Exfoliative Cytology and Genetic Analysis for a Non-Invasive Approach to the Diagnosis of White Sponge Nevus: Case Series
Abstract
:1. Introduction
2. Materials and Methods
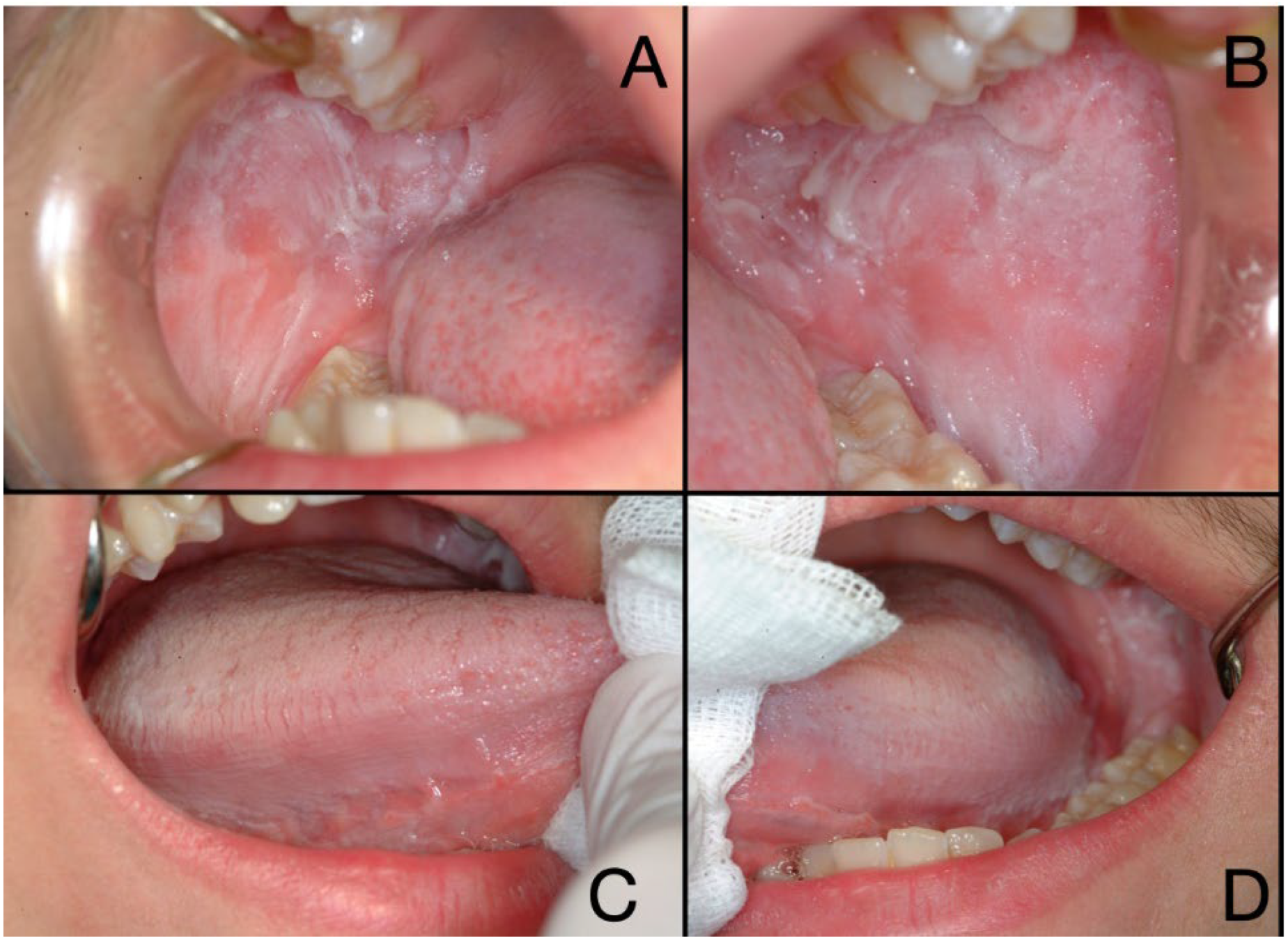
2.1. Patient Information and Clinical Findings
2.2. Timeline
2.3. Diagnostic Assessment
2.4. Laboratory Procedures
2.5. Genetic Analysis
2.6. In Silico Analysis
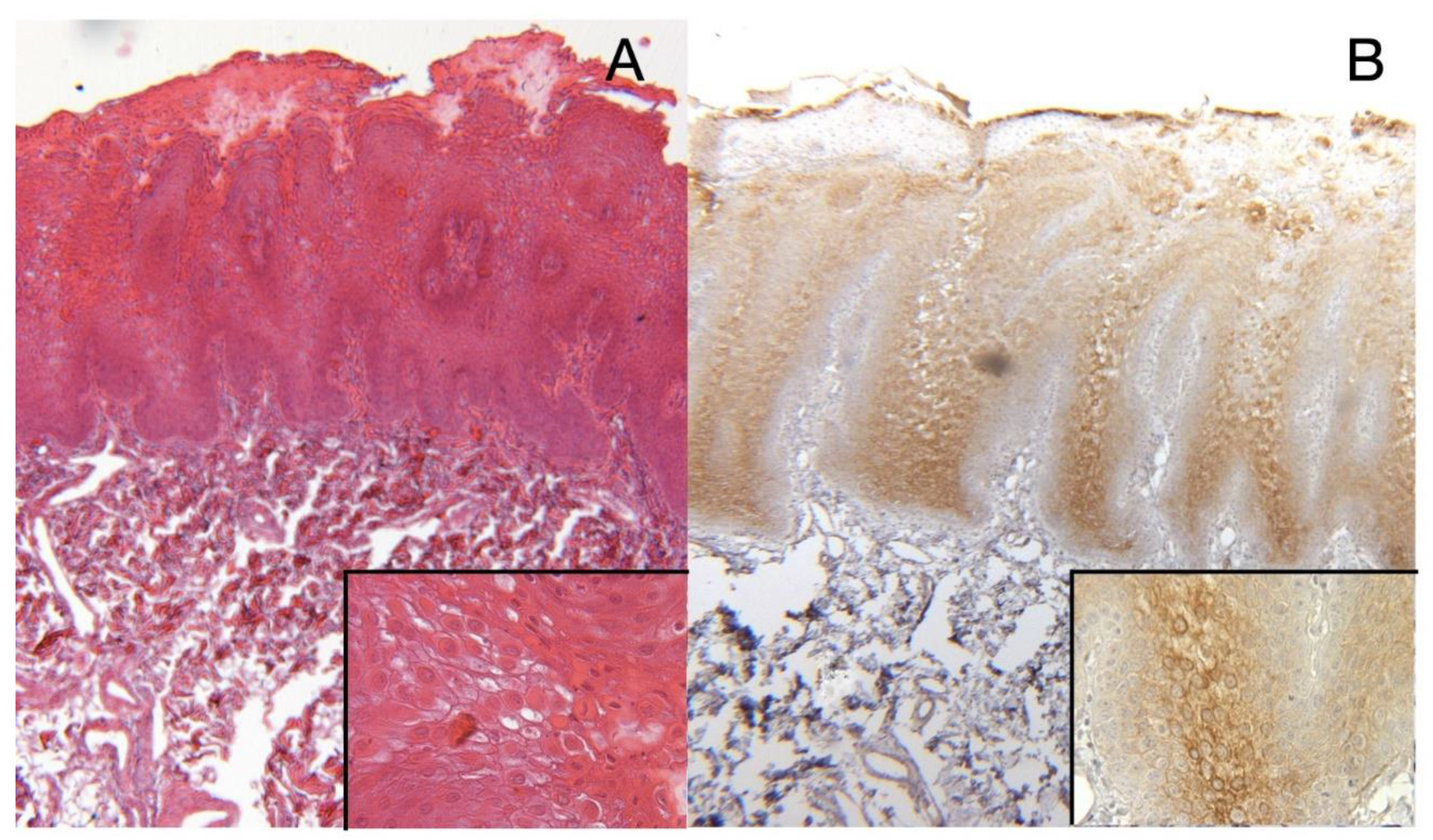
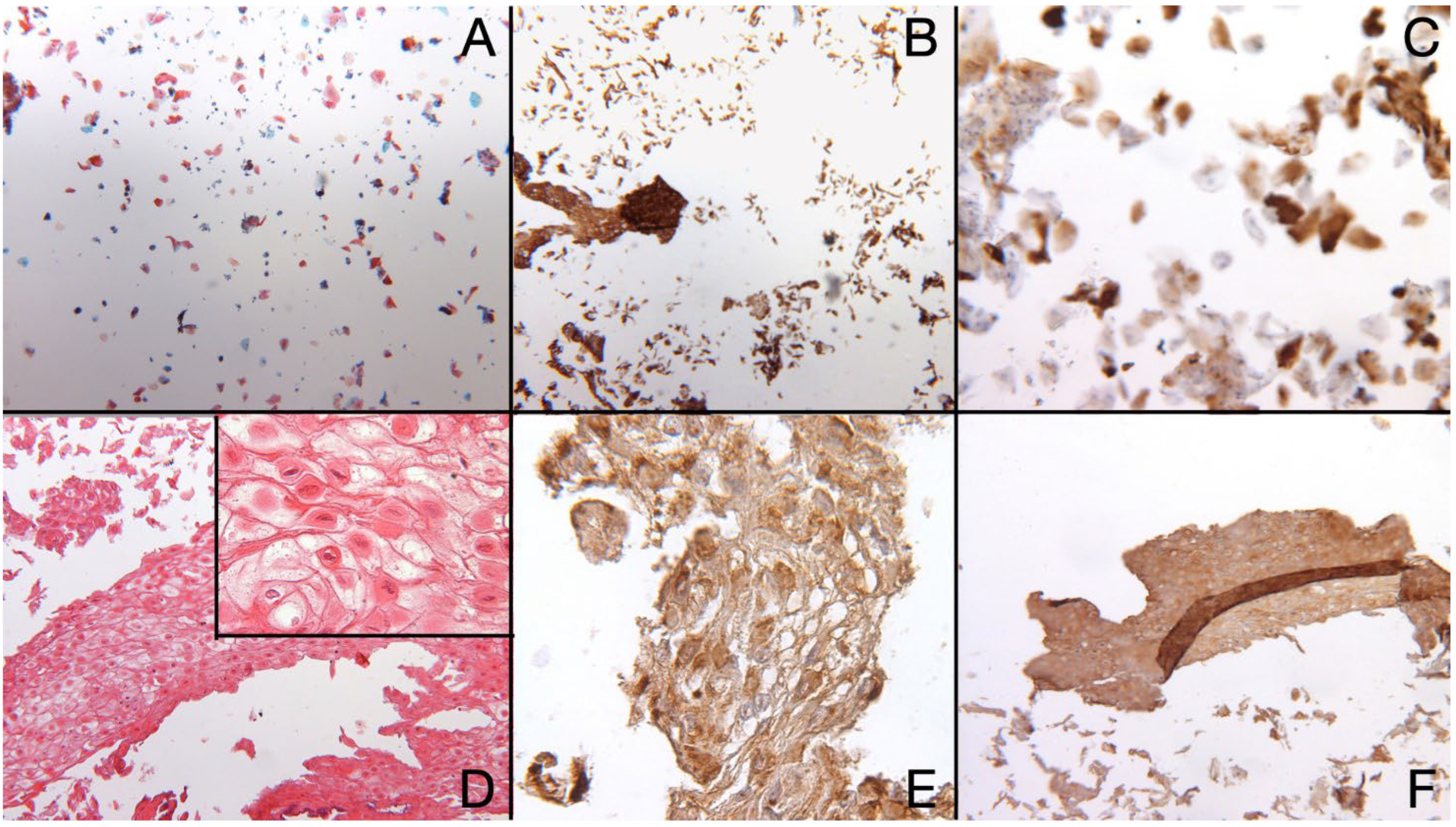
2.7. Results Histocytopathological, Immunocytochemistry and Cell Block Results
2.8. Genetic Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babu, N.A.; Rajesh, E.; Krupaa, J.; Gnananandar, G. Genodermatoses. J. Pharm. Bioallied Sci. 2015, 7, S203–S206. [Google Scholar] [CrossRef] [PubMed]
- Pinna, R.; Cocco, F.; Campus, G.; Conti, G.; Milia, E.; Sardella, A.; Cagetti, M.G. Genetic and developmental disorders of the oral mucosa: Epidemiology; molecular mechanisms; diagnostic criteria; management. Periodontology 2000 2019, 80, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.B.; Jordan, R. White lesions in the oral cavity: Clinical presentation, diagnosis, and treatment. Semin. Cutan. Med. Surg. 2015, 34, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, K.T.; Leite, T.C.; Roza, A.; Araujo, R.; Israel, M.S.; Canedo, N.H.S.; Agostini, M.; Benevenuto de Andrade, B.A.; Romanach, M.J. White sponge nevus: A condition not always clinically suspected. J. Cutan. Pathol. 2020, 47, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, A.; Santonocito, S.; Lo Giudice, A.; Alibrandi, A.; De Pasquale, R.; Isola, G. Analysis of the response to two pharmacological protocols in patients with oral lichen planus: A randomized clinical trial. Oral Dis. 2021; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, M.; Alirezaei, P.; Farshchian, M.; Eshghi, G.; Ghasemi Basir, H.R.; Khezrian, L. White Sponge Nevus: Report of a Case and Review of the Literature. Acta Med. Iran. 2017, 55, 533–535. [Google Scholar]
- Isola, G.; Matarese, M.; Ramaglia, L.; Iorio-Siciliano, V.; Cordasco, G.; Matarese, G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2019, 23, 2443–2453. [Google Scholar] [CrossRef]
- de Haseth, S.B.; Bakker, E.; Vermeer, M.H.; El Idrissi, H.; Bosse, T.; Smit, V.; Terron-Kwiatkowski, A.; McLean, W.H.I.; Peters, A.A.W.; Hes, F.J. A novel keratin 13 variant in a four-generation family with white sponge nevus. Clin. Case Rep. 2017, 5, 1503–1509. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Gao, Y.; Song, S.; Hua, H. Mutational analysis in familial and sporadic patients with white sponge naevus. Br. J. Dermatol. 2011, 165, 448–451. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, J.; Ren, Y.; Chen, M.; Yang, J.; Zhang, X. Keratin 4 regulates the development of human white sponge nevus. J. Oral Pathol. Med. 2018, 47, 598–605. [Google Scholar] [CrossRef]
- Westin, M.; Rekabdar, E.; Blomstrand, L.; Klintberg, P.; Jontell, M.; Robledo-Sierra, J. Mutations in the genes for keratin-4 and keratin-13 in Swedish patients with white sponge nevus. J. Oral Pathol. Med. 2018, 47, 152–157. [Google Scholar] [CrossRef]
- Songu, M.; Adibelli, H.; Diniz, G. White sponge nevus: Clinical suspicion and diagnosis. Pediatr. Dermatol. 2012, 29, 495–497. [Google Scholar] [CrossRef]
- Morris, R.; Gansler, T.S.; Rudisill, M.T.; Neville, B. White sponge nevus. Diagnosis by light microscopic and ultrastructural cytology. Acta Cytol. 1988, 32, 357–361. [Google Scholar]
- Huang, B.W.; Lin, C.W.; Lee, Y.P.; Chiang, C.P. Differential diagnosis between leukoedema and white spongy nevus. J. Dent. Sci. 2020, 15, 554–555. [Google Scholar] [CrossRef]
- Berrone, M.; Lajolo, C.; De Corso, E.; Settimi, S.; Rupe, C.; Crosetti, E.; Succo, G. Cooperation between ENT surgeon and dentist in head and neck oncology. Acta Otorhinolaryngol. Ital. 2021, 41, S124–S137. [Google Scholar] [CrossRef]
- Kurklu, E.; Ozturk, S.; Cassidy, A.J.; Ak, G.; Koray, M.; Cefle, K.; Palanduz, S.; Gulluoglu, M.G.; Tanyeri, H.; McLean, W.H. Clinical features and molecular genetic analysis in a Turkish family with oral white sponge nevus. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e144–e150. [Google Scholar] [CrossRef]
- Isola, G.; Anastasi, G.P.; Matarese, G.; Williams, R.C.; Cutroneo, G.; Bracco, P.; Piancino, M.G. Functional and molecular outcomes of the human masticatory muscles. Oral Dis. 2018, 24, 1428–1441. [Google Scholar] [CrossRef]
- Simonson, L.; Vold, S.; Mowers, C.; Massey, R.J.; Ong, I.M.; Longley, B.J.; Chang, H. Keratin 13 deficiency causes white sponge nevus in mice. Dev. Biol. 2020, 468, 146–153. [Google Scholar] [CrossRef]
- Cai, W.; Jiang, B.; Yu, F.; Yang, J.; Chen, Z.; Liu, J.; Wei, R.; Zhao, S.; Wang, X.; Liu, S. Current approaches to the diagnosis and treatment of white sponge nevus. Expert Rev. Mol. Med. 2015, 17, e9. [Google Scholar] [CrossRef]
- Alsarraf, A.H.; Kujan, O.; Farah, C.S. The utility of oral brush cytology in the early detection of oral cancer and oral potentially malignant disorders: A systematic review. J. Oral Pathol. Med. 2018, 47, 104–116. [Google Scholar] [CrossRef]
- Idrees, M.; Farah, C.S.; Sloan, P.; Kujan, O. Oral brush biopsy using liquid-based cytology is a reliable tool for oral cancer screening: A cost-utility analysis: Oral brush biopsy for oral cancer screening. Cancer Cytopathol. 2022, 130, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Zannoni, G.F.; Moncelsi, S.; Stigliano, E.; Santeusanio, G.; Lombardi, C.P.; Pontecorvi, A.; Fadda, G. Application of liquid-based cytology to fine-needle aspiration biopsies of the thyroid gland. Front. Endocrinol. 2012, 3, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-de-Oliveira, M.E.; Petersen Wagner, V.; do Amaral-Silva, G.K.; Almeida Leite, A.; Ajudarte Lopes, M.; Santos-Silva, A.R.; Jorge Junior, J.; de Almeida, O.P.; Agustin Vargas, P. An audit of cytopathology in the oral and maxillofacial region: 18 years of experience. Cytopathology 2020, 31, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.F.; Farag, A.M.; Treister, N.S. Chronic Oral Lesions. Dermatol. Clin. 2020, 38, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Curro, M.; Matarese, G.; Isola, G.; Caccamo, D.; Ventura, V.P.; Cornelius, C.; Lentini, M.; Cordasco, G.; Ientile, R. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral Dis. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- da Cunha Santos, G.; Saieg, M.A.; Troncone, G.; Zeppa, P. Cytological preparations for molecular analysis: A review of technical procedures, advantages and limitations for referring samples for testing. Cytopathology 2018, 29, 125–132. [Google Scholar] [CrossRef]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Lo Muzio, L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Lo Giudice, R.; Matarese, G.; Isola, G.; Cicciù, M.; Terranova, A.; Palaia, G.; Romeo, U. Evaluation of magnification systems in restorative dentistry. An in-vitro study. Dental Cadmos 2015, 83, 296–305. [Google Scholar] [CrossRef]
- Liu, D.; Sheng, C.; Gao, S.; Yao, C.; Li, J.; Jiang, W.; Chen, H.; Wu, J.; Pan, C.; Chen, S.; et al. SOCS3 Drives Proteasomal Degradation of TBK1 and Negatively Regulates Antiviral Innate Immunity. Mol. Cell. Biol. 2015, 35, 2400–2413. [Google Scholar] [CrossRef] [Green Version]
- Hosmani, J.V.; Pujari, V.K.; Kotrashetti, V.S.; Nayak, R.S.; Babji, D.V.; Patanshetti, S.M. Comparison of the Efficacy of Sediment Cytology over Oral Brush Cytology in Oral Leukoplakia. Acta Cytol. 2020, 64, 368–374. [Google Scholar] [CrossRef]
- Cannavale, R.; Matarese, G.; Isola, G.; Grassia, V.; Perillo, L. Early treatment of an ectopic premolar to prevent molar-premolar transposition. Am. J. Orthod. Dentofacial. Orthop. 2013, 143, 559–569. [Google Scholar] [CrossRef]
- Mortazavi, H.; Safi, Y.; Baharvand, M.; Jafari, S.; Anbari, F.; Rahmani, S. Oral White Lesions: An Updated Clinical Diagnostic Decision Tree. Dent. J. 2019, 7, 15. [Google Scholar] [CrossRef] [Green Version]

| Inheritance | Phenotype | OMIM | Location | Gene | OMIM |
|---|---|---|---|---|---|
| AD | White Sponge Nevus 1; WSN1 | #193900 | 12q13.13 | KRT4 | *123940 |
| AD | White Sponge Nevus 2; WSN2 | #615785 | 17q21.2 | KRT13 | *148065 |
| AD | Hereditary Benign Intraepithelial Dyskeratosis; HBID | %127600 | 4q35 | n.a. | n.a. |
| Pachyonychia congenita, PC | |||||
| AD | Pachyonychia congenita 1/PC1 | #167200 | 17q21.2 | KRT16 | *148067 |
| AD | Pachyonychia congenita 2/PC2 | #167210 | 17q21.2 | KRT17 | *148069 |
| AD | Pachyonychia congenita 3/PC3 | #615726 | 12q13.13 | KRT6A | *148041 |
| AD | Pachyonychia congenita 4/PC4 | #615728 | 12q13.13 | KRT6B | *148042 |
| AR | Pachyonychia congenita, autosomal recessive | 260130 | n.a. | n.a. | n.a. |
| Dyskeratosis congenita, DKC | |||||
| AD | DKC, autosomal dominant 1/DKCA1 | #127550 | 3q26.2 | TERC | *602322 |
| AD, AR | DKC, autosomal dominant 2/DKCA2 - autosomal recessive 4/DKCB4 | #613989 | 5p15.33 | TERT | *187270 |
| AD | DKC, autosomal dominant 3/DKCA3 - Revesz syndrome | #613990-#268130 | 14q12 | TINF2 | *604319 |
| AD, AR | DKC, autosomal dominant 4/DKCA4 - autosomal recessive 5/DKCB5 | #615190 | 20q13.33 | RTEL1 | *608833 |
| AD | DKC, autosomal dominant 6/DKCA6 | #616553 | 16q22.1 | ACD | *609377 |
| AR | DKC, autosomal recessive 1/DKCB1 | #224230 | 15q14 | NOP10 | *606471 |
| AR | DKC, autosomal recessive 2/DKCB2 | #613987 | 5q35.3 | NHP2 | *606470 |
| AR | DKC, autosomal recessive 3/DKCB3 | #613988 | 17p13.1 | WRAP53 | *612661 |
| AR | DKC, autosomal recessive 6/DKCB6 | #616353 | 16p13.12 | PARN | *604212 |
| AR | DKC, autosomal recessive 7/DKCB7 | #616553 | 16q22.1 | ACD | *609377 |
| XLR | DKC, X-linked | #305000 | Xq28 | DKC1 | *300126 |
| Primers for KRT4 Gene | Primer (5′ to 3′) | Annealing Temp. (C°) | Amplicon Size (bp) |
|---|---|---|---|
| CK4F1 | TGATAGCTCCCAGCTCGCT | 55° | 603 |
| CK4R1 | CCAGGGAAGTTCAGTGGTCT | ||
| CK4F2 | TGCCCTGGAGATGCAACATA | 55° | 464 |
| CK4R2 | CCTTCAGAGCCTGAGATTCT | ||
| CK4F3 | CCTGGCCTAAACGGGTACTT | 55° | 332 |
| CK4R3 | CCCATGACTTCAGCCAAAGA | ||
| CK4F4 | TTCCATGTCTTCAGGTGGCT | 55° | 402 |
| CK4R4 | GATGAGAACCCACTGCCCTA | ||
| CK4F5 | TCAGTGAAGGCTTTCCTGG | 55° | 668 (5F/6R) |
| CK4R6 | TCTGGAGATGTCTGCCTGAG | ||
| CK4F7 | TCAGGGAAAGTGGGCAGAA | 55° | 421 |
| CK47R | GGCATAAATAAGCTCATGGC | ||
| CK4F8 | GGTGCATTACGAACCAGGA | 55° | 650 (8F/9R) |
| CK4R9 | TCCCTGTCCCAGCACAGAA | ||
| Primers for KRT13 Gene | Primer (5′ to 3′) | Annealing Temp. (C°) | Amplicon Size (bp) |
| CK13F1 | GGGAAGGGAGGAGAGAAGAT | 55° | 715 |
| CK13R1 | CACACCTAGTCCCCCACAA | ||
| CK13F2 | TAGCGTATTTGATGTGTTGCC | 55° | 274 |
| CK13R2 | CCAGTGTCATTGGTCAGATG | ||
| CK13F3 | ACACTGGAGCATCCCAGGA | 55° | 668 (3F/4R) |
| CK13R4 | TATGGGATGGGCTATGTGGG | ||
| CK13F5 | CTTCCCCACCACCTTCTTC | 55° | 567 (5F/6R) |
| CK13R6 | TGACATGAGGGGGTGGATC | ||
| CK13F7 | GAAATGATAAGCCGAGGCAC | 55° | 276 |
| CK13R7 | CAGTGAGCGAATGACCACTT | ||
| CK13F8 | AATGAGGAGTTTGTGAGCCC | 55° | 301 |
| CK13R8 | ACTGAGCCTTGGGTCCAGC |
| Patient | Gene | Genetic Variant Effect—(Varsome Classification) | dbSNP (ClinVar) | Parental Origin | Variant Frequency (%) |
|---|---|---|---|---|---|
| P-1 | KRT4 | c.244_245insTTGGTGGCTTTGGTGCCGGCG GCTTCGGAGCTGGTTTCGGCA p.Gly81_Thr82insIleGlyGlyPheGlyAlaGlyGly PheGlyAlaGlyPheGly in frame insertion—(Uncertain Significance) | rs781060860 (n.a.) | Maternal | 2/47694 alleles |
| c.678-14G > A intronic variant–(Benign) | rs2307027 (309687) | Maternal | 234682/ 280914 alleles (84%) | ||
| c.1345A > G intronic variant–(Benign) | rs931479 (309671) | Paternal | 236494/ 282588 alleles (84%) | ||
| KRT13 | c.114C > T (p.Ser38 =) synonymous variant—(Benign) | rs8182306 (1300084) | Maternal | 260852/ 282488 alleles (92%) | |
| c.735 + 10A > G intronic variant—(Benign) | rs7211835 (323093) | Paternal | 257738/ 282656 alleles (91%) | ||
| c.897 + 6C > T intronic variant—(Benign) | rs4796698 (323084) | Paternal | 255407/ 282646 alleles (90%) | ||
| P-2 | KRT4 | c.244_245insTTGGTGGCTTTGGTGCCGGCG GCTTCGGAGCTGGTTTCGGCA p.Gly81_Thr82insIleGlyGlyPheGlyAlaGlyGly PheGlyAlaGlyPheGly in frame insertion—(Uncertain Significance) | rs781060860 (n.a.) | Paternal | 2/47694 alleles |
| c.678-14G > A intronic variant—(Benign) | rs2307027 (309687) | Maternal | 234682/ 280914 alleles (84%) | ||
| c.1020G > C (p.Ser340=) synonymous variant—(Benign) | rs7956809 (309677) | Paternal | 27040/ 281468 alleles (10%) | ||
| KRT13 | c.114C > T (p.Ser38=) synonymous variant—(Benign) | rs8182306 (1300084) | Maternal | 260852/ 282488 alleles (92%) | |
| c.340C > T (p.Arg114Cys) missense variant—(Likely pathogenic) | rs545085703 (n.a.) | Maternal | 1/251496 alleles | ||
| c.735 + 10A > G intronic variant—(Benign) | rs7211835 (323093) | Paternal | 257738/ 282656 alleles (91%) | ||
| c.897 + 6C > T intronic variant—(Benign) | rs4796698 (323084) | Maternal | 255407/ 282646 alleles (90%) | ||
| c.1335T > C (p.Ser445=) synonymous variant—(Likely benign) | rs772942425 (n.a.) | Paternal | 2/192196 alleles | ||
| P-3 | KRT4 | c.244_245insTTGGTGGCTTTGGTGCCGGCG GCTTCGGAGCTGGTTTCGGCA p.Gly81_Thr82insIleGlyGlyPheGlyAlaGlyGly PheGlyAlaGlyPheGly in frame insertion (Uncertain Significance) | rs781060860 (n.a.) | Maternal | 2/47694 alleles |
| c.678-14G > A intronic variant - (Benign) | rs2307027 (309687) | Paternal | 234682/ 280914 alleles (84%) | ||
KRT13 | c.114C > T (p.Ser38 =) synonymous variant - (Benign) | rs8182306 (1300084) | Paternal | 260852/ 282488 alleles (92%) | |
| c.437C > G (p.Ala146Gly) missense variant—(Benign) | rs760134 (323101) | Paternal | 15256/ 282858 alleles (5.4%) | ||
| c.735 + 10A > G intronic variant—(Benign) | rs7211835 (323093) | Maternal | 257738/ 282656 alleles (91%) | ||
| c.897 + 6C > T intronic variant—(Benign) | rs4796698 (323084) | Paternal | 255407/ 282646 alleles (90%) | ||
| P-4 | KRT4 | c.215C > T (p.Ala72Val) missense variant—(Benign) | rs2638525 (309709) | Paternal | 49870/ 229154 alleles (21.8%) |
| c.467A > G (p.Gln156Arg) missense variant—(Benign) | rs7959052 (309695) | Maternal | 53607/ 278106 alleles (19.3%) | ||
| KRT13 | c.114C > T (p.Ser38 =) synonymous variant—(Benign) | rs8182306 (1300084) | Paternal | 260852/ 282488 alleles (92%) | |
| c.560C > T (p.Ala187Val) missense variant—(Benign) | rs9891361 (323096) | Maternal | 239787/ 282512 alleles (85%) | ||
| c.735 + 10A > G intronic variant—(Benign) | rs7211835 (323093) | Paternal | 257738/ 282656 alleles (91%) | ||
| c.897 + 6C > T intronic variant—(Benign) | rs4796698 (323084) | Paternal | 255407/ 282646 alleles (90%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajolo, C.; Cafiero, C.; Stigliano, E.; Grippaudo, F.R.; Chiurazzi, P.; Grippaudo, C. Exfoliative Cytology and Genetic Analysis for a Non-Invasive Approach to the Diagnosis of White Sponge Nevus: Case Series. Bioengineering 2023, 10, 154. https://doi.org/10.3390/bioengineering10020154
Lajolo C, Cafiero C, Stigliano E, Grippaudo FR, Chiurazzi P, Grippaudo C. Exfoliative Cytology and Genetic Analysis for a Non-Invasive Approach to the Diagnosis of White Sponge Nevus: Case Series. Bioengineering. 2023; 10(2):154. https://doi.org/10.3390/bioengineering10020154
Chicago/Turabian StyleLajolo, Carlo, Concetta Cafiero, Egidio Stigliano, Francesca Romana Grippaudo, Pietro Chiurazzi, and Cristina Grippaudo. 2023. "Exfoliative Cytology and Genetic Analysis for a Non-Invasive Approach to the Diagnosis of White Sponge Nevus: Case Series" Bioengineering 10, no. 2: 154. https://doi.org/10.3390/bioengineering10020154
APA StyleLajolo, C., Cafiero, C., Stigliano, E., Grippaudo, F. R., Chiurazzi, P., & Grippaudo, C. (2023). Exfoliative Cytology and Genetic Analysis for a Non-Invasive Approach to the Diagnosis of White Sponge Nevus: Case Series. Bioengineering, 10(2), 154. https://doi.org/10.3390/bioengineering10020154









