An Alternating Magnetic Field-Controlled Drug Delivery System Based on 4,4′-Azobis (4-cyanovaleric Acid)-Functioned Fe3O4@Chitosan Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
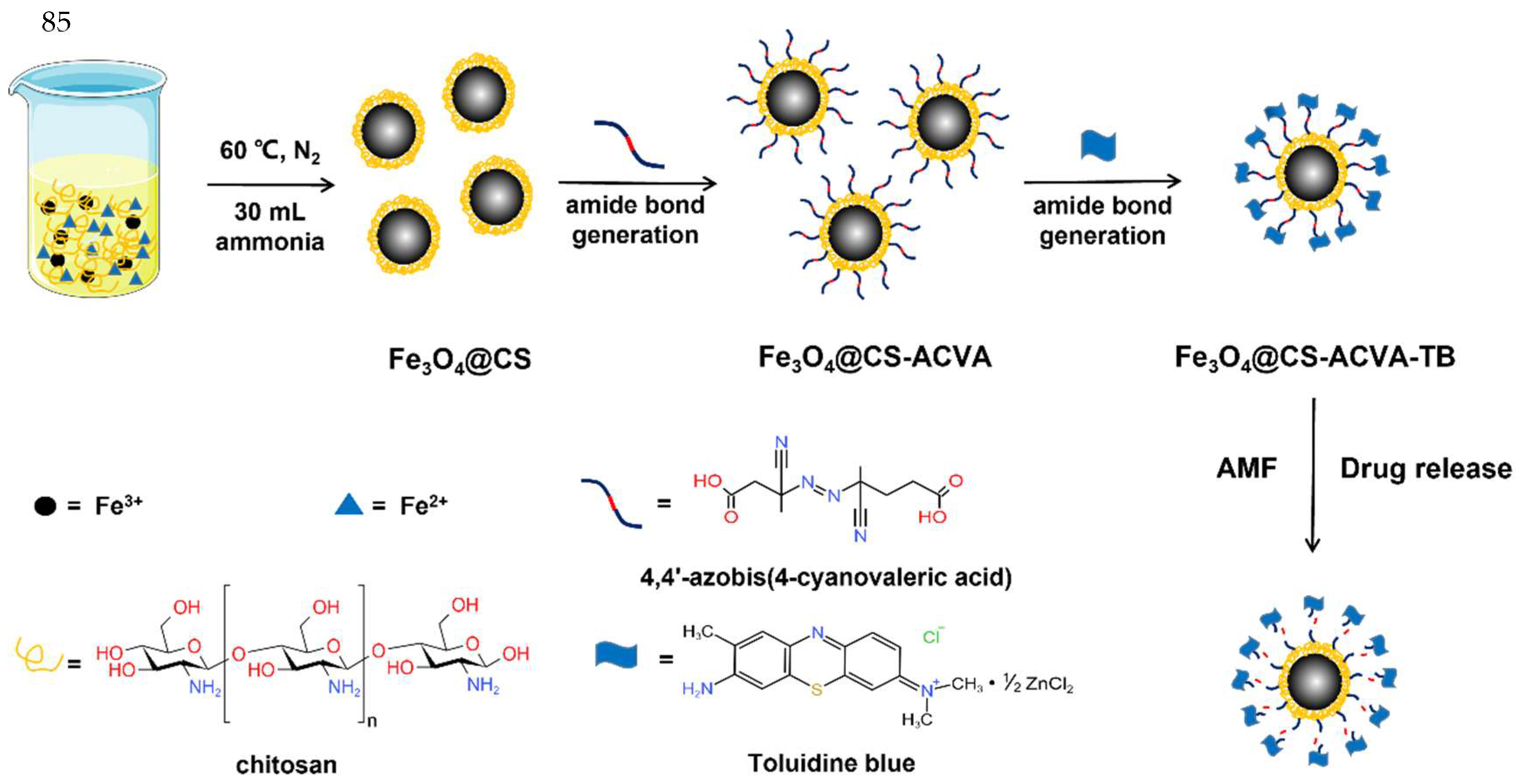
2.2. Synthesis of Fe3O4@CS Magnetic Nanoparticle
2.3. Synthesis of Fe3O4@CS-ACVA-TB Magnetic Nanoparticles
2.4. Characterization of the Magnetic Nanoparticles
2.5. Magneto-thermal Properties Analysis of Fe3O4@CS Nanoparticles
2.6. Hydrothermal Release of Fe3O4@CS-ACVA-TB Magnetic Nanoparticles
2.7. A Single Magnetic Heat Control Release of Fe3O4@CS-ACVA-TB Magnetic Nanoparticles
2.8. Multiple Magnetic Heat Control Releases of Fe3O4@CS-ACVA-TB Magnetic Nanoparticles
3. Results and Discussion
3.1. Synthesis and Characterization of Magnetic Fe3O4@CS Nanoparticles
3.2. The Magneto-Thermal Properties of Fe3O4@CS Magnetic Nanoparticles
3.3. In Vitro Release Study
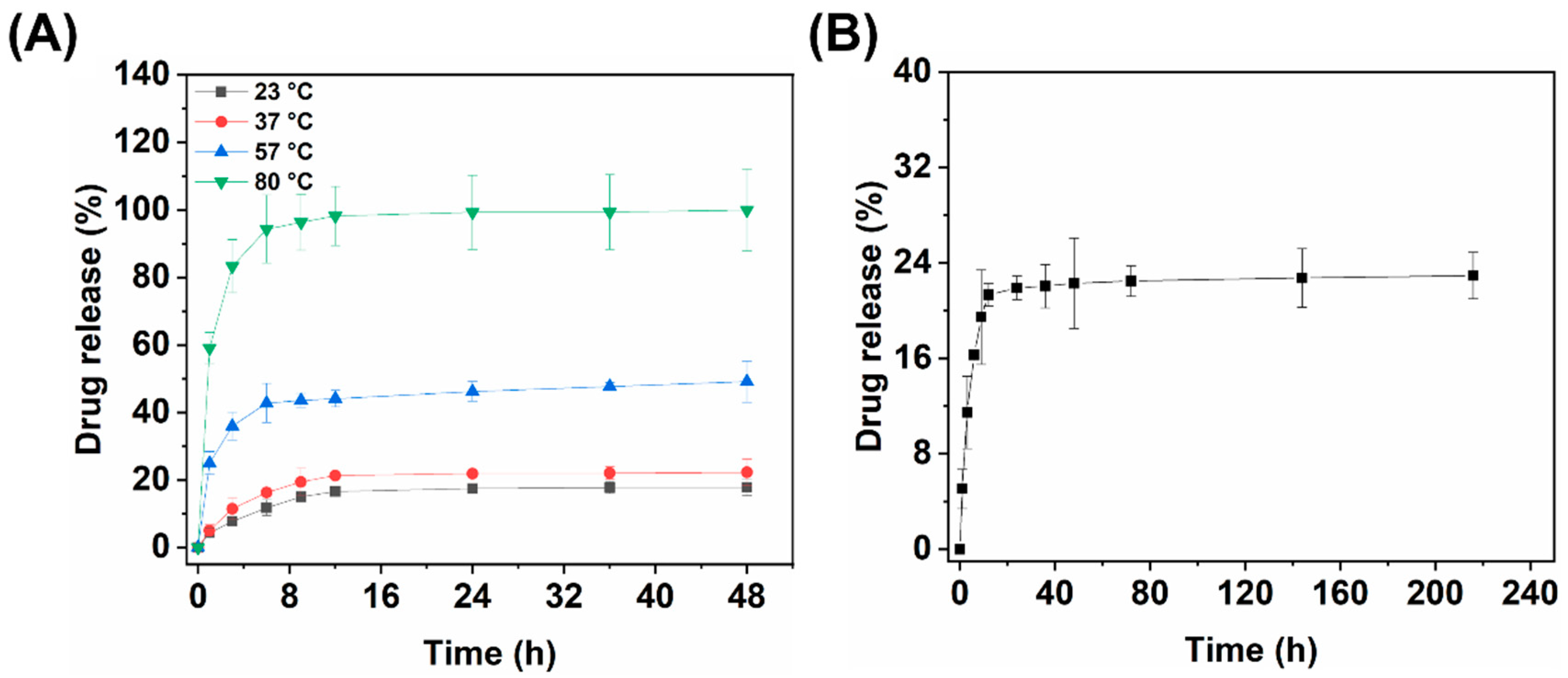
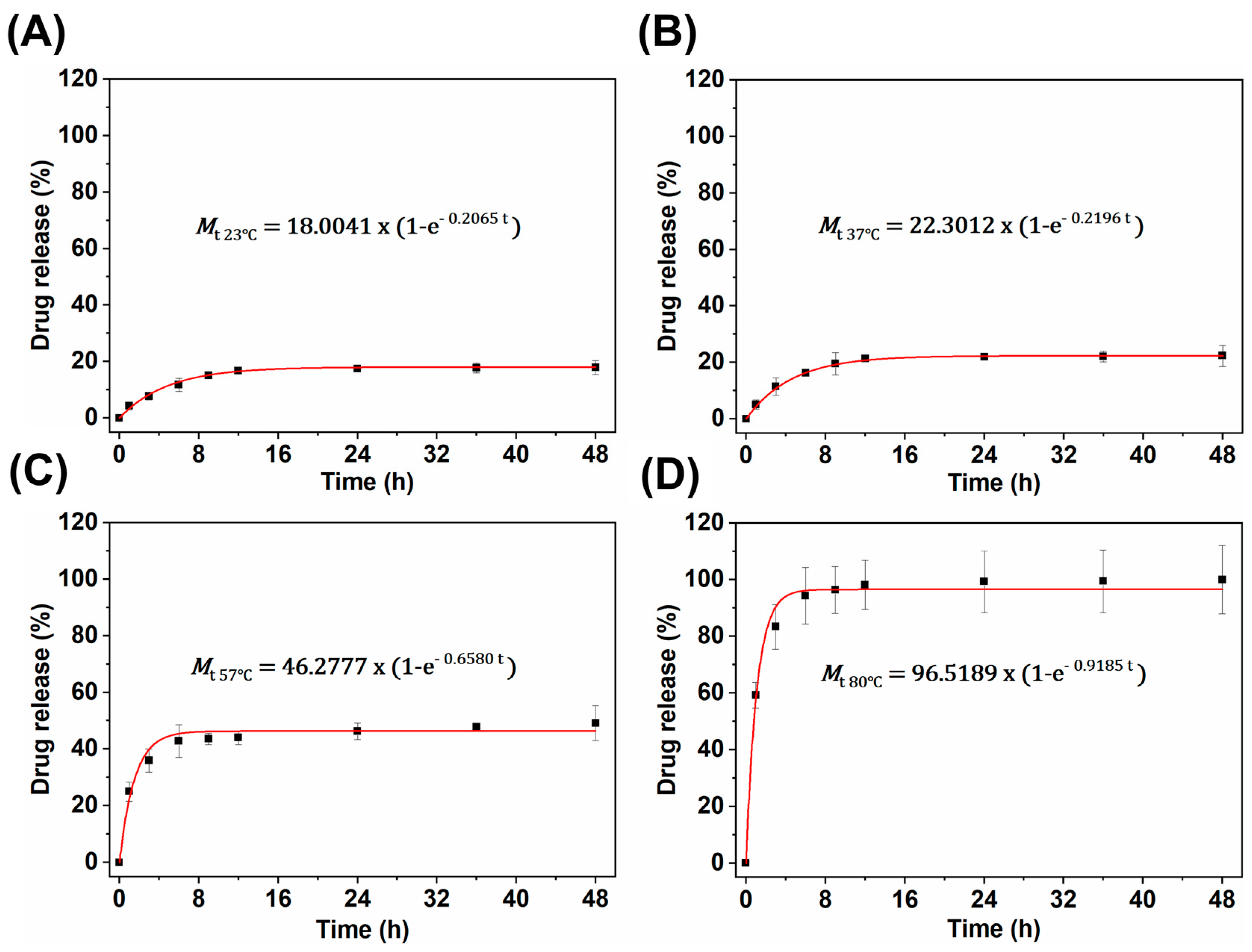
3.3.1. Hydrothermal Release of Fe3O4@CS-ACVA-TB Magnetic Nanoparticles
3.3.2. Kinetic-Analytic Studies of Drug Release
- (i)
- Zero-order:
- (ii)
- First-order:
- (iii)
- Higuchi:
- (iv)
- Korsemeyer–Peppas:where Mt is the total amount of TB released at time t, M∞ is the total amount of drug released as time goes to infinity (i.e., complete release), k is a release constant, and n is the release exponent that is used to characterize different release mechanisms. For spherical particles, the value of n indicates Fickian diffusion if n < 0.5, nonFickian or anomalous phenomena if 0.5 < n < 1, and n > 1 implies a lack of time dependence on release kinetics (i.e., zero-order kinetics) [33].
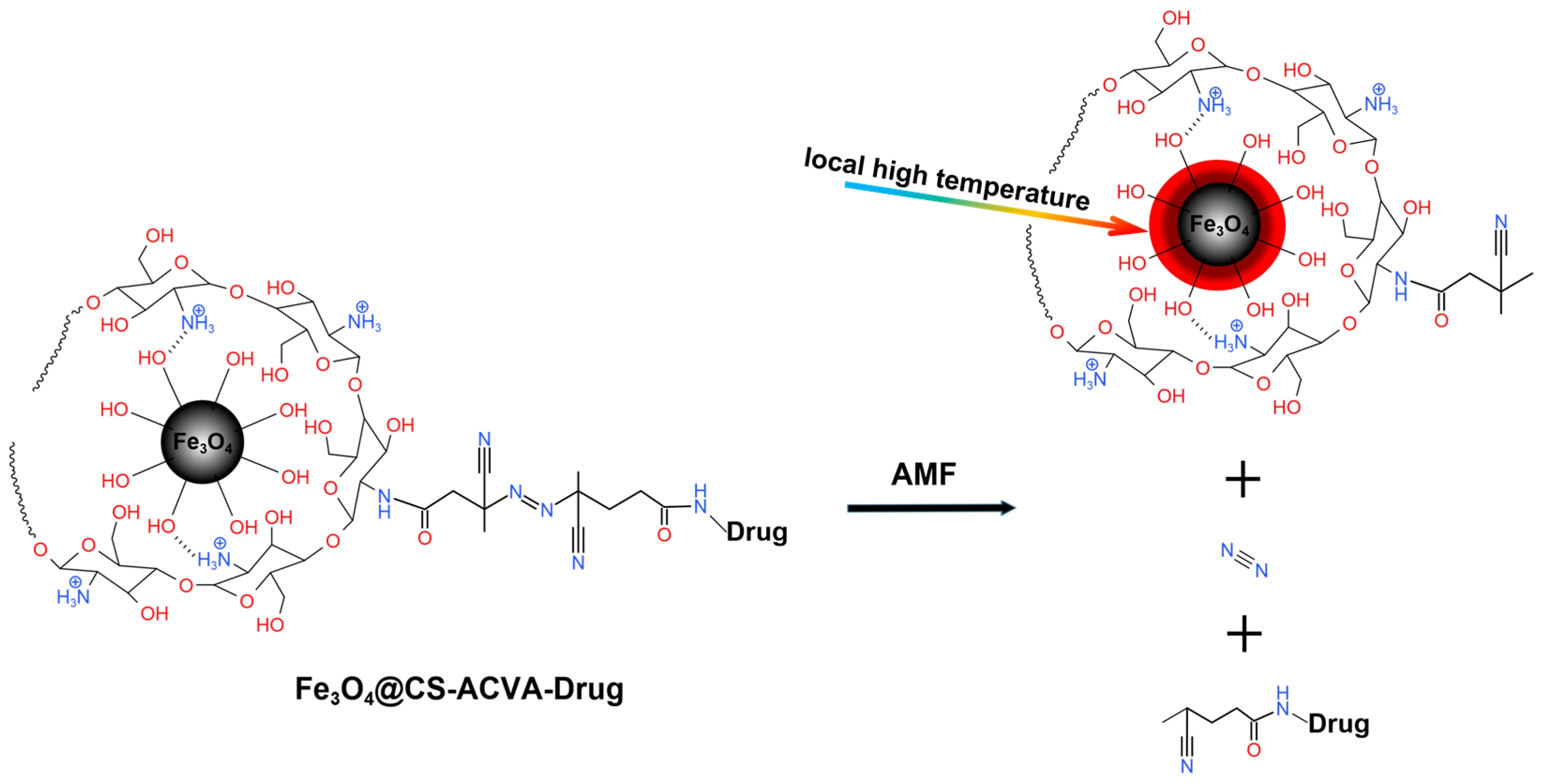
3.4. Magneto-thermal Controlled Release of Fe3O4@CS-ACVA-TB Nanoparticles
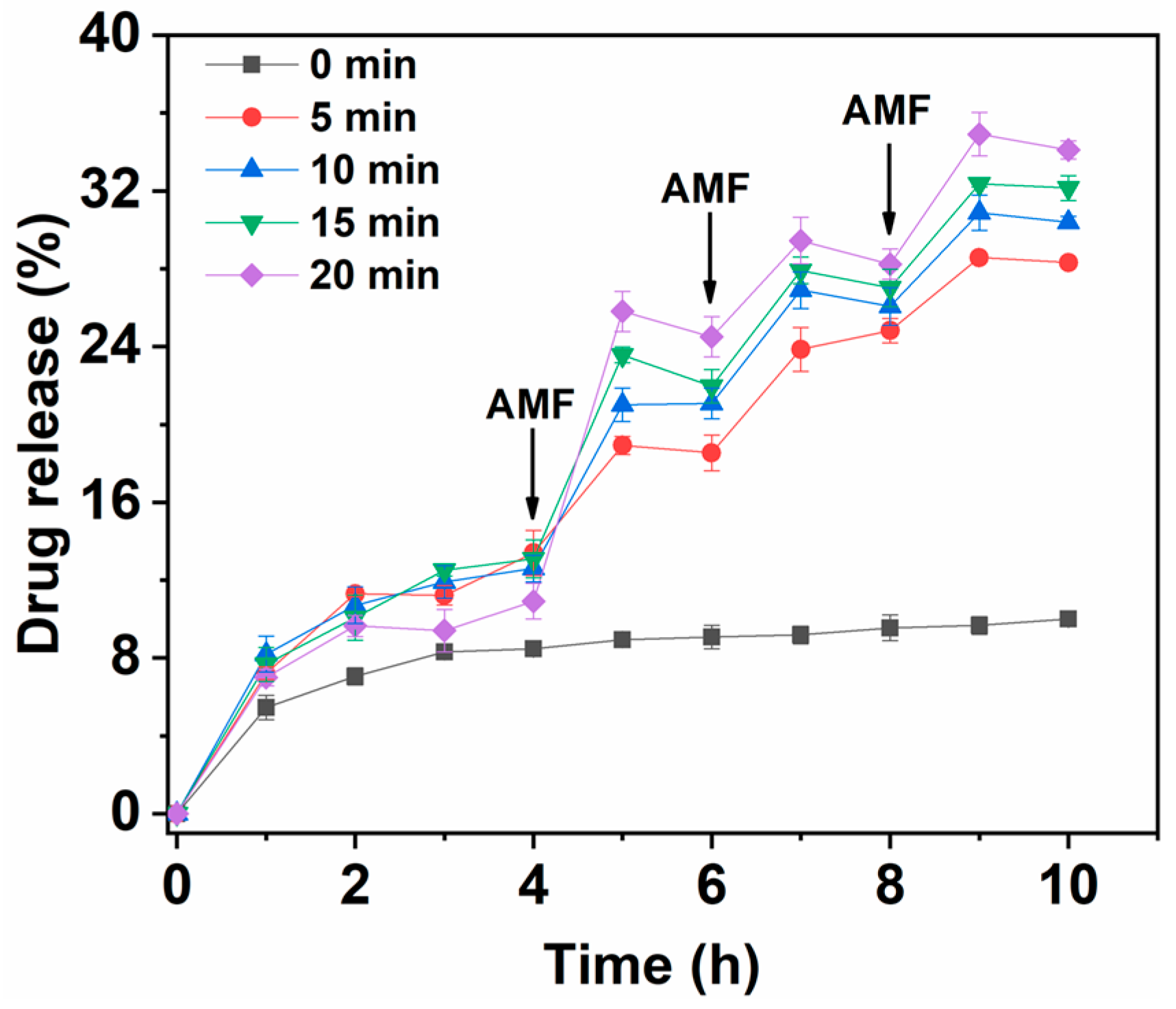
3.4.1. Single Magneto-thermal Controlled Release of Fe3O4@CS-ACVA-TB Nanoparticles
3.4.2. Multiple Magneto-thermal Controlled Release of Fe3O4@CS-ACVA-TB Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Li, S.; Guo, H.; You, W.; Tu, D.; Li, J.; Lu, C.; Yang, H. Chen, Enhancing Antitumor Efficacy by Simultaneous ATP-Responsive Chemodrug Release and Cancer Cell Sensitization Based on a Smart Nanoagent. Adv. Sci. 2018, 5, 1801201. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, Z.; Zhou, Z.; Ma, Y.; Kiesewetter, D.; Wang, Z.; Fan, W.; Zhu, S.; Zhang, M.; Tian, R.; et al. A Logic-Gated Modular Nanovesicle Enables Programmable Drug Release for On-Demand Chemotherapy. Theranostics 2019, 9, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, M.; Yan, W.; Liu, X.; Jiang, B.; Qian, Z.; Gao, Y.; Lu, X.; Chen, X.; Wang, Q. Delivery of a chemotherapeutic drug using novel hollow carbon spheres for esophageal cancer treatment. Int. J. Nanomed. 2017, 12, 6759–6769. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Awad, N.; Paul, V.; Kawak, P.; Al-Sayah, M.; Husseini, G. Transferrin-modified liposomes triggered with ultrasound to treat HeLa cells. Sci. Rep. 2021, 11, 11589. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Cao, X.; Ibnat, M.; Chen, G. Stimuli-responsive nanoformulations for CRISPR-Cas9 genome editing. J. Nanobiotechnol. 2022, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.; Pescador, J.; Pollok, N.; Beall, G.; Maeder, C.; Lewis, L. Impact of size; secondary structure, and counterions on the binding of small ribonucleic acids to layered double hydroxide nanoparticles. Biointerphases 2015, 10, 41007. [Google Scholar] [CrossRef]
- Usov, N.; Nesmeyanov, M.; Tarasov, V. Magnetic Vortices as Efficient Nano Heaters in Magnetic Nanoparticle Hyperthermia. Sci Rep. 2018, 8, 1224. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.; Nanda, K. Green synthesis of polysaccharide/gold nanoparticle nanocomposite: An efficient ammonia sensor. Carbohydr. Polym. 2013, 94, 229–234. [Google Scholar] [CrossRef]
- Saikia, C.; Gogoi, P. Chitosan: A Promising Biopolymer in Drug Delivery Applications. J. Mol. Genet. Med. 2015, 4, 899–910. [Google Scholar] [CrossRef]
- Tang, C.; Chen, N.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, X. Preparation and properties of chitosan nanocomposites with nanofillers of different dimensions. Polym. Degrad. Stab. 2009, 94, 124–131. [Google Scholar] [CrossRef]
- Ruta, S.; Chantrell, R.; Hovorka, O. Unified model of hyperthermia via hysteresis heating in systems of interacting magnetic nanoparticles. Sci. Rep. 2015, 5, 9090. [Google Scholar] [CrossRef] [PubMed]
- Suriyanto; Ng, E.; Kumar, S. Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Neelian and Brownian relaxation: A review. Biomed. Eng. Online 2017, 16, 36. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.; Birca, A.; Holban, A.; Urzica, I.; Avramescu, S.; Galateanu, B.; Hudita, A. Nanostructured Thin Coatings Containing Anthriscus sylvestris Extract with Dual Bioactivity. Molecules 2020, 25, 3866. [Google Scholar] [CrossRef]
- Chen, J.-P.; Yang, P.-C.; Ma, Y.-H.; Wu, T. Characterization of chitosan magnetic nanoparticles for in situ delivery of tissue plasminogen activator. Carbohydr. Polym. 2011, 84, 364–372. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef]
- Heydarifard, S.; Gao, W.; Fatehi, P. Impact of Counter Ions of Cationic Monomers on the Production and Characteristics of Chitosan-Based Hydrogel. ACS Omega 2019, 4, 15087–15096. [Google Scholar] [CrossRef]
- Pawde, D.; Viswanadh, M.; Mehata, A.; Sonkar, R.; Narendra; Poddar, S.; Burande, A.; Jha, A.; Vajanthri, K.; Mahto, S.; et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm. J. 2020, 28, 1616–1625. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Chen, Y.; Mi, Y.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis; Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts. Polymers 2019, 11, 1810. [Google Scholar] [CrossRef]
- Sridharan, G.; Shankar, A. Toluidine blue: A review of its chemistry and clinical utility. J. Oral Maxillofac. Pathol. 2012, 16, 251–255. [Google Scholar] [CrossRef]
- Kariminia, S.; Shamsipur, A.; Shamsipur, M. Analytical characteristics and application of novel chitosan coated magnetic nanoparticles as an efficient drug delivery system for ciprofloxacin. Enhanced drug release kinetics by low-frequency ultrasounds. J. Pharm. Biomed. Anal. 2016, 129, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Straczek, T.; Fiejdasz, S.; Rybicki, D.; Goc, K.; Przewoznik, J.; Mazur, W.; Nowakowska, M.; Zapotoczny, S.; Rumian, S.; Kapusta, C. Dynamics of Superparamagnetic Iron Oxide Nanoparticles with Various Polymeric Coatings. Materials 2019, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, C.; Zink, J. Spatial; Temporal, and Dose Control of Drug Delivery using Noninvasive Magnetic Stimulation. ACS Nano 2019, 13, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Ge, H.; Shi, C.; Long, S.; Sun, W.; Fan, J.; Peng, X. Ultrasound-degradable serum albumin nanoplatform for in situ controlled drug release. Chem. Commun. 2020, 56, 7503–7506. [Google Scholar] [CrossRef]
- Yildiz, A.; Bayramol, D.V.; Atav, R.; Ağirgan, A.; Kurç, M.A.; Ergünay, U.; Mayer, C.; Hadimani, R. Synthesis and characterization of Fe3O4@Cs@Ag nanocomposite and its use in the production of magnetic and antibacterial nanofibrous membranes. Appl. Surf. Sci. 2020, 521, 146332. [Google Scholar] [CrossRef]
- Santadkha, T.; Skolpap, W.; Thitapakorn, V. Diffusion Modeling and In Vitro Release Kinetics Studies of Curcumin-Loaded Superparamagnetic Nanomicelles in Cancer Drug Delivery System. J. Pharm. Sci. 2022, 111, 1690–1699. [Google Scholar] [CrossRef]
- Obaidat, I.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Gopi, C.M. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Soleymani, M.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Kalhori, M.; Hadjighassem, M.; Shaterabadi, Z.; Alizadeh, A. Effects of multiple injections on the efficacy and cytotoxicity of folate-targeted magnetite nanoparticles as theranostic agents for MRI detection and magnetic hyperthermia therapy of tumor cells. Sci. Rep. 2020, 10, 1695. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.; Rodriguez, E.M.; Sole, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, J.; Jia, N.; Zhao, Y.; Shen, H. Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: Preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2009, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mousavian, D.; Nafchi, A.M.; Nouri, L.; Abedinia, A. Physicomechanical properties; release kinetics, and antimicrobial activity of activated low-density polyethylene and orientated polypropylene films by Thyme essential oil active component. J. Food Meas. Charact. 2020, 15, 883–891. [Google Scholar] [CrossRef]
- Fahmi, M.; Prasetya, R.; Dzikri, M.; Sakti, S.; Yuliarto, B.; Irzaman, F. MnFe2O4 nanoparticles/cellulose acetate composite nanofiber for controllable release of naproxen. Mater. Chem. Phys. 2020, 250, 123055. [Google Scholar] [CrossRef]
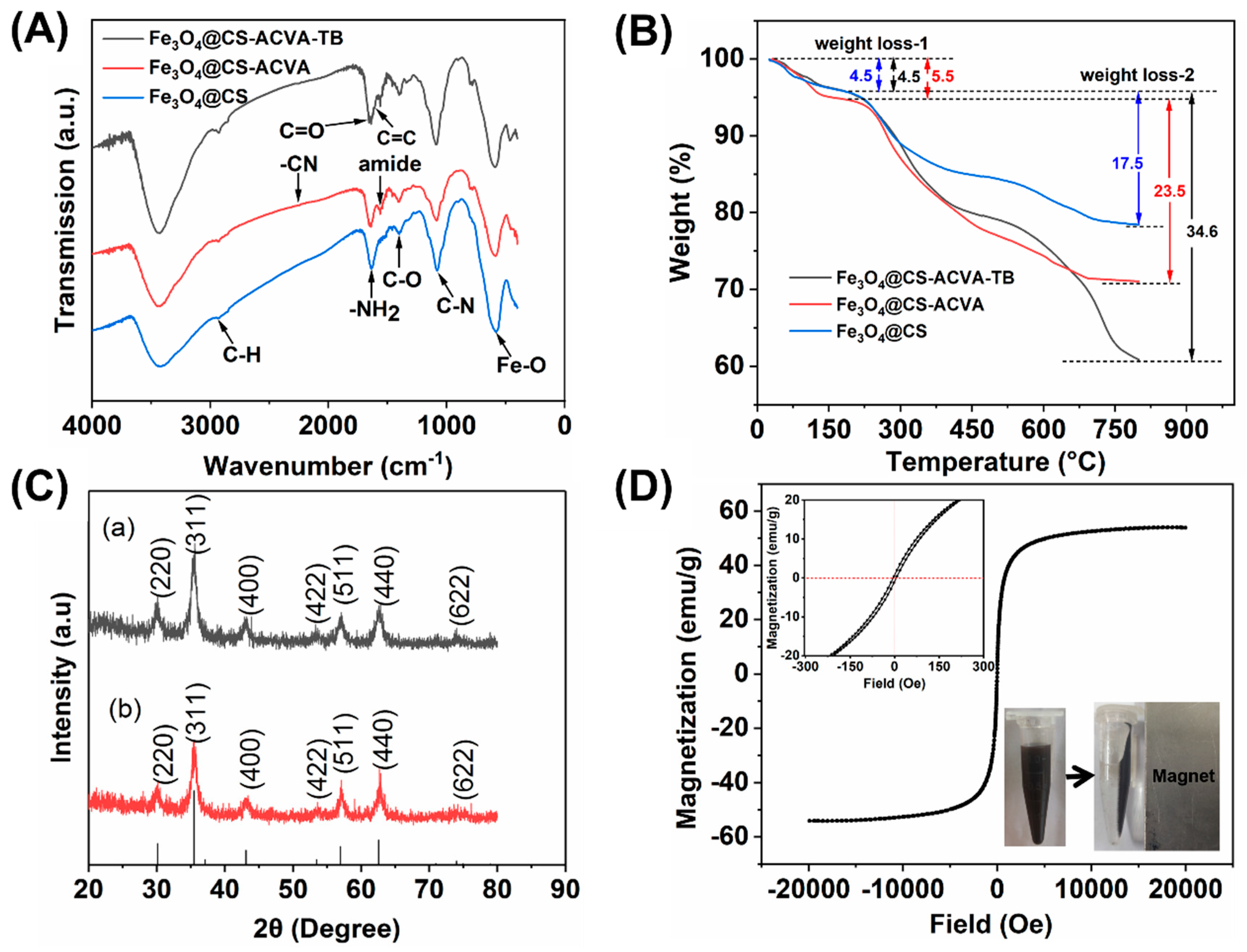



| Sample | Ms (emu/g) | Mr (emu/g) | Mr/Ms |
|---|---|---|---|
| Fe3O4@CS-1 | 54.0057 | 0.1395 | 0.0026 |
| The Drug Release Kinetics | Constants | Correlation Coefficients |
|---|---|---|
| Zero-order release kinetics | k23 °C = 1.1256 | R223 °C = 0.8499 |
| k37 °C = 1.4637 | R237 °C = 0.6784 | |
| k57 °C = 1.4679 | R257 °C = 0.7966 | |
| k80 °C = 3.5318 | R280 °C = 0.4294 | |
| First-order release kinetics | k23 °C = 0.2065 | R223 °C = 0.9997 |
| k37 °C = 0.2196 | R237 °C = 0.9998 | |
| k57 °C = 0.6580 | R257 °C = 0.9978 | |
| k80 °C = 0.9185 | R280 °C = 0.9985 | |
| Higuchi model | k23 °C = 4.5645 | R223 °C = 0.9787 |
| k37 °C = 5.9771 | R237 °C = 0.9628 | |
| k57 °C = 8.8708 | R257 °C = 0.9275 | |
| k80 °C = 23.5588 | R280 °C = 0.7873 | |
| Korsmeyer–Peppas model | k23 °C = 8.9168, n23 °C = 0.2378 | R223 °C = 0.9946 |
| k37 °C = 10.6153, n37 °C = 0.2370 | R237 °C = 0.9958 | |
| k57 °C = 31.2571, n57 °C = 0.1223 | R257 °C = 0.9975 | |
| k80 °C = 64.7859, n80 °C = 0.1415 | R280 °C = 0.9912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, W.; Nziengui Raby, R.B.; Li, Y.; Li, Z.; Sun, M.; Huang, Z. An Alternating Magnetic Field-Controlled Drug Delivery System Based on 4,4′-Azobis (4-cyanovaleric Acid)-Functioned Fe3O4@Chitosan Nanoparticles. Bioengineering 2023, 10, 129. https://doi.org/10.3390/bioengineering10020129
Yin W, Nziengui Raby RB, Li Y, Li Z, Sun M, Huang Z. An Alternating Magnetic Field-Controlled Drug Delivery System Based on 4,4′-Azobis (4-cyanovaleric Acid)-Functioned Fe3O4@Chitosan Nanoparticles. Bioengineering. 2023; 10(2):129. https://doi.org/10.3390/bioengineering10020129
Chicago/Turabian StyleYin, Wang, Randy Bachelard Nziengui Raby, Yuankai Li, Zuojun Li, Mengqing Sun, and Zhi Huang. 2023. "An Alternating Magnetic Field-Controlled Drug Delivery System Based on 4,4′-Azobis (4-cyanovaleric Acid)-Functioned Fe3O4@Chitosan Nanoparticles" Bioengineering 10, no. 2: 129. https://doi.org/10.3390/bioengineering10020129
APA StyleYin, W., Nziengui Raby, R. B., Li, Y., Li, Z., Sun, M., & Huang, Z. (2023). An Alternating Magnetic Field-Controlled Drug Delivery System Based on 4,4′-Azobis (4-cyanovaleric Acid)-Functioned Fe3O4@Chitosan Nanoparticles. Bioengineering, 10(2), 129. https://doi.org/10.3390/bioengineering10020129






