Endoscope Capsules: The Present Situation and Future Outlooks
Abstract
:1. Introduction
1.1. Organization of the Paper
1.2. Capsule Endoscopy Origins
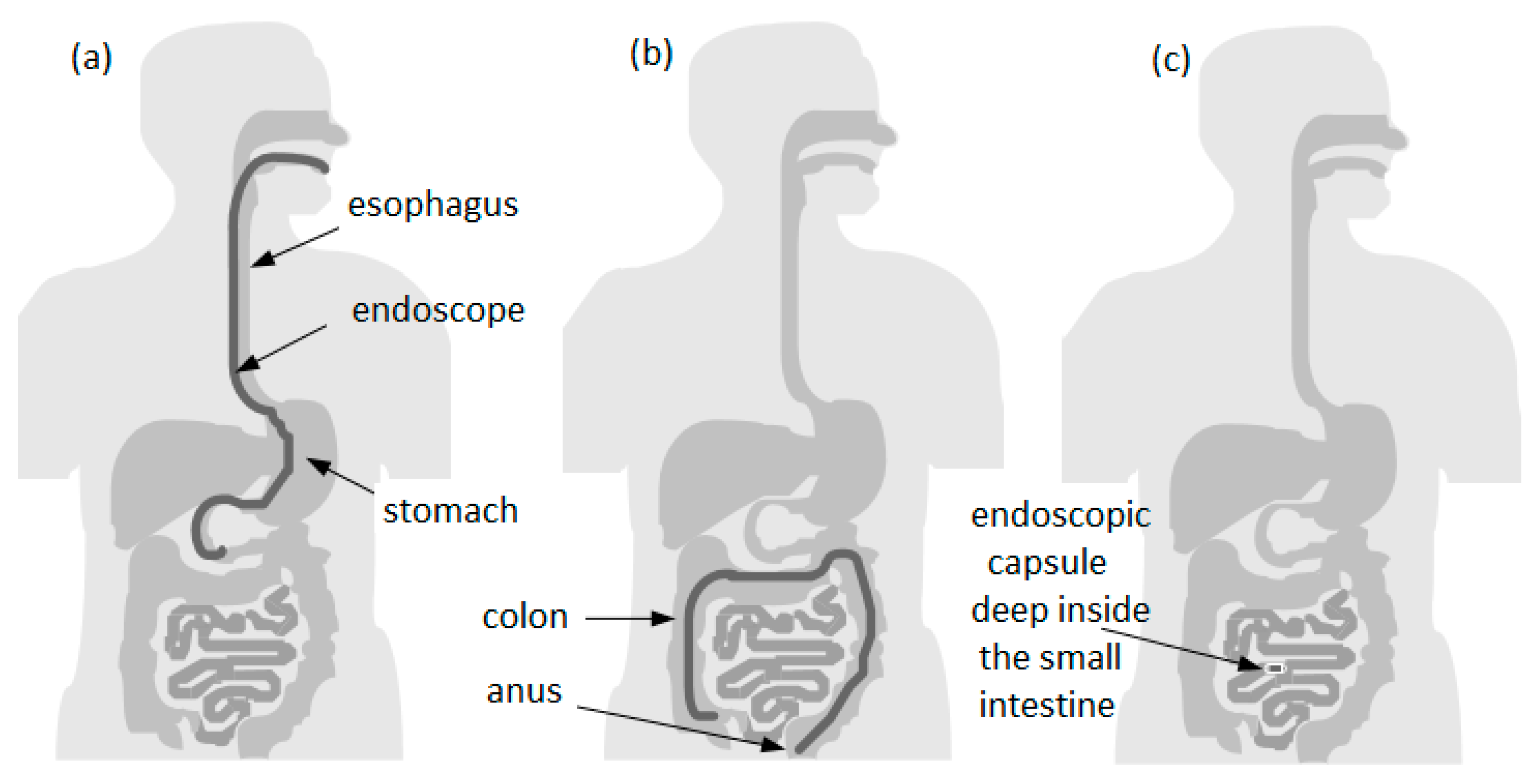
2. Capsule Endoscopy
2.1. Benefits of Capsule Endoscopy
2.2. Drawbacks of Capsule Endoscopy
2.3. Indications of the Capsule Endoscopy
2.4. Contraindications of Capsule Endoscopy
2.5. The Problem of Capsule Mobility
3. Indications of Esophageal Capsules
3.1. Screening for Barrett’s Esophagus
3.2. Screening for Esophageal Varices
4. Indications of Intestinal Capsules
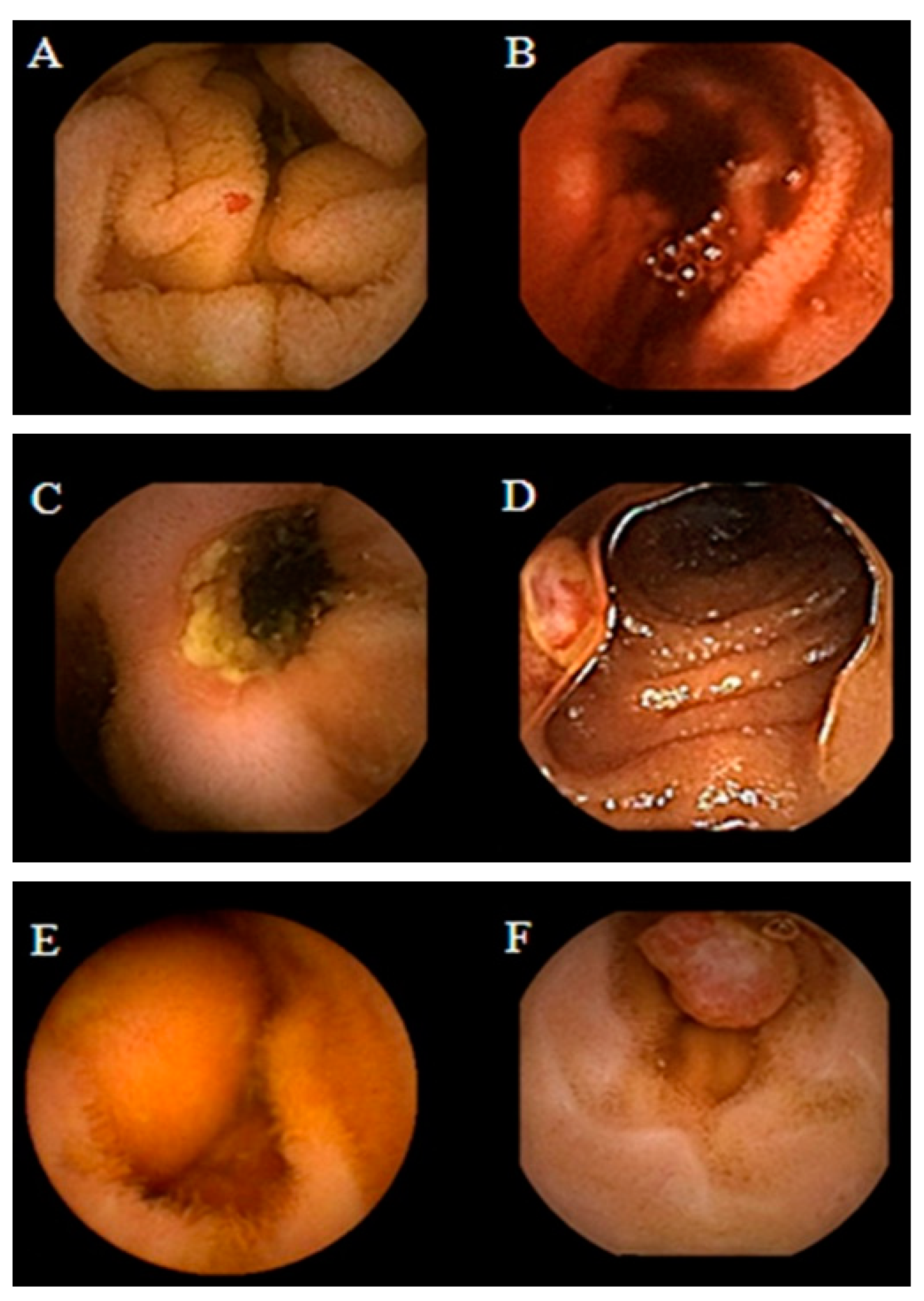
4.1. Intestinal Tumors
4.2. Obscure GI Bleeding
4.3. Crohn’s Disease
4.4. Celiac Disease
4.5. Genetic Disorders
5. Commercial Capsules
6. New Functionalities for Capsule Endoscopy
7. Photodynamic Therapy
8. Laser Endomicroscopy
9. Spectroscopy
10. Narrow-Band Imaging
10.1. Design and Fabrication of Optical Filters
10.2. Optical Filter Characterization and Results
10.3. Narrow-Band Imaging vs. CLE
10.4. Narrow-Band Imaging vs. FICE
10.5. Narrow-Band Imaging vs. BLI
10.6. Narrow-Band Imaging, FICE, and BLI Images Enhanced by i-SCAN
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Moltzer, E.; Noordman, B.J.; Renken, N.S.; Roos, D. Determination of Tumor Location in Rectosigmoid Carcinomas: Difficulties in Preoperative Diagnostics. Gastrointest. Disord. 2019, 1, 210–219. [Google Scholar] [CrossRef]
- Ortega, S.; Fabelo, H.; Iakovidis, D.K.; Koulaouzidis, A.; Callico, G.M. Use of Hyperspectral/Multispectral Imaging in Gastroenterology. Shedding Some–Different–Light into the Dark. J. Clin. Med. 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Beeley, J.; Melino, G.; Al-Rawahani, M.; Turcanu, M.; Stewart, F.; Cochran, S.; Cumming, D. Imaging Fluorophore-Labelled Intestinal Tissue via Fluorescence Endoscope Capsule. Proceedings 2018, 2, 766. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamamura, T.; Maeda, K.; Sawada, T.; Mizutani, Y.; Ishikawa, T.; Furukawa, K.; Ohno, E.; Kawashima, H.; Miyahara, R.; et al. Validity of Capsule Endoscopy in Monitoring Therapeutic Interventions in Patients with Crohn’s Disease. J. Clin. Med. 2018, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, G.R.; Singh, P.; Pandey, K.; Kala, C.; Pradhan, A. Improving Diagnosis of Cervical Pre-Cancer: Combination of PCA and SVM Applied on Fluorescence Lifetime Images. Photonics 2018, 5, 57. [Google Scholar] [CrossRef]
- Askoura, M.L.; Vaudelle, F.; L’Huillier, J.P. Experimental Study of Light Propagation in Apple Tissues Using a Multispectral Imaging System. Photonics 2016, 3, 50. [Google Scholar] [CrossRef]
- Rohrbach, D.J.; Salem, H.; Aksahin, M.; Sunar, U. Photodynamic Therapy-Induced Microvascular Changes in a Nonmelanoma Skin Cancer Model Assessed by Photoacoustic Microscopy and Diffuse Correlation Spectroscopy. Photonics 2016, 3, 48. [Google Scholar] [CrossRef]
- Peretti, R.; Braud, F.; Peytavit, E.; Dubois, E.; Lampin, J.F. Broadband Terahertz Light-Matter Interaction Enhancement for Precise Spectroscopy of Thin Films and Micro-Samples. Photonics 2018, 5, 11. [Google Scholar] [CrossRef]
- Tansu, N. Photonics-Advances in Fundamental Sciences and Engineering Technologies of Light. Photonics 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Chiu, P.W.Y. Second Look Endoscopy in Acute Non-Variceal Upper Gastrointestinal Bleeding. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 905–911. [Google Scholar] [CrossRef]
- Kahi, C.J.; Myers, L.J.; Slaven, J.E.; Haggstrom, D.; Pohl, H.; Robertson, D.J.; Imperiale, T.F. Lower Endoscopy Reduces Colorectal Cancer Incidence in Older Individuals. Gastroenterology 2014, 146, 718–725.e3. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Wang, L. Swallowable Wireless Capsule Endoscopy: Progress and Technical Challenges. Gastroenterol. Res. Pract. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Karargyris, A.; Bourbakis, N. Wireless Capsule Endoscopy and Endoscopy Imaging. IEEE Eng. Med. Biol. 2010, 29, 72–83. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless Capsule Endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Leighton, J.A.; Sharma, V.K. Capsule Endoscopy in the Evaluation of Obscure Gastrointestinal Bleeding: A Comprehensive Review. Gastroenterol. Hepatol. 2007, 3, 777–785. [Google Scholar]
- Schnoll-Sussman, F.; Kulkarni, K. Risks of Capsule Endoscopy. Tech. Gastrointest. Endosc. 2008, 10, 25–30. [Google Scholar] [CrossRef]
- Rondonotti, E.; Herrerias, J.M.; Pennazio, M.; Caunedo, A.; Mascarenhas-Saraiva, M.; De Franchis, R. Complications, Limitations, and Failures of Capsule Endoscopy: A Review of 733 Cases. Gastrointest. Endosc. 2005, 62, 712–716. [Google Scholar] [CrossRef]
- Spada, C.; Shah, S.K.; Riccioni, M.E.; Spera, G.; Marchese, M.; Iacopini, F.; Familiari, P.; Costamagna, G. Video Capsule Endoscopy in Patients With Known or Suspected Small Bowel Stricture Previously Tested With the Dissolving Patency Capsule. J. Clin. Gastroenterol. 2007, 41, 576–582. [Google Scholar] [CrossRef]
- Boivin, M.L.; Lochs, H.; Voderholzer, W.A. Does Passage of a Patency Capsule Indicate Small−Bowel Patency? A Prospective Clinical Trial? Endoscopy 2005, 37, 808–815. [Google Scholar] [CrossRef]
- Signorelli, C.; Rondonotti, E.; Villa, F.; Abbiati, C.; Beccari, G.; Avesani, E.C.; Vecchi, M.; de Franchis, R. Use of the Given® Patency System for the Screening of Patients at High Risk for Capsule Retention. Dig. Liver Dis. 2006, 38, 326–330. [Google Scholar] [CrossRef]
- Holden, J.P.; Dureja, P.; Pfau, P.R.; Schwartz, D.C.; Reichelderfer, M.; Judd, R.H.; Danko, I.; Iyer, L.V.; Gopal, D.V. Endoscopic Placement of the Small-Bowel Video Capsule by Using a Capsule Endoscope Delivery Device. Gastrointest. Endosc. 2007, 65, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Selby, W.; Sydney, F. Complete Small-Bowel Transit in Patients Undergoing Capsule Endoscopy: Determining Factors and Improvement with Metoclopramide. Gastrointest. Endosc. 2005, 61, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Fireman, Z.; Paz, D.; Elsevier, K.; Kopelman, Y. Capsule Endoscopy: Improving Transit Time and Image View. World J. Gastroenterol. 2005, 11, 5863–5866. [Google Scholar] [CrossRef]
- Moy, L.; Levine, J. Wireless Capsule Endoscopy in the Pediatric Age Group: Experience and Complications. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Ben-Soussan, E.; Savoye, G.; Antonietti, M.; Ramirez, S.; Ducrotté, P.; Lerebours, E. Is a 2-Liter PEG Preparation Useful Before Capsule Endoscopy? J. Clin. Gastroenterol. 2005, 39, 381–384. [Google Scholar] [CrossRef]
- Dai, N.; Gubler, C.; Hengstler, P.; Meyenberger, C.; Bauerfeind, P.; Gallen, S. Improved Capsule Endoscopy after Bowel Preparation. Gastrointest. Endosc. 2005, 61, 28–31. [Google Scholar] [CrossRef]
- Shiotani, A.; Opekun, A.R.; Graham, D.Y. Visualization of the Small Intestine Using Capsule Endoscopy in Healthy Subjects. Dig. Dis. Sci. 2007, 52, 1019–1025. [Google Scholar] [CrossRef]
- Pennazio, M. Capsule Endoscopy: Where Are We after 6 Years of Clinical Use? Dig. Liver Dis. 2006, 38, 867–878. [Google Scholar] [CrossRef]
- Jain, R.K.; Jain, S. Capsule Endoscopy: A Comprehensive Review. In New Techniques in Gastrointestinal Endoscopy; Pascu, O., Seicean, A., Eds.; IntechOpen: Rijeka, Croatia, 2011; pp. 85–102. [Google Scholar]
- Leighton, J.A.; Sharma, V.K.; Srivathsan, K.; Heigh, R.I.; McWane, T.L.; Post, J.K.; Robinson, S.R.; Bazzell, J.L.; Fleischer, D.E. Safety of Capsule Endoscopy in Patients with Pacemakers. Gastrointest. Endosc. 2004, 59, 567–569. [Google Scholar] [CrossRef]
- Leighton, J.A.; Srivathsan, K.; Carey, E.J.; Sharma, V.K.; Heigh, R.I.; Post, J.K.; Erickson, P.J.; Robinson, S.R.; Bazzell, J.L.; Fleischer, D.E. Safety of Wireless Capsule Endoscopy in Patients with Implantable Cardiac Defibrillators. Off. J. Am. Coll. Gastroenterol. |ACG 2005, 100, 1728–1731. [Google Scholar] [CrossRef]
- Swain, P. The future of wireless capsule endoscopy. World J. Gastroenterol. 2008, 14, 4142–4145. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.A. Current and future applications of the capsule camera. Nat. Rev. Drug Discov. 2004, 3, 447–480. [Google Scholar] [CrossRef]
- Carta, R.; Pateromichelakis, N.; Thoné, J.; Sfakiotakis, M.; Tsakiris, D.P.; Puers, R. A wireless powering system for a vibratory-actuated endoscopic capsule. In Proceedings of the Eurosensors XXII, Linz, Austria, 5–8 September 2010; pp. 1369–1372. [Google Scholar]
- Sfakiotakis, M.; Tsakiris, D.P. Pedundulatory robotic locomotion: Centipede and polychaete modes in unstructured substrates. In Proceedings of the IEEE International Conference on Robotics and Biomimetics (ROBIO’08), Bangkok, Thailand, 22–25 February 2009; pp. 651–658. [Google Scholar]
- Valdastri, P.; Webster, R.J.; Quaglia, C.; Quirini, M.; Menciassi, A.; Dario, P. A new mechanism for meso-scale legged locomotion in compliant tubular environments. IEEE Trans. Robot. 2009, 25, 1047–1057. [Google Scholar] [CrossRef]
- Carta, R.; Tortora, G.; Thoné, J.; Lenaerts, B.; Valdastri, P.; Menciassi, A.; Dario, P.; Puers, R. Wireless powering for a self-propelled and steerable endoscopic capsule for stomach inspection. Biosens. Bioelectron. 2009, 25, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Roth Net Standard Retrievers, US Endoscopy. Available online: www.usendoscopy.com (accessed on 12 March 2019).
- Zabulis, X.; Sfakiotakis, M.; Tsakiris, D.I. Effects of vibratory actuation on endoscopic capsule vision. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 5901–5902. [Google Scholar]
- Radley, B.; Elson, J.N.; Gervasoni, S.; Chiu, P.W.Y.; Hang, L.; Zemmar, A. Magnetically Actuated Medical Robots: An in vivo Perspective. Proc. IEEE 2022, 110, 1028–1037. [Google Scholar] [CrossRef]
- Carpi, F.; Galbiati, S.; Carpi, A. Controlled Navigation of Endoscopic Capsules: Concept and Preliminary Experimental Investigations. IEEE Trans. Biomed. Eng. 2007, 54, 2028–2036. [Google Scholar] [CrossRef]
- Silva, M.F.; Ribeiro, J.F.; Goncalves, L.M.; Carmo, J.P.; Silva, C.A.; Correia, J.H. Magnetic control platform for wireless endoscopic capsules. Procedia Eng. 2011, 25, 996–999. [Google Scholar] [CrossRef]
- Keller, H.; Juloski, A.; Kawano, H.; Bechtold, M.; Kimura, A.; Takizaw, H. Method for navigation and control of a magnetically guided capsule endoscope in the human stomach. In Proceedings of the 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 859–865. [Google Scholar]
- Tai, C.; Chen, M.; Luo, C. Study of a magnetic levitation technique for use in a wireless capsule endoscope. J. Med. Biol. Eng. 2007, 27, 57–64. [Google Scholar]
- Shaheen, N.J.; Richter, J.E. Barrett’s Oesophagus. Lancet 2009, 373, 850–861. [Google Scholar] [CrossRef]
- Spechler, S.J. Barrett’s Esophagus. New Engl. J. Med. 2002, 346, 836–842. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Crosby, M.A.; Bozymski, E.M.; Sandler, R.S. Is There Publication Bias in the Reporting of Cancer Risk in Barrett’s Esophagus? Gastroenterology 2000, 119, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; Devesa, S.S.; Kneller, R.W.; Fraumeni, J.F., Jr. Rising Incidence of Adenocarcinoma of the Esophagus and NGastric Cardia. JAMA 1991, 265, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Devesa, S.S.; Blot, W.J.; Fraumeni, J.F., Jr. Changing Patterns in the Incidence of Esophageal and Gastric Carcinoma in the United States. Cancer 1998, 83, 2049–2053. [Google Scholar] [CrossRef]
- Galmiche, J.P.; Coron, E.; Sacher-Huvelin, S. Recent Developments in Capsule Endoscopy. Gut 2008, 57, 695. [Google Scholar] [CrossRef]
- Gerson, L.; Lin, O.S. Cost-Benefit Analysis of Capsule Endoscopy Compared With Standard Upper Endoscopy for the Detection of Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2007, 5, 319–325.e3. [Google Scholar] [CrossRef]
- Alsayid, M.; Melson, J. Will magnet-assisted capsule endoscopy become a viable screening tool for Barrett’s esophagus and esophageal varices? Clin. Endosc. 2020, 91, 782–784. [Google Scholar] [CrossRef]
- Pena, L.R.; Cox, T.; Koch, A.G.; Bosch, A. Study Comparing Oesophageal Capsule Endoscopy versus EGD in the Detection of Varices. Dig. Liver Dis. 2008, 40, 216–223. [Google Scholar] [CrossRef]
- McCarty, T.R.; Afinogenova, Y.; Njei, B. Use of Wireless Capsule Endoscopy for the Diagnosis and Grading of Esophageal Varices in Patients With Portal Hypertension: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 174. [Google Scholar] [CrossRef]
- Eisen, R.; Zaman, A.; Schwartz, J.; Faigel, D.; Rondonotti, E.; Villa, F.; Weizman, E.; Yassin, K.; deFranchis, R. The Accuracy of PillCam ESO Capsule Endoscopy Versus Conventional Upper Endoscopy for the Diagnosis of Esophageal Varices: A Prospective Three-Center Pilot Study. Endoscopy 2006, 38, 31–35. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, R.; Liao, Z.; Hu, L.H.; Li, Z.S. Meta-Analysis of Capsule Endoscopy in Patients Diagnosed or Suspected with Esophageal Varices. World J. Gastroenterol 2009, 15, 1254–1258. [Google Scholar] [CrossRef]
- Beg, S.; Card, T.; Warburton, S.; Rahman, I.; Wilkes, E.; White, J.; Ragunath, K. Diagnosis of Barrett’s esophagus and esophageal varices using a magnetically assisted capsule endoscopy system. Gastrointest. Endosc. 2020, 91, 773–781. [Google Scholar] [CrossRef]
- Jensen, D.M.; Singh, B.; Chavalitdhamrong, D.; Kovacs, T.O.; Carrico, M.; Han, S.-H.B.; Durazo, F.A.; Saab, S. Is Capsule Endoscopy Accurate Enough to Screen Cirrhotics for High Risk Varices & Other Lesions? A Blinded Comparison of EGD & PillCam ESO. Gastrointest. Endosc. 2008, 67, AB122. [Google Scholar] [CrossRef]
- Smith, R.A.; Cokkinides, V.; von Eschenbach, A.C.; Levin, B.; Cohen, C.; Runowicz, C.D.; Sener, S.; Saslow, D.; Eyre, H.J. American Cancer Society Guidelines for the Early Detection of Cancer. CA Cancer J. Clin. 2002, 52, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Cobrin, G.M.; Pittman, R.H.; Lewis, B.S. Increased Diagnostic Yield of Small Bowel Tumors with Capsule Endoscopy. Cancer 2006, 107, 22–27. [Google Scholar] [CrossRef]
- Delvaux, M.; Gay, G. Capsule Endoscopy: Technique and Indications. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 813–837. [Google Scholar] [CrossRef] [PubMed]
- Alquist, D.; Fennerty, B.; Fleischer, D.; McDonnell, W.M.; McGill, D.B.; Waring, P.; Wilcox, C.M.; Winawer, S. American Gastroenterological Association Medical Position Statement: Evaluation and Management of Occult and Obscure Gastrointestinal Bleeding. Gastroenterology 2000, 118, 197–200. [Google Scholar] [CrossRef]
- Rockey, D.C. Occult and Obscure Gastrointestinal Bleeding: Causes and Clinical Management. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 265–279. [Google Scholar] [CrossRef]
- Pennazio, M.; Santucci, R.; Rondonotti, E.; Abbiati, C.; Beccari, G.; Rossini, F.P.; De Franchis, R. Outcome of Patients with Obscure Gastrointestinal Bleeding after Capsule Endoscopy: Report of 100 Consecutive Cases. Gastroenterology 2004, 126, 643–653. [Google Scholar] [CrossRef]
- Triester, S.L.; Leighton, J.A.; Leontiadis, G.I.; Gurudu, S.R.; Fleischer, D.E.; Hara, A.K.; Heigh, R.I.; Shiff, A.D.; Sharma, V.K. A Meta-Analysis of the Yield of Capsule Endoscopy Compared to Other Diagnostic Modalities in Patients with Non-Stricturing Small Bowel Crohn’s Disease. Off. J. Am. Coll. Gastroenterol. |ACG 2006, 101, 954–964. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sekine, Y.; Sato, Y.; Higashizawa, T.; Miyata, T.; Iino, S.; Ido, K.; Sugano, K. Total Enteroscopy with a Nonsurgical Steerable Double-Balloon Method. Gastrointest. Endosc. 2001, 53, 216–220. [Google Scholar] [CrossRef]
- Rondonotti, E.; Koulaouzidis, A.; Silvia, P.; Franco, R.; Pennazio, M. Obscure Gastrointestinal Bleeding and Iron-Deficiency Anemia-Where Does Capsule Endoscopy Fit? Tech. Gastrointest. Endosc. 2015, 17, 12–18. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. Classification of Inflammatory Bowel Disease. Scand J. Gastroenterol. 1989, 24, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, R.; Frascio, M.; Denegri, A.; Stabilini, C.; Imperatore, M.; Mandolfino, F.; Fabrizio, L.; Gianetta, E. Malattia Di Crohn e Cancro. Ann. Ital. Chir. 2009, 80, 119–125. [Google Scholar] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Schulmann, K.; Hollerbach, S.; Schmiegel, W. Diagnosing Small Bowel Crohn’s Disease with Wireless Capsule Endoscopy. Gut 2003, 52, 1531. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.G.; Martiny, F.; Krummenerl, A.; Stock, K.; Leßke, J.; Göbel, C.M.; Lotterer, E.; Nietsch, H.H.; Behrmann, C.; Fleig, W.E. Diagnosis of Small Bowel Crohn’s Disease: A Prospective Comparison of Capsule Endoscopy with Magnetic Resonance Imaging and Fluoroscopic Enteroclysis. Gut 2005, 54, 1721. [Google Scholar] [CrossRef]
- Odeyinka, O.; Alhashimi, R.; Thoota, S. The Role of Capsule Endoscopy in Crohn’s Disease: A Review. Cureus 2022, 14, e27242. [Google Scholar] [CrossRef]
- Leighton, J.A.; Helper, D.J.; Gralnek, I.M.; Dotan, I.; Fernandez-Urien, I.; Lahat, A.; Malik, P.; Mullin, G.E.; Rosa, B. Comparing Diagnostic Yield of a Novel Pan-Enteric Video Capsule Endoscope with Ileocolonoscopy in Patients with Active Crohn’s Disease: A Feasibility Study. Gastrointest. Endosc. 2017, 85, 196–205.e1. [Google Scholar] [CrossRef]
- Fry, L.; Carey, E.; Shiff, A.; Heigh, R.; Sharma, V.; Post, J.; Hentz, J.; Fleischer, D.; Leighton, J. The Yield of Capsule Endoscopy in Patients with Abdominal Pain or Diarrhea. Endoscopy 2006, 38, 498–502. [Google Scholar] [CrossRef]
- Petroniene, R.; Dubcenco, E.; Baker, J.P.; Ottaway, C.A.; Tang, S.-J.; Zanati, S.A.; Streutker, C.J.; Gardiner, G.W.; Warren, R.E.; Jeejeebhoy, K.N. Given® Capsule Endoscopy in Celiac Disease: Evaluation of Diagnostic Accuracy and Interobserver Agreement. Off. J. Am. Coll. Gastroenterol.|ACG 2005, 100, 685–694. [Google Scholar] [CrossRef]
- Green, P.H.R.; Rubin, M. Capsule Endoscopy in Celiac Disease. Gastrointest. Endosc. 2005, 62, 797–799. [Google Scholar] [CrossRef]
- Lewis, S.K.; Semrad, C.E. Capsule Endoscopy and Enteroscopy in Celiac Disease. Gastroenterol. Clin. North Am. 2019, 48, 73–84. [Google Scholar] [CrossRef]
- Luján-Sanchis, M.; Pérez-Cuadrado-Robles, E.; García-Lledó, J.; Fernández, J.F.J.; Elli, L.; Jiménez-García, V.A.; Egea-Valenzuela, J.; Valle-Muñoz, J.; Carretero-Ribón, C.; Fernández-Urién-Sainz, I.; et al. Role of Capsule Endoscopy in Suspected Celiac Disease: A European Multi-Centre Study. World J. Gastroenterol. 2017, 23, 703–711. [Google Scholar] [CrossRef]
- Cellier, P.H.R.; Collin, P.; Murray, J. ICCE Consensus for Celiac Disease. Endoscopy 2005, 37, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.; Vilas Boas, G.; Pinho, C.J.L. Wireless Capsule Endoscopy for Evaluation of Phenotypic Expression of Small-Bowel Polyps in Patients with Peutz-Jeghers Syndrome and in Symptomatic First-Degree Relatives. Endoscopy 2004, 36, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.P.; Gong, F.; Mills, T.N. Wireless Transmission of a Colour Television Moving Image from the Stomach Using a Miniature CCD Camera, Light Source and Microwave Transmitter. Gastrointest. Endosc. 1997, 45, AB40. [Google Scholar] [CrossRef]
- Appleyard, M.; Glukhovsky, A.; Swain, P. Wireless-Capsule Diagnostic Endoscopy for Recurrent Small-Bowel Bleeding. N. Engl. J. Med. 2001, 344, 232–233. [Google Scholar] [CrossRef]
- Kornbluth, A.; Legnani, P.; Lewis, B.S. Video Capsule Endoscopy in Inflammatory Bowel Disease: Past, Present, and Future. Inflamm. Bowel. Dis. 2004, 10, 278–285. [Google Scholar] [CrossRef]
- Cave, D.; Legnani, P.; De Franchis, R.; Lewis, B.S. ICCE Consensus for Capsule Retention. Endoscopy 2005, 37, 1065–1067. [Google Scholar] [CrossRef]
- Lewis, B.S.; Rey, J.F.; Seidman, E.G. Capsule Endoscopy 2005: Results of the 2005 International Consensus Conference–Introduction. Endoscopy 2005, 37, 1038–1039. [Google Scholar] [CrossRef]
- De Franchis, A.; Barkin, J.; Cave, D.; Filoche, B.R.A. ICCE Consensus for Bowel Preparation and Prokinetics. Endoscopy 2005, 37, 1040–1045. [Google Scholar] [CrossRef]
- Pennazio, G.; Goldfarb, N.M.E. ICCE Consensus for Obscure Gastrointestinal Bleeding. Endoscopy 2005, 37, 1046–1050. [Google Scholar] [CrossRef]
- Kornbluth, J.F.; Leighton, J.A.; Loftus, E.A.C. ICCE Consensus for Inflammatory Bowel Disease. Endoscopy 2005, 37, 1051–1054. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, P.; Faigel, D.V.K.E. ICCE Consensus for Esophageal Capsule Endoscopy. Endoscopy 2005, 37, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Gurudu, S.R.; Vargas, H.E.; Leighton, J.A. New Frontiers in Small-Bowel Imaging: The Expanding Technology of Capsule Endoscopy and Its Impact in Clinical Gastroenterology. Rev. Gastroenterol. Disord. 2008, 8, 1–14. [Google Scholar]
- PillCamTM SB 3 Capsule Endoscopy System|Medtronic. Available online: https://www.medtronic.com/covidien/en-us/products/capsule-endoscopy/pillcam-sb3-system.html (accessed on 1 September 2023).
- Galmiche, J.P.; Sacher-Huvelin, S.; Coron, E.; Cholet, F.; Soussan, E.B.; Sébille, V.; Filoche, B.; D’Abrigeon, G.; Antonietti, M.; Robaszkiewicz, M.; et al. Screening for Esophagitis and Barrett’s Esophagus with Wireless Esophageal Capsule Endoscopy: A Multicenter Prospective Trial in Patients with Reflux Symptoms. Am. J. Gastroenterol. 2008, 103, 538–545. [Google Scholar] [CrossRef]
- Eliakim, R.; Yassin, K.; Shlomi, I.; Suissa, A.; Eisen, G.M. A Novel Diagnostic Tool for Detecting Oesophageal Pathology: The PillCam Oesophageal Video Capsule. Aliment. Pharmacol. Ther. 2004, 20, 1083–1089. [Google Scholar] [CrossRef]
- Eliakim, R.; Sharma, V.K.; Yassin, K.; Adler, S.N.; Jacob, H.; Cave, D.R.; Sachdev, R.; Mitty, R.D.; Hartmann, D.; Schilling, D.; et al. A Prospective Study of the Diagnostic Accuracy of PillCam ESO Esophageal Capsule Endoscopy versus Conventional Upper Endoscopy in Patients with Chronic Gastroesophageal Reflux Diseases. J. Clin. Gastroenterol. 2005, 39, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Römmele, C.; Brueckner, J.; Messmann, H.; Gölder, S.K. Clinical Experience with the PillCam Patency Capsule Prior to Video Capsule Endoscopy: A Real-World Experience. Gastroenterol. Res. Pract. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Spada, C.; Spera, G.; Riccioni, M.; Biancone, L.; Petruzziello, L.; Tringali, A.; Familiari, P.; Marchese, M.; Onder, G.; Mutignani, M.; et al. A Novel Diagnostic Tool for Detecting Functional Patency of the Small Bowel: The given Patency Capsule. Endoscopy 2005, 37, 793–800. [Google Scholar] [CrossRef]
- Caunedo-Álvarez, Á.; Romero-Vazquez, J.; Herrerias-Gutierrez, J.M. Patency© and Agile© Capsules. World J. Gastroenterol. 2008, 14, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhang, B.L.; Chen, C.X.; Li, Y.M. OMOM Capsule Endoscopy in Diagnosis of Small Bowel Disease. J. Zhejiang Univ. Sci. B 2008, 9, 857–862. [Google Scholar] [CrossRef]
- Moglia, A.; Menciassi, A.; Schurr, M.O.; Dario, P. Wireless Capsule Endoscopy: From Diagnostic Devices to Multipurpose Robotic Systems. Biomed. Microdevices 2007, 9, 235–243. [Google Scholar] [CrossRef]
- Bang, S.; Park, J.Y.; Jeong, S.; Kim, Y.H.; Shim, H.B.; Kim, T.S.; Lee, D.H.; Song, S.Y. First Clinical Trial of the “MiRo” Capsule Endoscope by Using a Novel Transmission Technology: Electric-Field Propagation. Gastrointest. Endosc. 2009, 69, 253–259. [Google Scholar] [CrossRef]
- Capsule Endoscopy|ENDOCAPSULE 10 System|Olympus Medical Systems. Available online: https://www.olympus.co.uk/medical/en/Products-and-solutions/Products/Product/ENDOCAPSULE-10-System.html (accessed on 1 September 2023).
- Hartmann, D.; Eickhoff, A.; Damian, U.; Riemann, J.F. Diagnosis of Small-Bowel Pathology Using Paired Capsule Endoscopy with Two Different Devices: A Randomized Study. Endoscopy 2007, 39, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Mussetto, A.; Fuccio, L.; Dari, S.; Gasperoni, S.; Cantoni, F.; Brancaccio, M.L.; Triossi, O.; Casetti, T. MiroCam Capsule for Obscure Gastrointestinal Bleeding: A Prospective, Single Centre Experience. Dig. Liver Dis. 2013, 45, 124–128. [Google Scholar] [CrossRef]
- Esaki, M.; Matsumoto, T. Capsule Endoscopy. In Endoscopy in the Diagnosis of Small Intestine Diseases; Matsui, T., Matsumoto, T., Aoyagi, K., Eds.; Springer: Tokyo, Japan, 2014; pp. 19–23. ISBN 978-4-431-54352-7. [Google Scholar]
- Uchiyama, A.; Takizawa, H.; Yokoi, T.; Mizuno, H. Encapsulated Endoscope System in Which Endoscope Moves in Lumen by Itself and Rotation of Image of Region to Be Observed Is Ceased. U.S. Patent 2003 0 229 268 A1, 11 December 2003. [Google Scholar]
- Hu, C.; Gao, M.; Chen, Z.; Zhang, H.; Liu, S. Magnetic Analysis and Simulations of a Self-Propelled Capsule Endoscope. In Proceedings of the 2010 11th International Conference on Thermal, Mechanical and Multi-Physics Simulation, and Experiments in Microelectronics and Microsystems, EuroSimE 2010, Linz, Austria, 26–28 April 2010. [Google Scholar]
- Matsui, T.; Murata, S.; Honda, T. Fabrication of Magnetically Driven Biopsy Mechanism Applicable to Capsule-Type Medical Device. J. Robot. Mechatron. 2018, 30, 292–299. [Google Scholar] [CrossRef]
- Carpi, F.; Galbiati, S.; Carpi, A. Magnetic shells for gastrointestinal endoscopic capsules as a means to control their motion. Biomed Pharmacother. 2006, 60, 370–374. [Google Scholar] [CrossRef]
- Casanovas, O.A. Enabling Active Locomotion and Advanced Features in Capsule Endoscopy; LAP LAMBERT Academic Publishing: London, UK, 2012. [Google Scholar]
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. Aust. New Zealand J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem Photobiol 2007, 74, 656–669. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Dhaneshwar, S.; Patil, K.; Bulbule, M.; Kinjawadekar, V.; Joshi, D.; Joshi, V. Photodynamic Therapy for Cancer. Int. J. Pharm. Sci Rev. Res. 2014, 27, 125–141. [Google Scholar]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of Singlet Oxygen as the Cytotoxic Agent in Photo-Inactivation of a Murine Tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef]
- McCaughan, J.S.; Hicks, W.; Laufman, L.; May, E.; Roach, R. Palliation of Esophageal Malignancy with Photoradiation Therapy. Cancer 1984, 54, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, V.G.; Bologna, S.; Batra, S.K. Head and Neck and Plastic Surgery A Targeted Problem and Its Solution. Laryngoscope 1993, 103, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Qumseya, B.J.; David, W.; Wolfsen, H.C. Photodynamic Therapy for Barrett’s Esophagus and Esophageal Carcinoma. Clin. Endosc. 2013, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, K.; Dixon, K.; Thorpe, J.A.C.; Stringer, M.; Moore, P.J. The Role of Photodynamic Therapy (PDT) in Inoperable Oesophageal Cancer. Eur. J. Cardio-Thorac. Surg. 2000, 17, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Sibille, A.; Lambert, R.; Souquet, J.C.; Sabben, G.; Descos, F. Long-Term Survival after Photodynamic Therapy for Esophageal Cancer. Gastroenterology 1995, 108, 337–344. [Google Scholar] [CrossRef]
- Grosjean, P.; Savary, J.F.; Mizeret, J.; Wagnieres, G.; Woodtli, A.; Theumann, J.F.; Fontolliet, C.; Van Den Bergh, H.; Monnier, P. Photodynamic Therapy for Cancer of the Upper Aerodigestive Tract Using Tetra(m-Hydroxyphenyl)Chlorin. J. Clin. Laser Med. Surg. 1996, 14, 281–287. [Google Scholar] [CrossRef]
- Brown, L.M.; Devesa, S.S. Epidemiologic Trends in Esophageal and Gastric Cancer in the United States. Surg. Oncol. Clin. N Am. 2002, 11, 235–256. [Google Scholar] [CrossRef]
- Overholt, B.F.; Panjehpour, M. Photodynamic Therapy in Barrett’s Esophagus. J. Clin. Laser Med. Surg. 1996, 14, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Overholt, B.F.; Panjehpour, M.; Haydek, J.M. Photodynamic Therapy for Barrett’s Esophagus: Follow-up in 100 Patients. Gastrointest. Endosc. 1999, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.G.; Gomes, J.M.; Wolffenbuttel, R.F.; Correia, J.H. Optical Microsystem Design and Fabrication for Medical Image Magnification. Microsyst. Technol. 2016, 22, 1747–1755. [Google Scholar] [CrossRef]
- Gounella, R.H.; Leite, I.S.; Inada, N.M.; Do Carmo, J.P.P. Wireless Portable Evaluation Platform for Photodynamic Therapy: In Vitro Assays on Human Gastric Adenocarcinoma Cells. IEEE Sens. J. 2020, 20, 13950–13958. [Google Scholar] [CrossRef]
- Teubner, D.; Kiesslich, R.; Matsumoto, T.; Rey, J.W.; Hoffman, A. Beyond Standard Image-Enhanced Endoscopy Confocal Endomicroscopy. Gastrointest. Endosc. Clin. N Am. 2014, 24, 427–434. [Google Scholar] [CrossRef]
- Clark, C.; Turner, J. Diagnostic Modalities for Inflammatory Bowel Disease. Serologic Markers and Endoscopy. Surg. Clin. North Am. 2015, 95, 1123–1141. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Costa, A.C.; Gomes, J.M.; Costa, C.G.; Goncalves, S.B.; Wolffenbuttel, R.F.; Correia, J.H. PDMS Microlenses for Optical Biopsy Microsystems. IEEE Trans. Ind. Electron. 2017, 64, 9683–9690. [Google Scholar] [CrossRef]
- Tabatabaei, N.; Kang, D.; Wu, T.; Kim, M.; Carruth, R.W.; Leung, J.; Sauk, J.S.; Shreffler, W.; Yuan, Q.; Katz, A.; et al. Tethered Confocal Endomicroscopy Capsule for Diagnosis and Monitoring of Eosinophilic Esophagitis. Biomed. Opt. Express 2014, 5, 197–207. [Google Scholar] [CrossRef]
- Hou, R.; Le, T.; Murgu, S.D.; Chen, Z.; Brenner, M. Recent Advances in Optical Coherence Tomography for the Diagnoses of Lung Disorders. Expert Rev. Respir. Med. 2011, 5, 711–724. [Google Scholar] [CrossRef]
- Bouma, B.E.; Tearney, G.J. Handbook of Optical Coherence Tomography; Marcel Dekker: New York, NU, USA, 2002; ISBN 0824705580. [Google Scholar]
- Pawley, J.B.; Masters, B.R. Handbook of Biological Confocal Microscopy, Third Edition. J. Biomed. Opt. 2008, 13, 029902. [Google Scholar] [CrossRef]
- Gora, M.J.; Quénéhervé, L.; Carruth, R.W.; Lu, W.; Rosenberg, M.; Sauk, J.S.; Fasano, A.; Lauwers, G.Y.; Nishioka, N.S.; Tearney, G.J. Tethered Capsule Endomicroscopy for Microscopic Imaging of the Esophagus, Stomach, and Duodenum without Sedation in Humans (with Video). Gastrointest. Endosc. 2018, 88, 830–840.e3. [Google Scholar] [CrossRef]
- Maciel, M.J.; Costa, C.G.; Silva, M.F.; Peixoto, A.C.; Wolffenbuttel, R.F.; Correia, J.H. A Wafer-Level Miniaturized Michelson Interferometer on Glass Substrate for Optical Coherence Tomography Applications. Sens. Actuators A Phys. 2016, 242, 210–216. [Google Scholar] [CrossRef]
- Maciel, M.J.; Rosa, C.C.; Wolffenbuttel, R.F.; Correia, J.H. Optical Coherence Tomography within a Single Microsystem. J. Phys. D Appl. Phys. 2018, 51, 365401. [Google Scholar] [CrossRef]
- Pimenta, S.; Castanheira, E.M.S.; Minas, G. Optical Microsystem for Analysis of Diffuse Reflectance and Fluorescence Signals Applied to Early Gastrointestinal Cancer Detection. Sensors 2015, 15, 3138–3153. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Lau, C.; O’Donoghue, G.; Mirkovic, J.; McGee, S.; Galindo, L.; Elackattu, A.; Stier, E.; Grillone, G.; Badizadegan, K.; et al. Quantitative Spectroscopic Imaging for Non-Invasive Early Cancer Detection. Opt. Express 2008, 16, 16227–16239. [Google Scholar] [CrossRef]
- Georgakoudi, I. The Color of Cancer. J. Lumin. 2006, 119–120, 75–83. [Google Scholar] [CrossRef]
- Brown, J.Q.; Vishwanath, K.; Palmer, G.M.; Ramanujam, N. Advances in Quantitative UV-Visible Spectroscopy for Clinical and Pre-Clinical Application in Cancer. Curr. Opin. Biotechnol. 2009, 20, 119–131. [Google Scholar] [CrossRef]
- Pimenta, S.; Carmo, J.P.; Correia, R.G.; Minas, G.; Castanheira, E.M.S. Characterization of Silicon Photodiodes for Diffuse Reflectance Signal Extraction. In Proceedings of the Proceedings-2015 IEEE 4th Portuguese Meeting on Bioengineering, ENBENG 2015, Porto, Portugal, 26–28 February 2015. [Google Scholar]
- Ell, C. Improving Endoscopic Resolution and Sampling: Fluorescence Techniques. Gut 2003, 52, iv30. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Jacobson, B.C.; Van Dam, J.; Backman, V.; Wallace, M.B.; Müller, M.G.; Zhang, Q.; Badizadegan, K.; Sun, D.; Thomas, G.A.; et al. Fluorescence, Reflectance, and Light-Scattering Spectroscopy for Evaluating Dysplasia in Patients with Barrett’s Esophagus. Gastroenterology 2001, 120, 1620–1629. [Google Scholar] [CrossRef]
- Mayinger, B.; Jordan, M.; Horner, P.; Gerlach, C.; Muehldorfer, S.; Bittorf, B.R.; Matzel, K.E.; Hohenberger, W.; Hahn, E.G.; Guenther, K. Endoscopic Light-Induced Autofluorescence Spectroscopy for the Diagnosis of Colorectal Cancer and Adenoma. J. Photochem. Photobiol. B 2003, 70, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.R.; Chen, G.N.; Wu, S.S.; Chen, R. Distinguishing Human Normal or Cancerous Esophagus Tissue Ex Vivo Using Multiphoton Microscopy. J. Opt. (UK) 2014, 16, 025301. [Google Scholar] [CrossRef]
- Lo, J.Y.; Yu, B.; Fu, H.L.; Bender, J.E.; Palmer, G.M.; Kuech, T.F.; Ramanujam, N. A Strategy for Quantitative Spectral Imaging of Tissue Absorption and Scattering Using Light Emitting Diodes and Photodiodes. Opt Express 2009, 17, 1372–1384. [Google Scholar] [CrossRef]
- Yu, B.; Lo, J.Y.; Kuech, T.F.; Palmer, G.M.; Bender, J.E.; Ramanujam, N. Cost-Effective Diffuse Reflectance Spectroscopy Device for Quantifying Tissue Absorption and Scattering in Vivo. J. Biomed. Opt. 2008, 13, 060505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Luo, J.C.; Zeng, F.; Wang, Q.Z.; Alfano, S.A.; Katz, A.; Zevallos, M.; Alfano, R.R. Wireless Spectroscopic Compact Photonic Explorer for Diagnostic Optical Imaging. Biomed Microdevices 2005, 7, 111–115. [Google Scholar] [CrossRef]
- Alfano, R.R.; Alfano, S.; Wang, Q.; Ho, P.P. Remote-Controllable, Micro-Scale Device for Use in in Vivo Medical Diagnosis and/or Treatment. U.S. Patent No. 6,240,312, 29 May 2001. [Google Scholar]
- Alfano, R.; Katz, A.; Alfano, S. Micro-Scale Compact Device for in Vivo Medical Diagnosis Combining Optical Imaging and Point Fluorescence Spectroscopy. U.S. Patent No. 8,082,024, 20 December 2011. [Google Scholar]
- Gono, K.; Obi, T.; Yamaguchi, M.; Oyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; Hamamoto, Y.; Endo, T. Appearance of Enhanced Tissue Features in Narrow-Band Endoscopic Imaging. J. Biomed. Opt. 2004, 9, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Gono, K. An Introduction to High-Resolution Endoscopy and Narrowband Imaging. In Comprehensive Atlas of High-Resolution Endoscopy and Narrowband Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 7–15. ISBN 9781118705940. [Google Scholar]
- Van den Broek, F.J.C.; Fockens, P.; Dekker, E. Review Article: New Developments in Colonic Imaging. Aliment. Pharmacol. Ther. 2007, 26, 91–99. [Google Scholar] [CrossRef]
- Machida, Y.; Hamamoto, Y.; Muto, M.; Kozu, T.; Tajiri, H.; Yoshida, S. Narrow-Band Imaging in the Diagnosis of Colorectal Mucosal Lesions: A Pilot Study. Endoscopy 2004, 36, 1094–1098. [Google Scholar] [CrossRef]
- Muto, M.; Horimatsu, T.; Ezoe, Y.; Morita, S.; Miyamoto, S. Improving Visualization Techniques by Narrow Band Imaging and Magnification Endoscopy. J. Gastroenterol. Hepatol. 2009, 24, 1333–1346. [Google Scholar] [CrossRef]
- Kuznetsov, R.; Rey, J.-F. Narrow-Band Imaging: Potential and Limitations. Endoscopy 2006, 38, 76–81. [Google Scholar] [CrossRef]
- Gono, K.; Yamazaki, K.; Doguchi, N.; Nonami, T.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; et al. Endoscopic Observation of Tissue by Narrowband Illumination. Opt. Rev. 2003, 10, 211–215. [Google Scholar] [CrossRef]
- Wong Kee Song, L.M.; Adler, D.G.; Conway, J.D.; Diehl, D.L.; Farraye, F.A.; Kantsevoy, S.V.; Kwon, R.; Mamula, P.; Rodriguez, B.; Shah, R.J.; et al. Narrow Band Imaging and Multiband Imaging. Gastrointest. Endosc. 2008, 67, 581–589. [Google Scholar] [CrossRef]
- Yao, M.; Fujisaki, J. Techniques Using the Hemoglobin Index of the Gastric Mucosa. Endoscopy 2005, 37, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nakagawa, S.; Shinmizu, Y.; Sugiyama, T.; Asaka, M. The efficacy of magnifying endoscopy with adaptive index of hemoglobin enhancement for diagnosis of helicobacter pylori-induced gastritis. Dig. Endosc. 2002, 14, S72–S75. [Google Scholar] [CrossRef]
- Toyota, Y.; Honda, H.; Omoya, T.; Inayama, K.; Suzuki, M.; Kubo, K.; Nakasono, M.; Muguruma, N.; Okamura, S.; Shimizu, I.; et al. Usefulness of a Hemoglobin Index Determined by Electronic Endoscopy in the Diagnosis of Helicobacter Pylori Gastritis. Dig. Endosc. 2002, 14, 156–162. [Google Scholar] [CrossRef]
- Macleod, H.A. Thin-Film Optical Filters, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781351982245. [Google Scholar]
- Correia, J.H.G. Optical Microsystems in Silicon Based on a Fabry-Perot Resonance Cavity: Application for Spectral Analysis of Visible Light; Delft University Press: Delft, The Netherlands, 1999. [Google Scholar]
- Gounella, R.H.; Granado, T.C.; da Costa, J.P.C.; Carmo, J.P. Optical Filters for Narrow Band Light Adaptation on Imaging Devices. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 1–8. [Google Scholar] [CrossRef]
- Ragunath, K.; Chiu, P. A primer to image enhanced endoscopy. Transl. Gastroenterol. Hepatol. 2022, 7, 1. [Google Scholar] [CrossRef]
- Akarsu, C.; Sahbaz, N.A.; Dural, A.C.; Unsal, M.G.; Kones, O.; Kocatas, A.; Halicioglu, I.; Alis, H. FICE vs Narrow Band Imaging for In Vivo Histologic Diagnosis of Polyps. JSLS 2016. [Google Scholar] [CrossRef]
- Subramaniam, S.; Kandiah, K.; Schoon, E.; Aepli, P.; Hayee, B.; Pischel, A.; Stefanovic, M.; Alkandari, A.; Coron, E.; Omae, M.; et al. Development and validation of the international Blue Light Imaging for Barrett’s Neoplasia Classification. Gastrointest. Endosc. 2020, 91, 310–320. [Google Scholar] [CrossRef]
- Osawa, H.; Yamamoto, H.; Miura, Y.; Sasao, W.; Ino, Y.; Satoh, H.; Satoh, K.; Sugano, K. Blue Laser Imaging Provides Excellent Endoscopic Images of Upper Gastrointestinal Lesions. Video, J. Encycl. GI Endosc. 2014, 1, 607–610. [Google Scholar] [CrossRef]
- Lee, C.K.; Lee, S.H.; Hwangbo, Y. Narrow-band imaging versus I-SCAN for the real-time histological prediction of diminutive colonic polyps: A prospective comparative study by using the simple unified endoscopic classification. Gastrointest. Endosc. 2011, 74, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Sparano, L. Image-Enhanced Endoscopy with I-SCAN Technology for the Evaluation of Duodenal Villous Patterns. Dig. Dis. Sci. 2013, 58, 1287–1293. [Google Scholar] [CrossRef] [PubMed]











| Capsule | Company | Indication | Imaging System | Size (Diam. × Length) | References |
|---|---|---|---|---|---|
| PillCam SB | Given Imaging Inc. | For pediatric patients; diagnosis of small bowel pathologies | 1 video camera; 2 fps | 11.4 mm × 26.4 mm | [65,91] |
| PillCam SB2 | Given Imaging Inc. | For diagnosis of small bowel pathologies | 1 video camera with fixed high frame rate; 2 or 4 fps | 11.4 mm × 26.3 mm | [92] |
| PillCam SB3 | Given Imaging Inc. | For diagnosis of small bowel pathologies | 1 video camera; enhanced imaging capabilities and AFR; 2 fps or 2–6 fps | 11.4 mm × 26.2 mm | [92] |
| PillCam ESO 2 | Given Imaging Inc. | For investigation of esophageal disorders | 2 video cameras; 19 fps | 11.4 mm × 26.4 mm | [92,93,94,95] |
| PillCam ESO 3 | Given Imaging Inc. | For investigation of esophageal disorders | 2 video cameras; 35 fps | 11.6 mm × 31.5 mm | [92,93,94,95] |
| PillCam COLON | Given Imaging Inc. | For investigation of intestinal disorders | 2 video cameras and AFR; 4 fps | 11.4 mm × 31 mm | [92] |
| PillCam COLON 2 | Given Imaging Inc. | For investigation of intestinal disorders | 2 video cameras and AFR; 4–35 fps | 11.6 mm × 31.5 mm | [92] |
| Patency System | Given Imaging Inc. | For detecting obstructions or strictures in the GI tract | None (radiofrequency identification (RFID) detected by an RFID scanner) | 11.4 mm × 26.4 mm | [97] |
| OMOM | Jinshan Science and Technology Company | For investigation of OGIB, abdominal pain or diarrhea, inflammatory bowel disease, and tumors | 1 video camera; 2 fps | 13 mm × 27.9 mm | [99] |
| EndoCapsule | Olympus Medical Systems | Detection of GI bleeding | 1 video camera and high-resolution image chip | 11 mm × 26 mm | [28,100,101,102] |
| MiRoCam | Intromedic | For investigation of stomach, small bowel, and colon | 1 video camera; 2 fps | 10.8 mm × 24 mm | [102,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gounella, R.; Granado, T.C.; Hideo Ando Junior, O.; Luporini, D.L.; Gazziro, M.; Carmo, J.P. Endoscope Capsules: The Present Situation and Future Outlooks. Bioengineering 2023, 10, 1347. https://doi.org/10.3390/bioengineering10121347
Gounella R, Granado TC, Hideo Ando Junior O, Luporini DL, Gazziro M, Carmo JP. Endoscope Capsules: The Present Situation and Future Outlooks. Bioengineering. 2023; 10(12):1347. https://doi.org/10.3390/bioengineering10121347
Chicago/Turabian StyleGounella, Rodrigo, Talita Conte Granado, Oswaldo Hideo Ando Junior, Daniel Luís Luporini, Mario Gazziro, and João Paulo Carmo. 2023. "Endoscope Capsules: The Present Situation and Future Outlooks" Bioengineering 10, no. 12: 1347. https://doi.org/10.3390/bioengineering10121347








