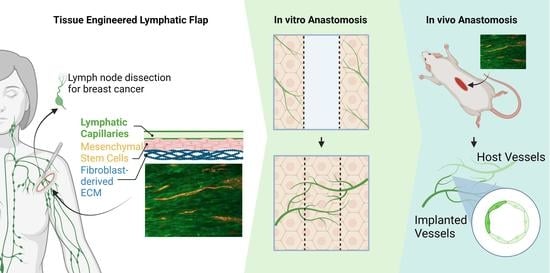
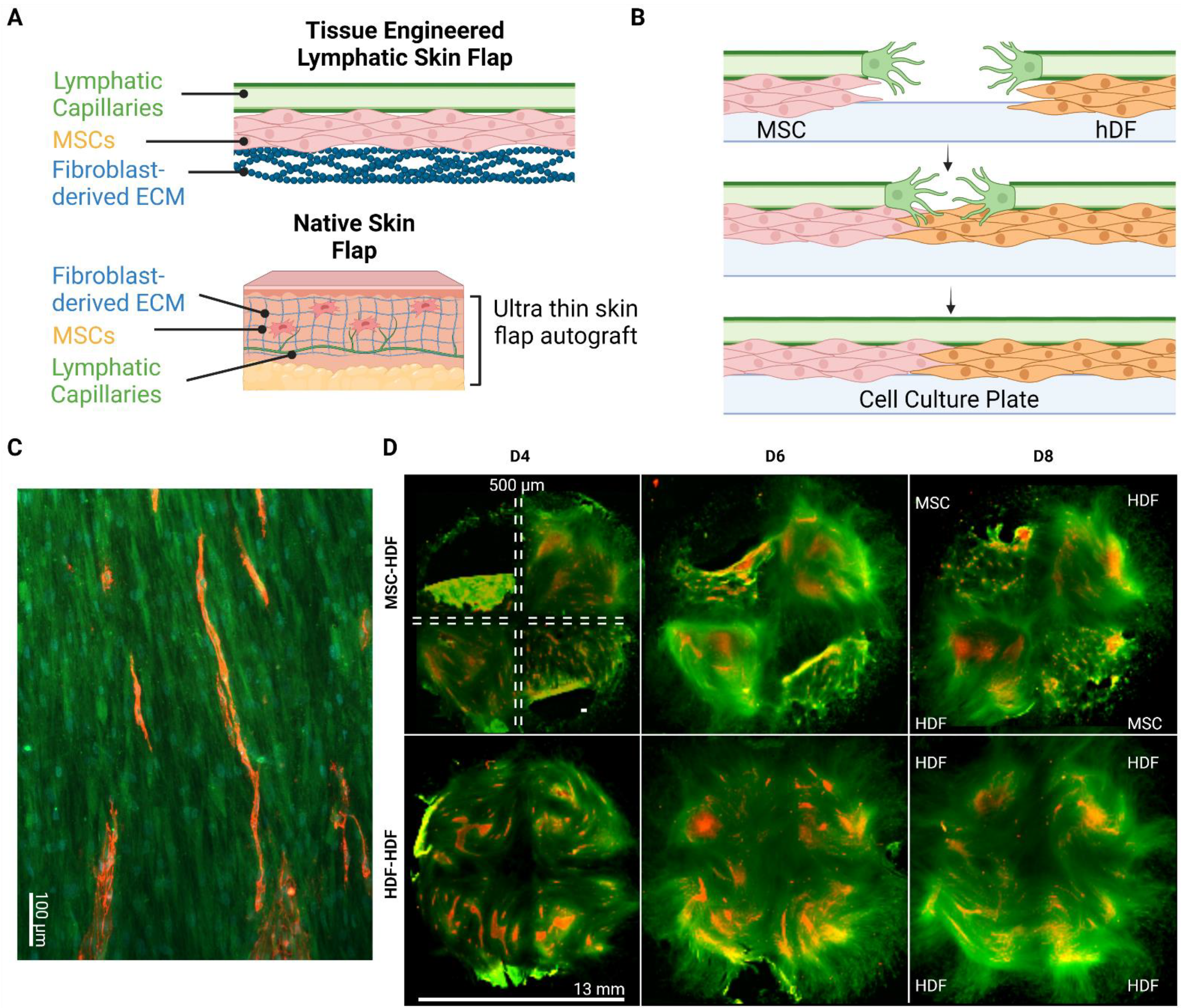
Fibroblast-Generated Extracellular Matrix Guides Anastomosis during Wound Healing in an Engineered Lymphatic Skin Flap
Abstract
1. Introduction
2. Materials & Methods
2.1. Cell Culture
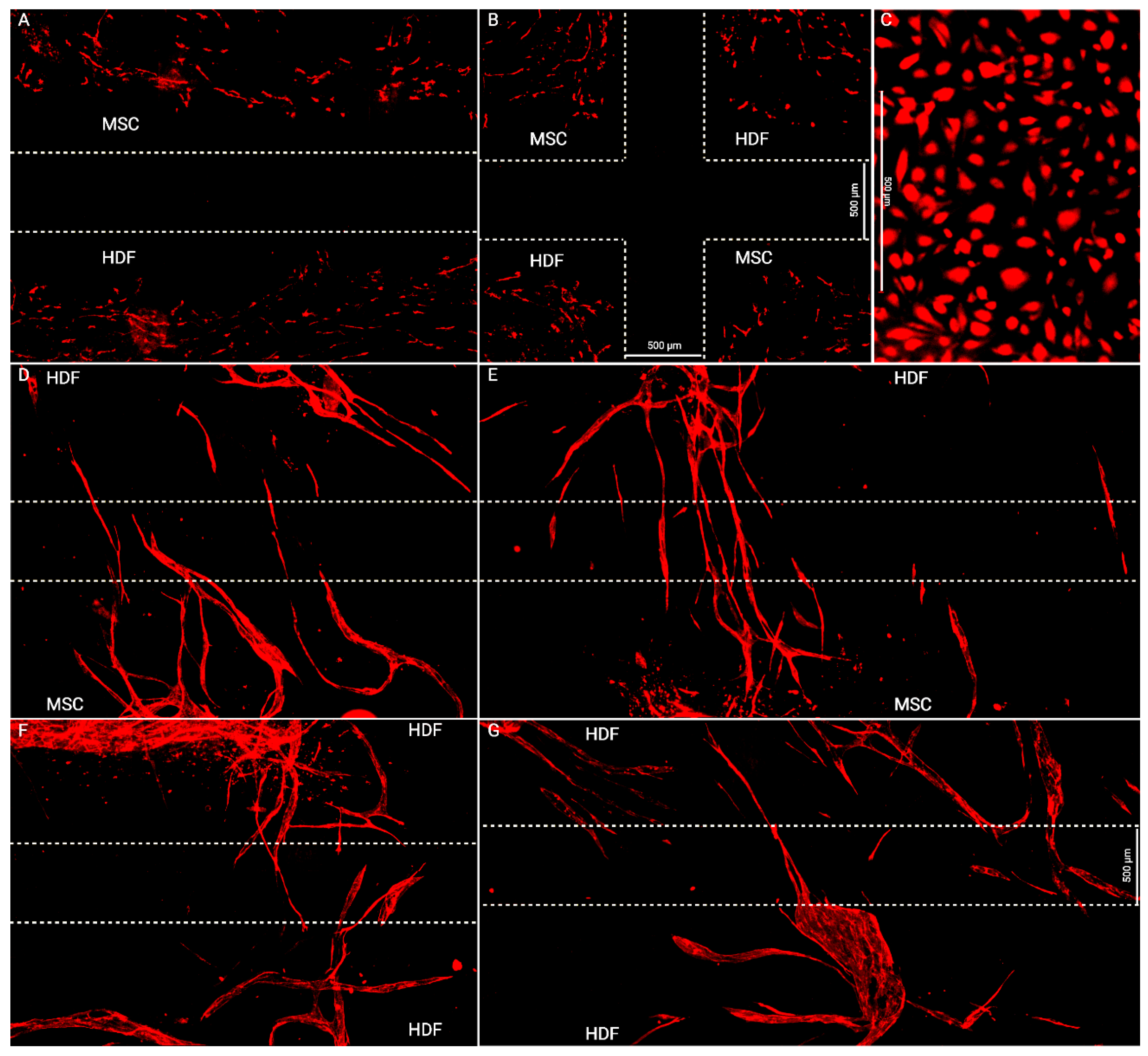
2.2. Wound Healing Model
2.3. Immunofluorescence Staining
2.4. Quantitating Endothelial Invasion
2.5. Lymphatic Skin Flap Replacement Fabrication
2.6. Animal Model
2.7. Statistical Analysis
3. Results
3.1. Flap Anastomoses with Self-Assembled Lymphatic Capillaries
3.2. Engineered Flap Spontaneously Anastomoses with Rat Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Otsubo, R.; Shinohara, S.; Morita, M.; Kuba, S.; Matsumoto, M.; Yamanouchi, K.; Yano, H.; Eguchi, S.; Nagayasu, T. Lymphedema After Axillary Lymph Node Dissection in Breast Cancer: Prevalence and Risk Factors—A Single-Center Retrospective Study. Lymphat. Res. Biol. 2022, 20, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Liu, J.-E.; Mak, Y.W.; Zhu, Y.; Qiu, H.; Liu, L.-H.; Yang, S.-S.; Chen, S.-H. Prevalence and predictors of breast cancer-related arm lymphedema over a 10-year period in postoperative breast cancer patients: A cross-sectional study. Eur. J. Oncol. Nurs. 2021, 51, 101909. [Google Scholar] [CrossRef] [PubMed]
- Togawa, K.; Ma, H.; Smith, A.W.; Neuhouser, M.L.; George, S.M.; Baumgartner, K.B.; McTiernan, A.; Baumgartner, R.; Ballard, R.M.; Bernstein, L. Self-reported symptoms of arm lymphedema and health-related quality of life among female breast cancer survivors. Sci. Rep. 2021, 11, 10701. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-C.T.; Xu, Y.; Cormier, J.N.; Giordano, S.H.; Ridner, S.H.; Buchholz, T.A.; Perkins, G.H.; Elting, L.S. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J. Clin. Oncol. 2009, 27, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, B.C.; Manna, B. Lymphedema. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zaleska, M.T.; Olszewski, W.L. The Effectiveness of Intermittent Pneumatic Compression in Therapy of Lymphedema of Lower Limbs: Methods of Evaluation and Results. Lymphat. Res. Biol. 2019, 17, 60–69. [Google Scholar] [CrossRef]
- Paramanandam, V.S.; Dylke, E.; Clark, G.M.; Daptardar, A.A.; Kulkarni, A.M.; Nair, N.S.; Badwe, R.A.; Kilbreath, S.L. Prophylactic Use of Compression Sleeves Reduces the Incidence of Arm Swelling in Women at High Risk of Breast Cancer–Related Lymphedema: A Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Wiser, I.; Baik, J.E.; Park, H.J.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Fibrosis and secondary lymphedema: Chicken or egg? Transl. Res. 2019, 209, 68–76. [Google Scholar] [CrossRef]
- Yoshimatsu, H.; Visconti, G.; Karakawa, R.; Hayashi, A. Lymphatic System Transfer for Lymphedema Treatment: Transferring the Lymph Nodes with Their Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2721. [Google Scholar] [CrossRef]
- Ito, R.; Zelken, J.; Yang, C.-Y.; Lin, C.-Y.; Cheng, M.-H. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol. Oncol. 2016, 141, 182–188. [Google Scholar] [CrossRef]
- Moon, K.-C.; Kim, H.-K.; Lee, T.-Y.; You, H.-J.; Kim, D.-W. Vascularized lymph node transfer for surgical treatments of upper versus lower extremity lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Narushima, M.; Mihara, M.; Yamamoto, T.; Hara, H.; Ohshima, A.; Kikuchi, K.; Todokoro, K.; Seki, Y.; Iida, T.; Nakagawa, M.; et al. Lymphadiposal Flaps and Lymphaticovenular Anastomoses for Severe Leg Edema: Functional Reconstruction for Lymph Drainage System. J. Reconstr. Microsurg. 2015, 32, 050–055. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Ramaut, L.; De Baerdemaeker, R.; Zeltzer, A. Decreasing donor site morbidity after groin vascularized lymph node transfer with lessons learned from a 12-year experience and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2020, 74, 540–548. [Google Scholar] [CrossRef]
- Ciudad, P.; Manrique, O.J.; Date, S.; Sacak, B.; Bs, W.-L.C.; Kiranantawat, K.; Lim, S.Y.; Chen, H.-C. A head-to-head comparison among donor site morbidity after vascularized lymph node transfer: Pearls and pitfalls of a 6-year single center experience. J. Surg. Oncol. 2016, 115, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pons, G.; Masia, J.; Loschi, P.; Nardulli, M.L.; Duch, J. A case of donor-site lymphoedema after lymph node–superficial circumflex iliac artery perforator flap transfer. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Babchenko, O.; Chen, W.F. Microsurgery: Vascularized Lymph Vessel Transfer. In Peripheral Lymphedema: Pathophysiology, Modern Diagnosis and Management; Liu, N., Ed.; Springer Singapore: Singapore, 2021; pp. 211–222. [Google Scholar]
- Orfahli, L.M.; Fahradyan, V.; Chen, W.F. Vascularized lymph vessel transplant (VLVT): Our experience and lymphedema treatment algorithm. Ann. Breast Surg. 2022, 6, 8. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iida, T.; Yoshimatsu, H.; Fuse, Y.; Hayashi, A.; Yamamoto, N. Lymph Flow Restoration after Tissue Replantation and Transfer: Importance of Lymph Axiality and Possibility of Lymph Flow Reconstruction without Lymph Node Transfer or Lymphatic Anastomosis. Plast. Reconstr. Surg. 2018, 142, 796–804. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Kageyama, T.; Sakai, H.; Fuse, Y.; Tsukuura, R. Lymph-interpositional-flap transfer (LIFT) based on lymph-axiality concept: Simultaneous soft tissue and lymphatic reconstruction without lymph node transfer or lymphatic anastomosis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2604–2612. [Google Scholar] [CrossRef]
- Pandey, S.K.; Fahradyan, V.; Orfahli, L.M.; Chen, W.F. Supermicrosurgical lymphaticovenular anastomosis vs. vascularized lymph vessel transplant—Technical optimization and when to perform which. Plast. Aesthetic Res. 2021, 8, 47. [Google Scholar] [CrossRef]
- Hartiala, P.; Suominen, S.; Suominen, E.; Kaartinen, I.; Kiiski, J.; Viitanen, T.; Alitalo, K.; Saarikko, A.M. Phase 1 LymfactinⓇ Study: Short-term Safety of Combined Adenoviral VEGF-C and Lymph Node Transfer Treatment for Upper Extremity Lymphedema. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1612–1621. [Google Scholar] [CrossRef]
- Visuri, M.T.; Honkonen, K.M.; Hartiala, P.; Tervala, T.V.; Halonen, P.J.; Junkkari, H.; Knuutinen, N.; Ylä-Herttuala, S.; Alitalo, K.; Saarikko, A.M. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: A large animal study. Angiogenesis 2015, 18, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Zaitseva, T.S.; Zhou, A.; Rochlin, D.; Sue, G.; Deptula, P.; Tabada, P.; Wan, D.; Loening, A.; Paukshto, M.; et al. Lymphatic regeneration after implantation of aligned nanofibrillar collagen scaffolds: Preliminary preclinical and clinical results. J. Surg. Oncol. 2021, 125, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ogino, R.; Hayashida, K.; Yamakawa, S.; Morita, E. Adipose-Derived Stem Cells Promote Intussusceptive Lymphangiogenesis by Restricting Dermal Fibrosis in Irradiated Tissue of Mice. Int. J. Mol. Sci. 2020, 21, 3885. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Yan, A.; Zampell, J.C.; Daluvoy, S.V.; Haimovitz-Friedman, A.; Cordeiro, A.P.; Mehrara, B.J. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am. J. Physiol.-Cell Physiol. 2010, 299, C589–C605. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, L.; Schaupper, M.; Mühleder, S.; Schimek, K.; Hasenberg, T.; Marx, U.; Priglinger, E.; Redl, H.; Holnthoner, W. Engineering Blood and Lymphatic Microvascular Networks in Fibrin Matrices. Front. Bioeng. Biotechnol. 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Gibot, L.; Galbraith, T.; Bourland, J.; Rogic, A.; Skobe, M.; Auger, F.A. Tissue-engineered 3D human lymphatic microvascular network for in vitro studies of lymphangiogenesis. Nat. Protoc. 2017, 12, 1077–1088. [Google Scholar] [CrossRef]
- Landau, S.; Newman, A.; Edri, S.; Michael, I.; Ben-Shaul, S.; Shandalov, Y.; Ben-Arye, T.; Kaur, P.; Zheng, M.H.; Levenberg, S. Investigating lymphangiogenesis in vitro and in vivo using engineered human lymphatic vessel networks. Proc. Natl. Acad. Sci. USA 2021, 118, e2101931118. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Schaverien, M.V.; Hanson, S.E.; Chang, E.I.; Butler, C.E. Abstract 128: Engineering Lymphatic Vessels For Secondary Lymphedema Treatment. Plast. Reconstr. Surg.–Glob. Open 2020, 8, 85–86. [Google Scholar] [CrossRef]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Koda, I.; Song, M.; Chen, G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 2011, 32, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Sharma, D.; Jia, W.; Radke, D.; Kamp, T.; Zhao, F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics 2019, 9, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qian, Z.; Tahtinen, M.; Qi, S.; Zhao, F. Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications. J. Tissue Eng. Regen. Med. 2017, 12, e1325–e1336. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Willman, M.A.; Orfei, C.P.; Colombini, A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar fat pad-derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation. Sci. Rep. 2019, 9, 10864. [Google Scholar] [CrossRef]
- Robering, J.W.; Weigand, A.; Pfuhlmann, R.; Horch, R.E.; Beier, J.P.; Boos, A.M. Mesenchymal stem cells promote lymphangiogenic properties of lymphatic endothelial cells. J. Cell. Mol. Med. 2018, 22, 3740–3750. [Google Scholar] [CrossRef]
- Chen, L.; Xing, Q.; Zhai, Q.; Tahtinen, M.; Zhou, F.; Chen, L.; Xu, Y.; Qi, S.; Zhao, F. Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics 2017, 7, 117–131. [Google Scholar] [CrossRef]
- Kim, H.S.; Hwang, H.J.; Kim, H.J.; Choi, Y.; Lee, D.; Jung, H.H.; Do, S.H. Effect of Decellularized Extracellular Matrix Bioscaffolds Derived from Fibroblasts on Skin Wound Healing and Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 865545. [Google Scholar] [CrossRef]
- Xing, Q.; Vogt, C.; Leong, K.W.; Zhao, F. Highly Aligned Nanofibrous Scaffold Derived from Decellularized Human Fibroblasts. Adv. Funct. Mater. 2014, 24, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Uttayarat, P.; Toworfe, G.K.; Dietrich, F.; Lelkes, P.I.; Composto, R.J. Topographic guidance of endothelial cells on silicone surfaces with micro- to nanogrooves: Orientation of actin filaments and focal adhesions. J. Biomed. Mater. Res. Part A 2005, 75, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Montero, R.B.; Vial, X.; Nguyen, D.T.; Farhand, S.; Reardon, M.; Pham, S.M.; Tsechpenakis, G.; Andreopoulos, F.M. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012, 8, 1778–1791. [Google Scholar] [CrossRef]
- Doillon, C.J.; Dunn, M.G.; Bender, E.; Silver, F.H. Collagen Fiber Formation in Repair Tissue: Development of Strength and Toughness. Collagen Relat. Res. 1985, 5, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in Wound Healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Chiu, L.L.Y.; Montgomery, M.; Liang, Y.; Liu, H.; Radisic, M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc. Natl. Acad. Sci. USA 2012, 109, E3414–E3423. [Google Scholar] [CrossRef] [PubMed]
- O’Ceallaigh, S.; Herrick, S.E.; Bluff, J.E.; McGrouther, D.A.; Ferguson, M.W.J. Quantification of Total and Perfused Blood Vessels in Murine Skin Autografts Using a Fluorescent Double-Labeling Technique. Plast. Reconstr. Surg. 2006, 117, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.J.; Lu, J.; Sharma, R.; Mandell, S.P.; Wang, R.K. Imaging human skin autograft integration with optical coherence tomography. Quant. Imaging Med. Surg. 2021, 11, 784–796. [Google Scholar] [CrossRef]
- Tefft, J.B.; Chen, C.S.; Eyckmans, J. Reconstituting the dynamics of endothelial cells and fibroblasts in wound closure. APL Bioeng. 2021, 5, 016102. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, A.; Jia, W.; Sun, Y.; Goldman, J.; Zhao, F. Fibroblast-Generated Extracellular Matrix Guides Anastomosis during Wound Healing in an Engineered Lymphatic Skin Flap. Bioengineering 2023, 10, 149. https://doi.org/10.3390/bioengineering10020149
Chiu A, Jia W, Sun Y, Goldman J, Zhao F. Fibroblast-Generated Extracellular Matrix Guides Anastomosis during Wound Healing in an Engineered Lymphatic Skin Flap. Bioengineering. 2023; 10(2):149. https://doi.org/10.3390/bioengineering10020149
Chicago/Turabian StyleChiu, Alvis, Wenkai Jia, Yumeng Sun, Jeremy Goldman, and Feng Zhao. 2023. "Fibroblast-Generated Extracellular Matrix Guides Anastomosis during Wound Healing in an Engineered Lymphatic Skin Flap" Bioengineering 10, no. 2: 149. https://doi.org/10.3390/bioengineering10020149
APA StyleChiu, A., Jia, W., Sun, Y., Goldman, J., & Zhao, F. (2023). Fibroblast-Generated Extracellular Matrix Guides Anastomosis during Wound Healing in an Engineered Lymphatic Skin Flap. Bioengineering, 10(2), 149. https://doi.org/10.3390/bioengineering10020149