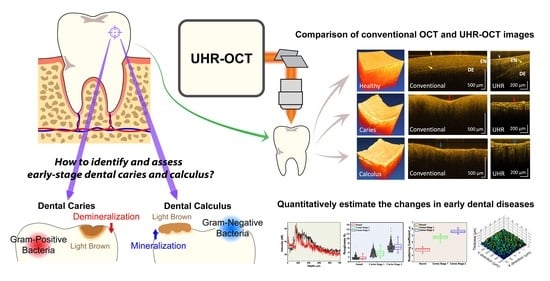
Quantitative Evaluation of Caries and Calculus with Ultrahigh-Resolution Optical Coherence Tomography
Abstract
:1. Introduction
2. Methods
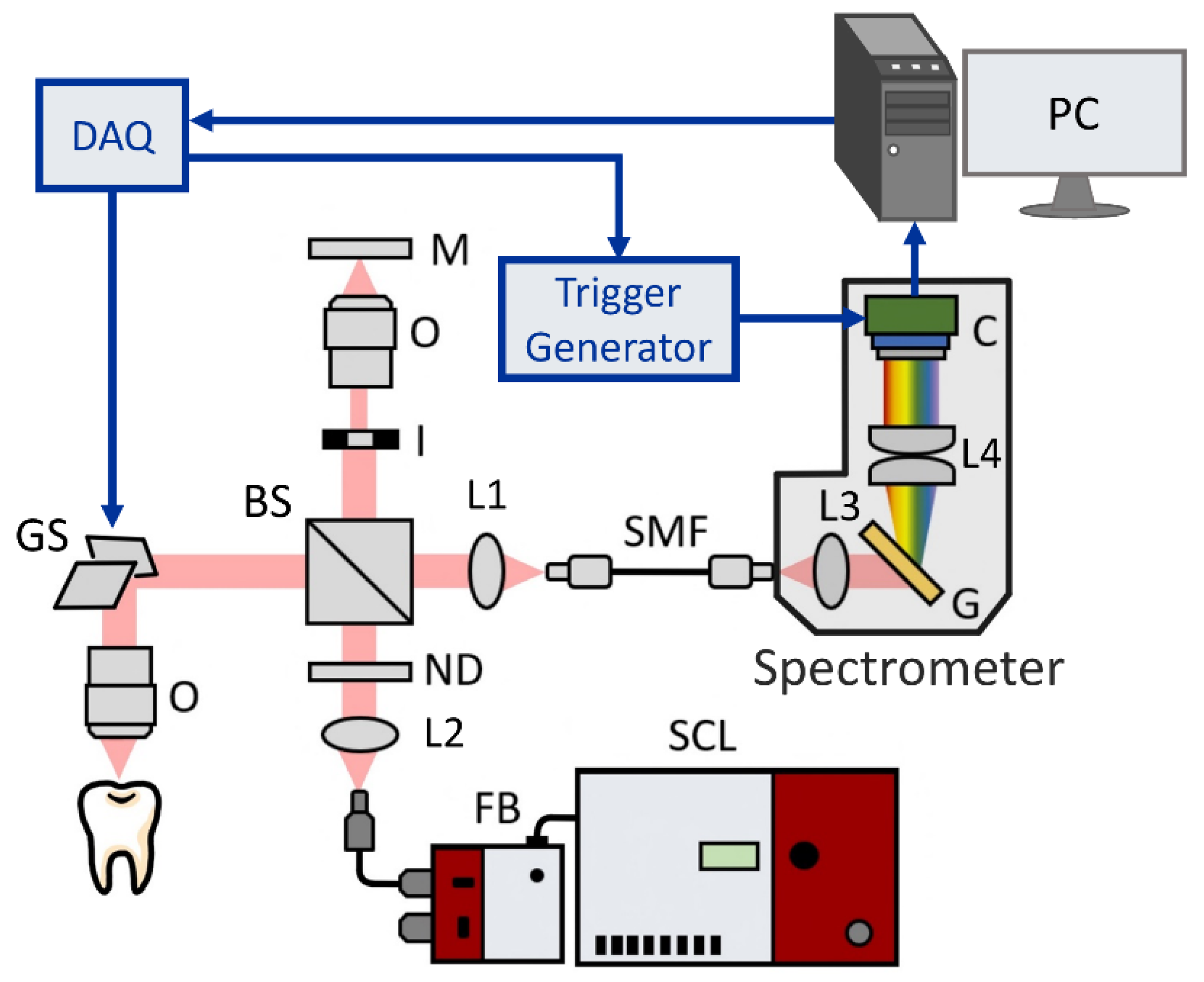
2.1. Setup of the UHR-OCT System
2.2. Sample Collection and Experimental Procedure
2.3. Data Analysis
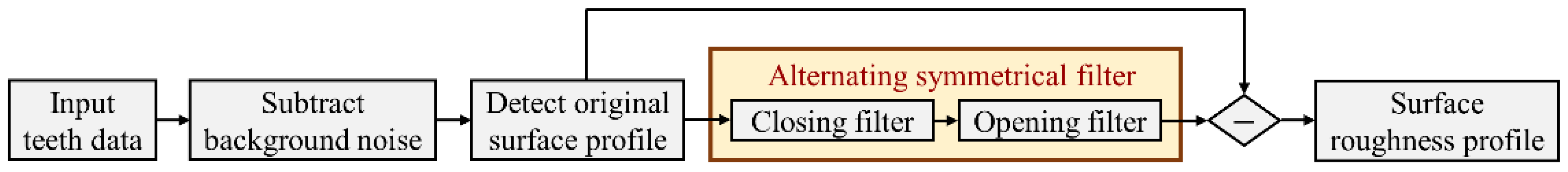
2.3.1. Surface Roughness of Dental Caries
2.3.2. Scattering Coefficient of Dental Caries
2.3.3. Segmentation of Dental Calculus
3. Results
3.1. System Characterization
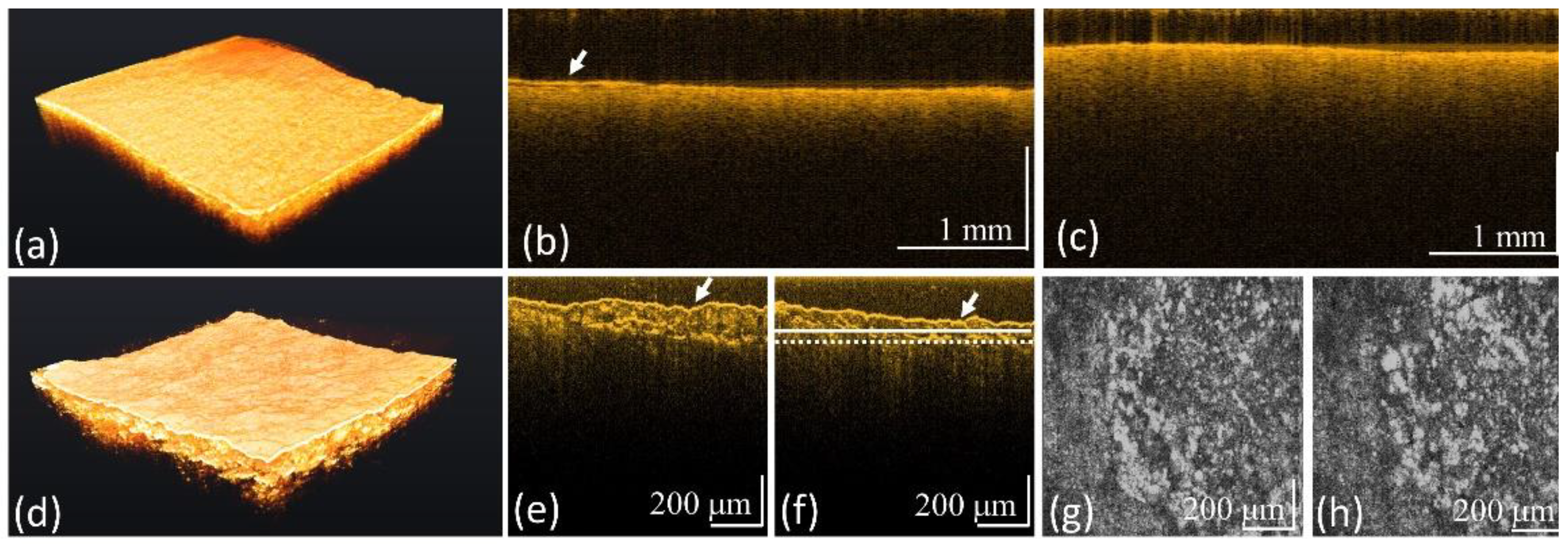
3.2. Surface Roughness of Carious Teeth
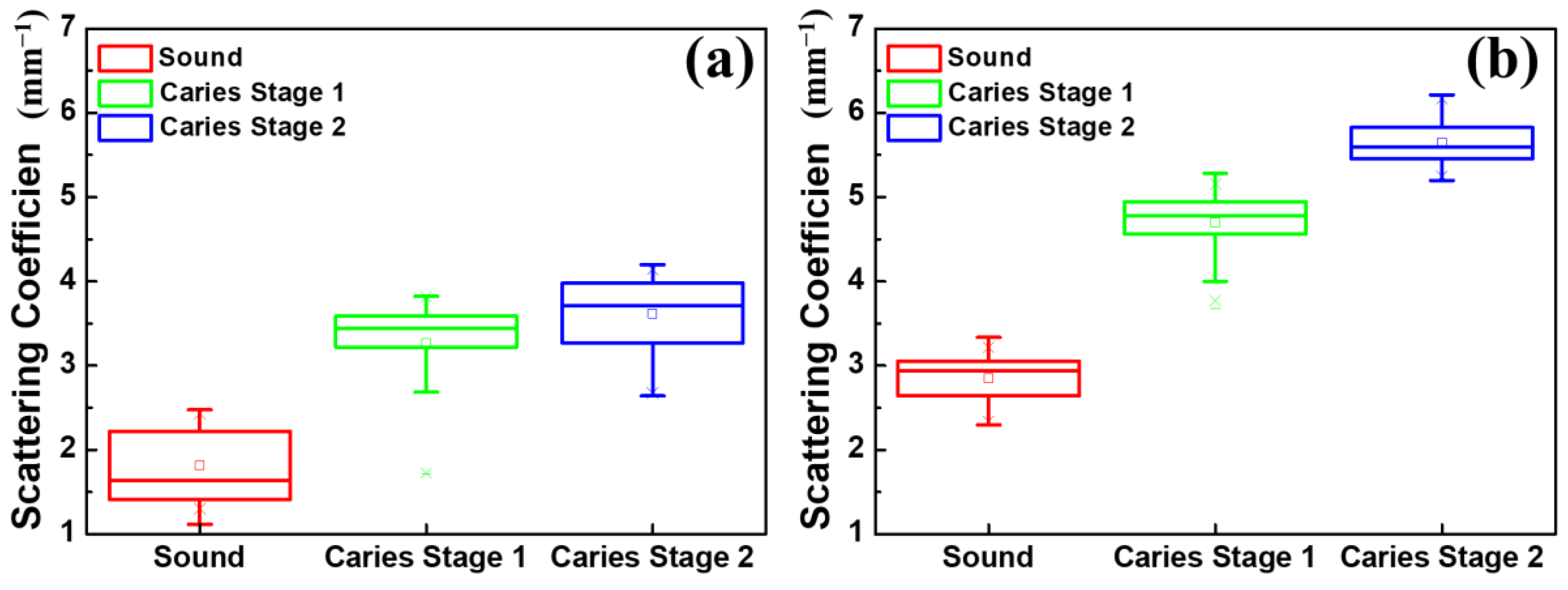
3.3. Scattering Coefficient of Carious Teeth
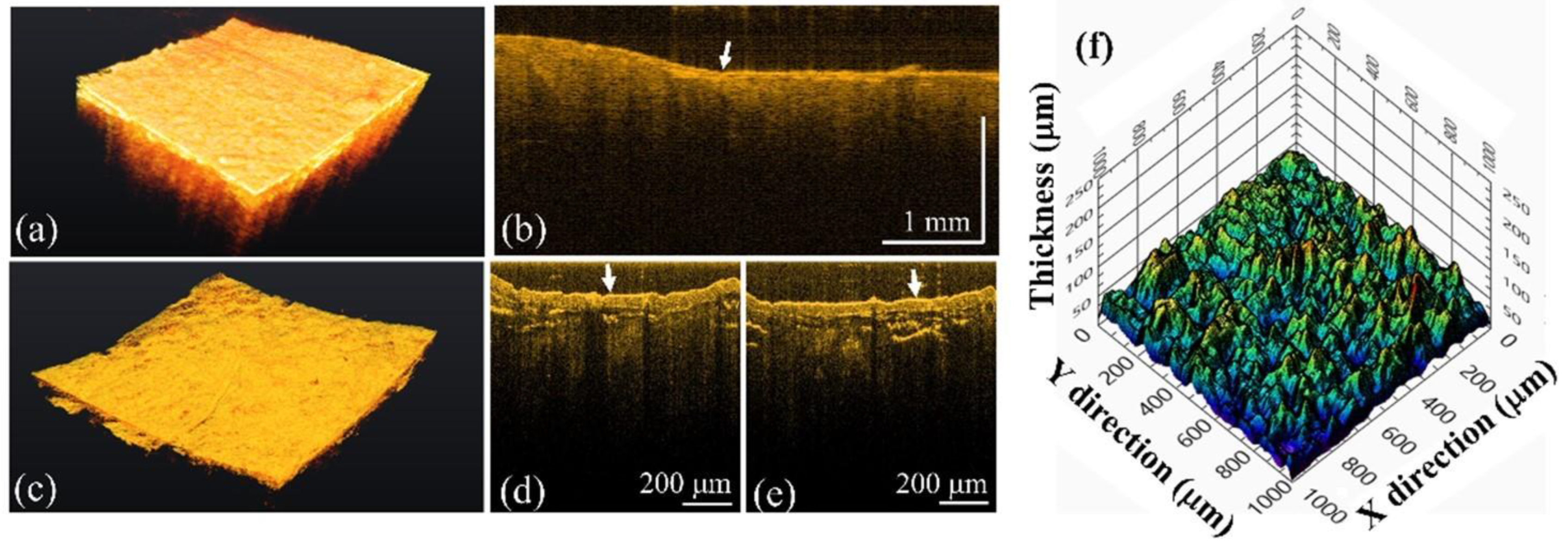
3.4. Calculus Segmentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dietrich, T.; Webb, I.; Stenhouse, L.; Pattni, A.; Ready, D.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence summary: The relationship between oral and cardiovascular disease. Br. Dent. J. 2017, 222, 381–385. [Google Scholar] [CrossRef]
- Franco, E.; Saunders, C.P.; Roberts, G.J.; Suwanprasit, A. Dental disease, caries related microflora and salivary IgA of children with severe congenital cardiac disease: An epidemiological and oral microbial survey. Pediatr. Dent. 1996, 18, 228–235. [Google Scholar]
- Chang, P.S.; Huang, C.J.; Hsiang, C.L.; Lai, H.; Tsai, A.I. Prevalence of dental caries and periodontal disease of high school students aged 15 to 18 years in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 9967. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers. 2017, 3, 17030. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers. 2017, 3, 17038. [Google Scholar]
- Forshaw, R. Dental calculus–Oral health, forensic studies and archaeology: A review. Br. Dent. J. 2022, 233, 961–967. [Google Scholar]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Schneider, H.; Park, K.J.; Rueger, C.; Ziebolz, D.; Krause, F.; Haak, R. Imaging resin infiltration into non-cavitated carious lesions by optical coherence tomography. J. Dent. 2017, 60, 94–98. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, T.; Inoue, G.; Nakagawa, H.; Tabata, T.; Zhou, Y.; Hiraishi, N.; Gondo, T.; Takano, S.; Ushijima, K.; et al. Evaluation of Incipient Enamel Caries at Smooth Tooth Surfaces Using SS-OCT. Materials 2022, 15, 5947. [Google Scholar] [CrossRef]
- Aldossari, G.S.; Alasmari, A.A.; Aldossary, M.S. Dental Caries Detection: The State of the Art. J. Appl. Dent. Med. 2019, 5, 17–30. [Google Scholar]
- Chang, J.J.; Chen, C.; Chang, J.; Koka, S.; Jokerst, J.V. A narrative review of imaging tools for imaging subgingival calculus. Front. Oral Maxillofac. Med. 2021, 5, 4. [Google Scholar] [CrossRef]
- Clark, D.J.; Sheets, C.G.; Paquette, J.M. Definitive diagnosis of early enamel and dentin cracks based on microscopic evaluation. J. Esthet. Restor. Dent. 2003, 15, 391–401. [Google Scholar] [CrossRef]
- Douglass, C.W.; Valachovic, R.W.; Wijesinha, A.; Chauncey, H.H.; Kapur, K.K.; McNeil, B.J. Clinical efficacy of dental radiography in the detection of dental caries and periodontal diseases. Oral Surg. Oral Med. Oral Pathol. 1986, 62, 330–339. [Google Scholar]
- Xiang, X.; Sowa, M.G.; Iacopino, A.M.; Maev, R.G.; Hewko, M.D.; Man, A.; Liu, K.-Z. An update on novel non-invasive approaches for periodontal diagnosis. J. Periodontol. 2010, 81, 186–198. [Google Scholar]
- Oh, S.H.; Lee, S.R.; Choi, J.Y.; Choi, Y.S.; Kim, S.H.; Yoon, H.C.; Nelson, G. Detection of dental caries and cracks with quantitative light-induced fluorescence in comparison to radiographic and visual examination: A retrospective case study. Sensors 2021, 21, 1741. [Google Scholar]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Tsai, M.T.; Lee, H.C.; Lee, C.K.; Yu, C.H.; Chen, H.M.; Chiang, C.P.; Chang, C.C.; Wang, Y.M.; Yang, C.C. Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt. Express 2008, 16, 15847–15862. [Google Scholar]
- Tsai, T.Y.; Chen, T.H.; Chen, H.C.; Chueh, C.B.; Huang, Y.P.; Hung, Y.P.; Tsai, M.T.; Baumann, B.; Wang, C.H.; Lee, H.C. Quantitative spectroscopic comparison of the optical properties of mouse cochlea microstructures using optical coherence tomography at 1.06 μm and 1.3 μm wavelengths. Biomed. Opt. Express 2021, 12, 2339–2352. [Google Scholar] [CrossRef]
- De Boer, J.F.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography—A review. Biomed. Opt. Express 2017, 8, 1838–1873. [Google Scholar]
- Villiger, M.; Braaf, B.; Lippok, N.; Otsuka, K.; Nadkarni, S.K.; Bouma, B.E. Optic axis mapping with catheter-based polarization-sensitive optical coherence tomography. Optica 2018, 5, 1329–1337. [Google Scholar]
- Golde, J.; Tetschke, F.; Walther, J.; Rosenauer, T.; Hempel, F.; Hannig, C.; Koch, E.; Kirsten, L. Detection of carious lesions utilizing depolarization imaging by polarization sensitive optical coherence tomography. J. Biomed. Opt. 2018, 23, 71203. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.Y.; Lee, S.Y.; Sun, C.W. Optical Polarimetric Detection for Dental Hard Tissue Diseases Characterization. Sensors 2019, 19, 4971. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Lu, J.; Tang, P.; Chung, K.-H.; Subhash, H.; Kilpatrick-Liverman, L.; Wang, R.K. Intraoral optical coherence tomography and angiography combined with autofluorescence for dental assessment. Biomed. Opt. Express 2022, 13, 3629–3646. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Chen, S.; Chen, S.; Liu, L. High Resolution Optical Coherence Tomography. J. Light. Technol. 2021, 39, 3824–3835. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Sapeta, S.; Schmidl, S.; Garhöfer, G.; Schmidinger, G.; Dos Santos, V.A.; Aschinger, G.C.; Baumgartner, I.; Pircher, N.; Schwarzhans, F.; et al. Ultrahigh-resolution OCT imaging of the human cornea. Biomed. Opt. Express 2017, 8, 1221–1239. [Google Scholar]
- Wang, T.A.; Chan, M.C.; Lee, H.C.; Lee, C.Y.; Tsai, M.T. Ultrahigh-resolution optical coherence tomography/angiography with an economic and compact supercontinuum laser. Biomed. Opt. Express 2019, 10, 5687–5702. [Google Scholar]
- Drexler, W.; Chen, Y.; Aguirre, A.D.; Považay, B.; Unterhuber, A.; Fujimoto, J.G. Ultrahigh resolution optical coherence tomography. In Optical Coherence Tomography—Technology and Applications, 2nd ed.; Drexler, W., Fujimoto, J.G., Eds.; Springer: Cham, Switzerland; New York, NY, USA, 2015; pp. 277–318. [Google Scholar]
- Wijesinghe, R.E.; Cho, N.H.; Park, K.; Jeon, M.; Kim, J. Biophotonic detection and quantitative evaluation method for the progression of dental caries using optical frequency-domain imaging method. Sensors 2016, 16, 2076. [Google Scholar] [CrossRef]
- Lee, C.K.; Tsai, M.T.; Lee, H.C.; Chen, H.M.; Chiang, C.P.; Wang, Y.M.; Yang, C.C. Diagnosis of oral submucous fibrosis with optical coherence tomography. J. Biomed. Opt. 2009, 14, 54008. [Google Scholar] [CrossRef]
- Kashiwa, M.; Shimada, Y.; Sadr, A.; Yoshiyama, M.; Sumi, Y.; Tagamin, J. Diagnosis of occlusal tooth wear using 3D imaging of optical coherence tomography ex vivo. Sensors 2020, 20, 6016. [Google Scholar] [CrossRef]
- Machoy, M.; Seeliger, J.; Szyszka-Sommerfeld, L.; Koprowski, R.; Gedrange, T.; Woźniak, K. The Use of Optical Coherence Tomography in Dental Diagnostics: A State-of-the-Art Review. J. Healthc. Eng. 2017, 2017, 7560645. [Google Scholar]
- Tsai, M.T.; Wang, Y.L.; Yeh, T.W.; Lee, H.C.; Chen, W.J.; Ke, J.L.; Lee, Y.J. Early detection of enamel demineralization by optical coherence tomography. Sci. Rep. 2019, 9, 17154. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Ho, Y.C.; Lee, S.Y.; Lu, C.W.; Jiang, C.P.; Chuang, C.C.; Wang, C.Y.; Sun, C.W. Subgingival calculus imaging based on swept-source optical coherence tomography. J. Biomed. Opt. 2011, 16, 071409. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Burrow, M.F.; Araki, K.; Zhou, Y.; Hosaka, K.; Sadr, A.; Yoshiyama, M.; Miyazaki, T.; Sumi, Y.; Tagami, J. 3D imaging of proximal caries in posterior teeth using optical coherence tomography. Sci. Rep. 2020, 11, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, R.M.; Huntington, E. On the relationship between calculus and caries. Monogr. Oral Sci. 2006, 19, 1–28. [Google Scholar]
- Ng, C.Y.; Wang, T.A.; Lee, H.C.; Huang, B.H.; Tsai, M.T. In vivo identification of skin photodamage induced by fractional CO2 and picosecond Nd:YAG lasers with optical coherence tomography. Diagnostics 2022, 12, 822. [Google Scholar] [CrossRef]
- Holmen, L.; Thylstrup, A.; Ogaard, B.; Kragh, F. A scanning electron microscopic study of progressive stages of enamel caries in vivo. Caries Res. 1985, 19, 355–367. [Google Scholar] [CrossRef]
- Myers, N.O. Characterization of surface roughness. Wear 1962, 5, 182–189. [Google Scholar] [CrossRef]
- Raja, J.; Muralikrishnan, B.; Fu, S. Recent advances in separation of roughness, waviness and form. Precis. Eng. 2002, 26, 222–235. [Google Scholar] [CrossRef]
- Lou, S.; Zeng, W.H.; Jiang, X.Q.; Scott, P.J. Robust filtration techniques in geometrical metrology and their comparison. Int. J. Autom. Comput. 2013, 10, 1–8. [Google Scholar] [CrossRef]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; De Boer, J.F. Depth-resolved model-based reconstruction of attenuation coefficients in optical coherence tomography. Biomed. Opt. Express 2014, 5, 322–337. [Google Scholar] [CrossRef]
- Faber, D.J.; van der Meer, F.J.; Aalders, M.C.G.; van Leeuwen, T.G. Quantitative measurement of attenuation coefficients of weakly scattering media using optical coherence tomography. Opt. Express 2004, 12, 4353–4365. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Srinivasan, V.J.; Ko, T.H.; Fujimoto, J.G.; Kowalczyk, A.; Duker, J.S. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Biomed. Opt. Express 2004, 12, 2404–2422. [Google Scholar] [CrossRef]
- Wang, X.J.; Milner, T.E.; de Boer, J.F.; Zhang, Y.; Pashley, D.H.; Nelson, J.S. Characterization of dentin and enamel by use of optical coherence tomography. Appl. Opt. 1999, 38, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.A.; de Freitas, A.Z.; Campello, S.d.L.; Gomes, A.S.L.; Karlsson, L. Evaluation of dental enamel caries assessment using Quantitative Light Induced Fluorescence and Optical Coherence Tomography. J. Biophotonics 2016, 9, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Darling, C.L.; Huynh, G.D.; Fried, D. Light scattering properties of natural and artificially demineralized dental enamel at 1310 nm. J. Biomed. Opt. 2006, 11, 34023. [Google Scholar] [CrossRef]
- Fried, D.; Glena, R.E.; Featherstone, J.D.; Seka, W. Nature of light scattering in dental enamel and dentin at visible and near-infrared wavelengths. Appl. Opt. 1995, 34, 1278–1285. [Google Scholar] [CrossRef]
- Popescu, D.P.; Sowa, M.G.; Hewko, M.D.; Choo-Smith, L.P. Assessment of early demineralization in teeth using the signal attenuation in optical coherence tomography images. J. Biomed. Opt. 2008, 13, 54053. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Yue, L.; Ling, J.; Fan, M.; Yang, D.; Huang, Z.; Niu, Y.; Liu, J.; Zhao, J.; et al. Expert consensus on dental caries management. Int. J. Oral Sci. 2022, 14, 17. [Google Scholar] [CrossRef]
- Luong, M.N.; Shimada, Y.; Araki, K.; Yoshiyama, M.; Tagami, J.; Sadr, A. Diagnosis of occlusal caries with dynamic slicing of 3D optical coherence tomography images. Sensors 2020, 20, 1659. [Google Scholar] [CrossRef]
- Tsubokawa, M.; Aoki, A.; Kakizaki, S.; Taniguchi, Y.; Ejiri, K.; Mizutani, K.; Koshy, G.; Akizuki, T.; Oda, S.; Sumi, Y.; et al. In vitro and clinical evaluation of optical coherence tomography for the detection of subgingival calculus and root cementum. J. Oral Sci. 2018, 60, 418–427. [Google Scholar] [CrossRef]
- Won, J.; Huang, P.C.; Spillman, D.R.; Chaney, E.J.; Adam, R.; Klukowska, M.; Barkalifa, R.; Boppart, S.A. Handheld optical coherence tomography for clinical assessment of dental plaque and gingiva. J. Biomed. Opt. 2020, 25, 116011. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, B.; Lindenmaier, A.; Standish, B.A.; Allo, G.; Bizheva, K.; Vitkin, A. Noninvasive in vivo structural and vascular imaging of human oral tissues with spectral domain optical coherence tomography. Biomed. Opt. Express 2012, 3, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Jang, W.H.; Xiao, P.; Kim, B.; Wang, T.; Li, Q.; Lee, J.Y.; Chung, E.; Kim, K.H. In vivo wide-field reflectance/fluorescence imaging and polarization-sensitive optical coherence tomography of human oral cavity with a forward-viewing probe. Biomed. Opt. Express 2015, 6, 524–535. [Google Scholar] [CrossRef] [PubMed]
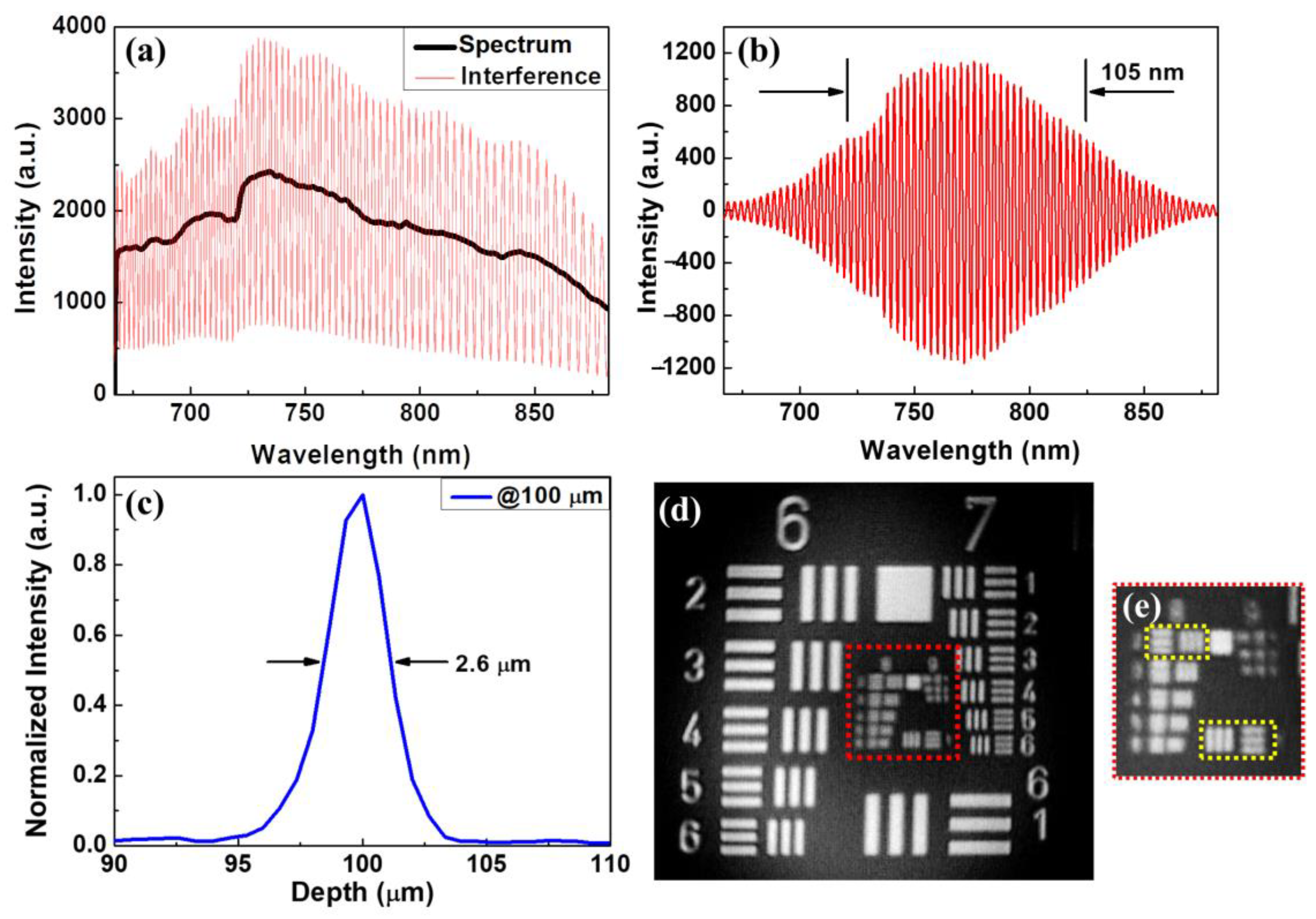


| Center Wavelength | FWHM | Axial Resolution | Transverse Resolution | Image Area | Frame Rate | |
|---|---|---|---|---|---|---|
| Conventional-resolution OCT | 840 nm | 45 nm | ~7 μm | ~10 μm | 25 mm2 | 50 frames/s |
| UHR-OCT | 775 nm | 105 nm | ~2.6 μm | ~1.8 μm | 1 mm2 | 50 frames/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-A.; Trung, N.H.; Lee, H.-C.; Lee, C.-K.; Tsai, M.-T.; Wang, Y.-L. Quantitative Evaluation of Caries and Calculus with Ultrahigh-Resolution Optical Coherence Tomography. Bioengineering 2023, 10, 1317. https://doi.org/10.3390/bioengineering10111317
Wang T-A, Trung NH, Lee H-C, Lee C-K, Tsai M-T, Wang Y-L. Quantitative Evaluation of Caries and Calculus with Ultrahigh-Resolution Optical Coherence Tomography. Bioengineering. 2023; 10(11):1317. https://doi.org/10.3390/bioengineering10111317
Chicago/Turabian StyleWang, Tai-Ang, Nguyễn Hoàng Trung, Hsiang-Chieh Lee, Cheng-Kuang Lee, Meng-Tsan Tsai, and Yen-Li Wang. 2023. "Quantitative Evaluation of Caries and Calculus with Ultrahigh-Resolution Optical Coherence Tomography" Bioengineering 10, no. 11: 1317. https://doi.org/10.3390/bioengineering10111317
APA StyleWang, T.-A., Trung, N. H., Lee, H.-C., Lee, C.-K., Tsai, M.-T., & Wang, Y.-L. (2023). Quantitative Evaluation of Caries and Calculus with Ultrahigh-Resolution Optical Coherence Tomography. Bioengineering, 10(11), 1317. https://doi.org/10.3390/bioengineering10111317