Two Operational Modes of Cardio-Respiratory Coupling Revealed by Pulse-Respiration Quotient
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Protocol
2.3. Data Acquisition
2.4. Data Processing
2.5. Statistics
2.6. Two Types of Correlations
- The degree of positive correlation between BBI and PRQ determines whether and to what extent the number of intra-BBI RR intervals increases with the increase in BBI;
- The degree of positive correlation between BBI and mRRI indicates whether and to what extent the mean intra-BBI RR interval is increasing with the increase in BBI.
2.6.1. Correlations between BBI and PRQ
2.6.2. Correlations between BBI and mRRI
Aggregation Measures
2.7. Cardio-Respiratory Synchronization
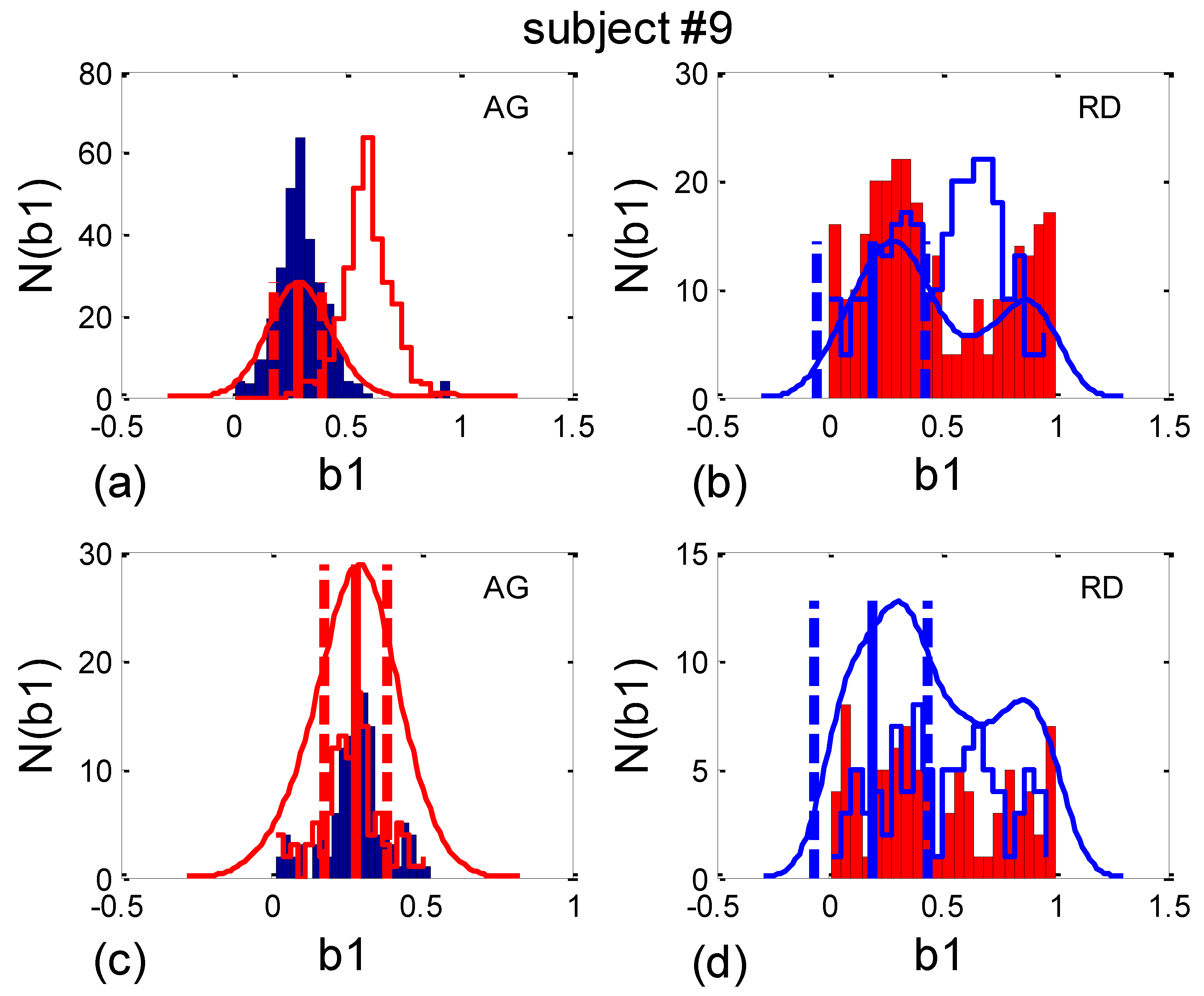
2.7.1. β1. Locking
- b1max, the value of b1 where the maximum of PDE or its histogram occurs. Using this analogy, b1max signifies the most probable “phase shift”, or, more precisely, the most probable part of the RR interval between respiration onset and the first occurring R impulse. However, since, in some cases, there is more than one PDE or histogram maximum, it is more reliable to use the angular mean value, according to Equation (10);
- histogram-derived standard deviation may be denoted with b1hst and may be used as a reciprocal measure for the degree of “b1 locking” or “β1 locking” (further details will be explained in Section 2.7.2). Note that it is not necessary to compute analogous quantities for b2, since, except for their first and last appearances in the signal, their relationship is complementary: b1(i) + b2(i − 1) = 1, i being the ith BB interval.
2.7.2. Circular Correction
3. Results
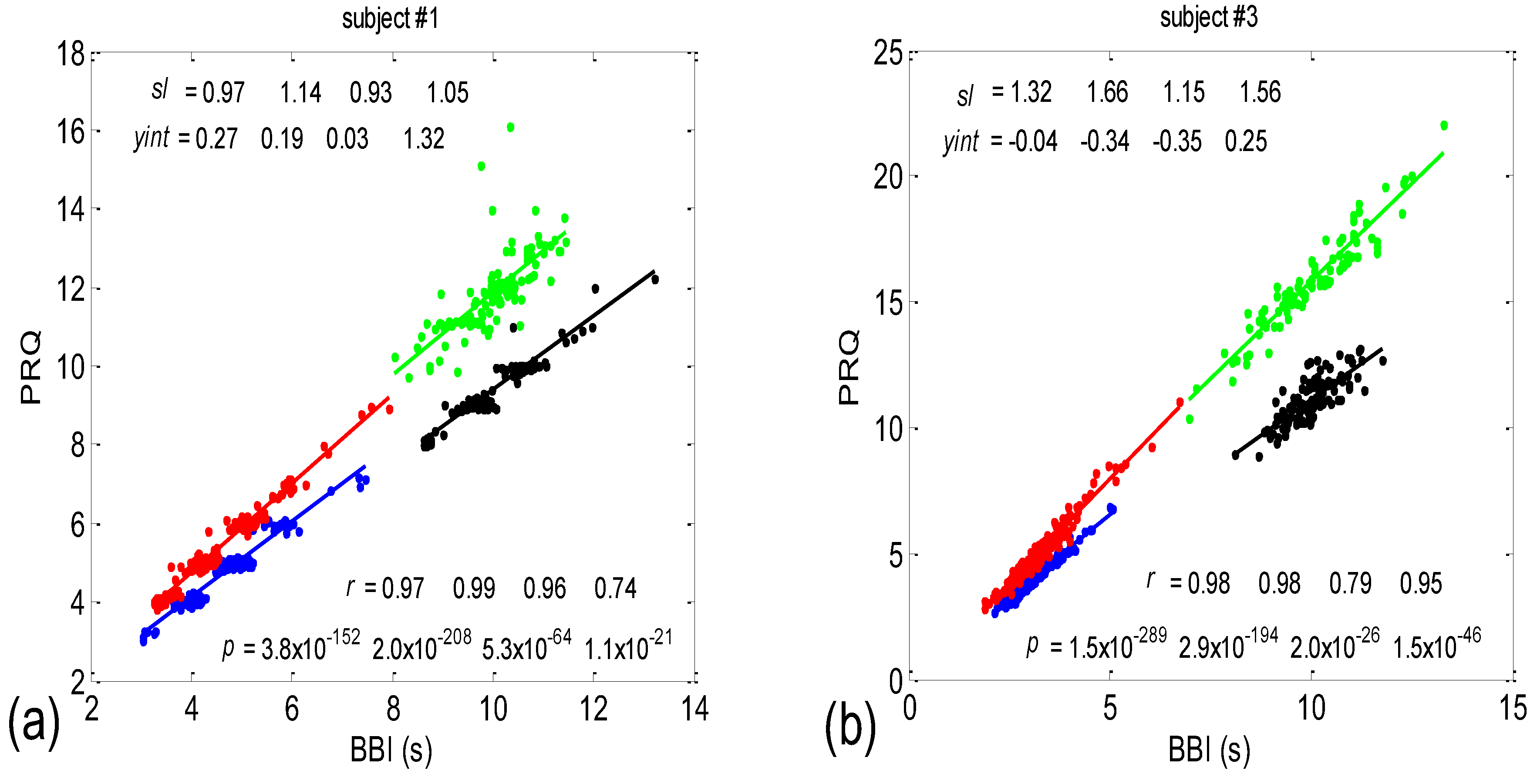
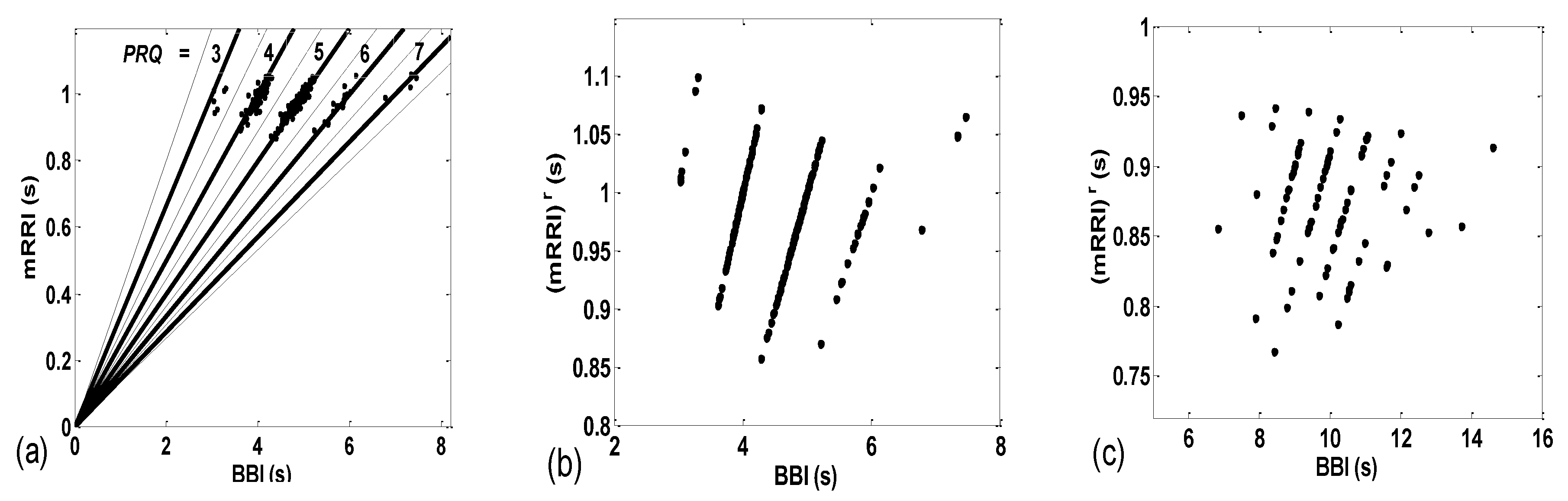
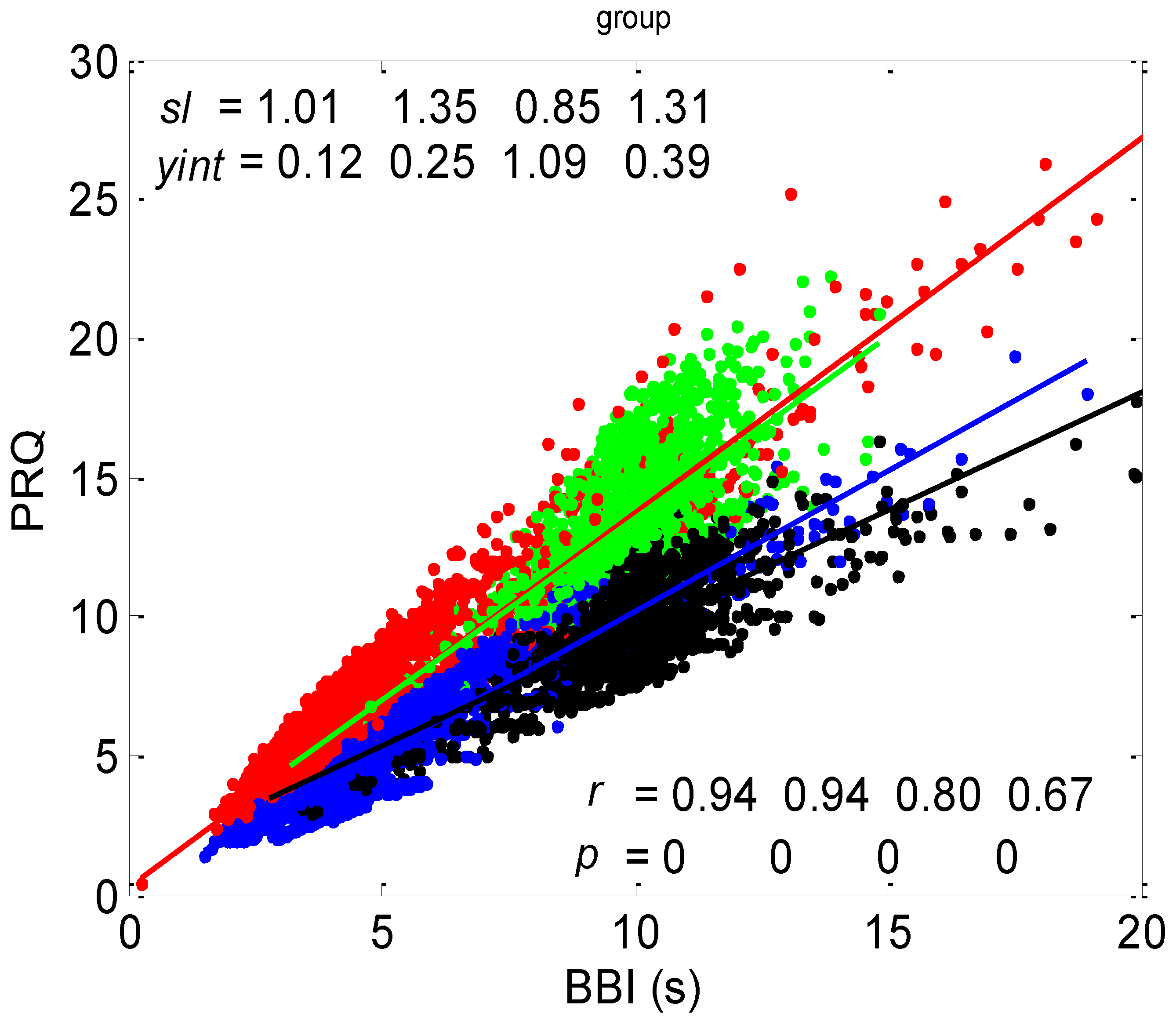
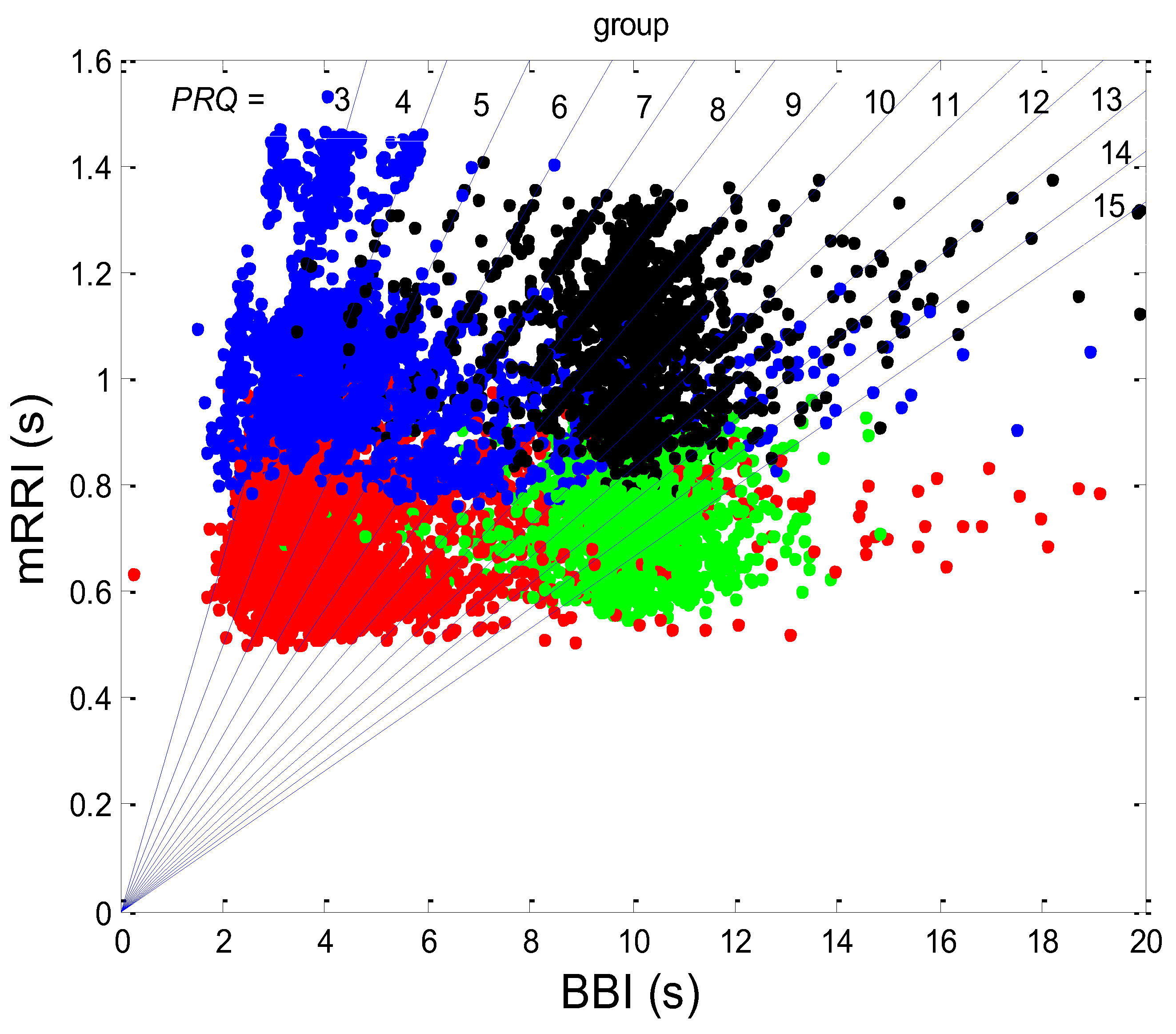
3.1. Correlations between BBI and PRQ
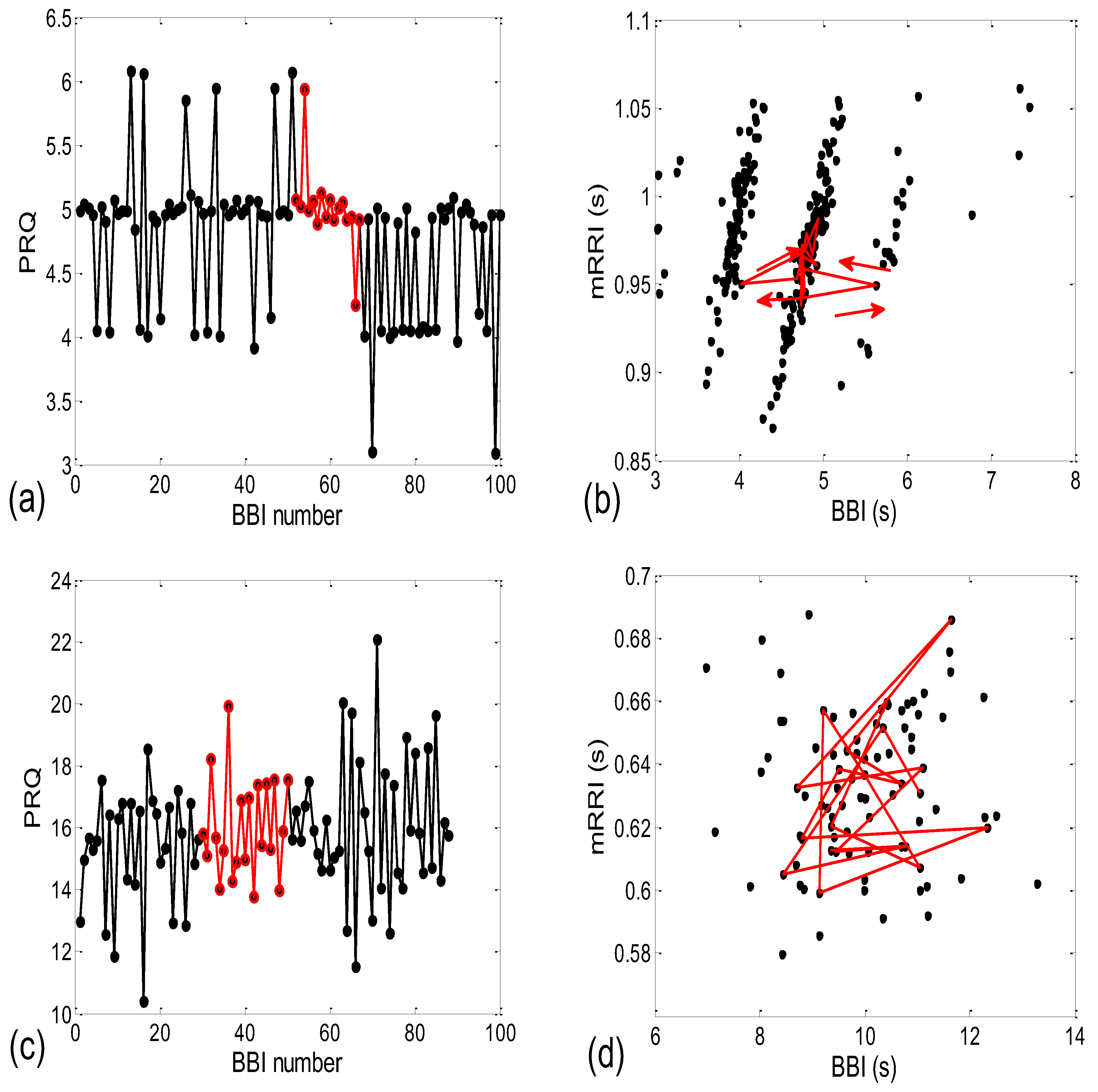
3.1.1. Positions of Points and Linear Regression Slopes
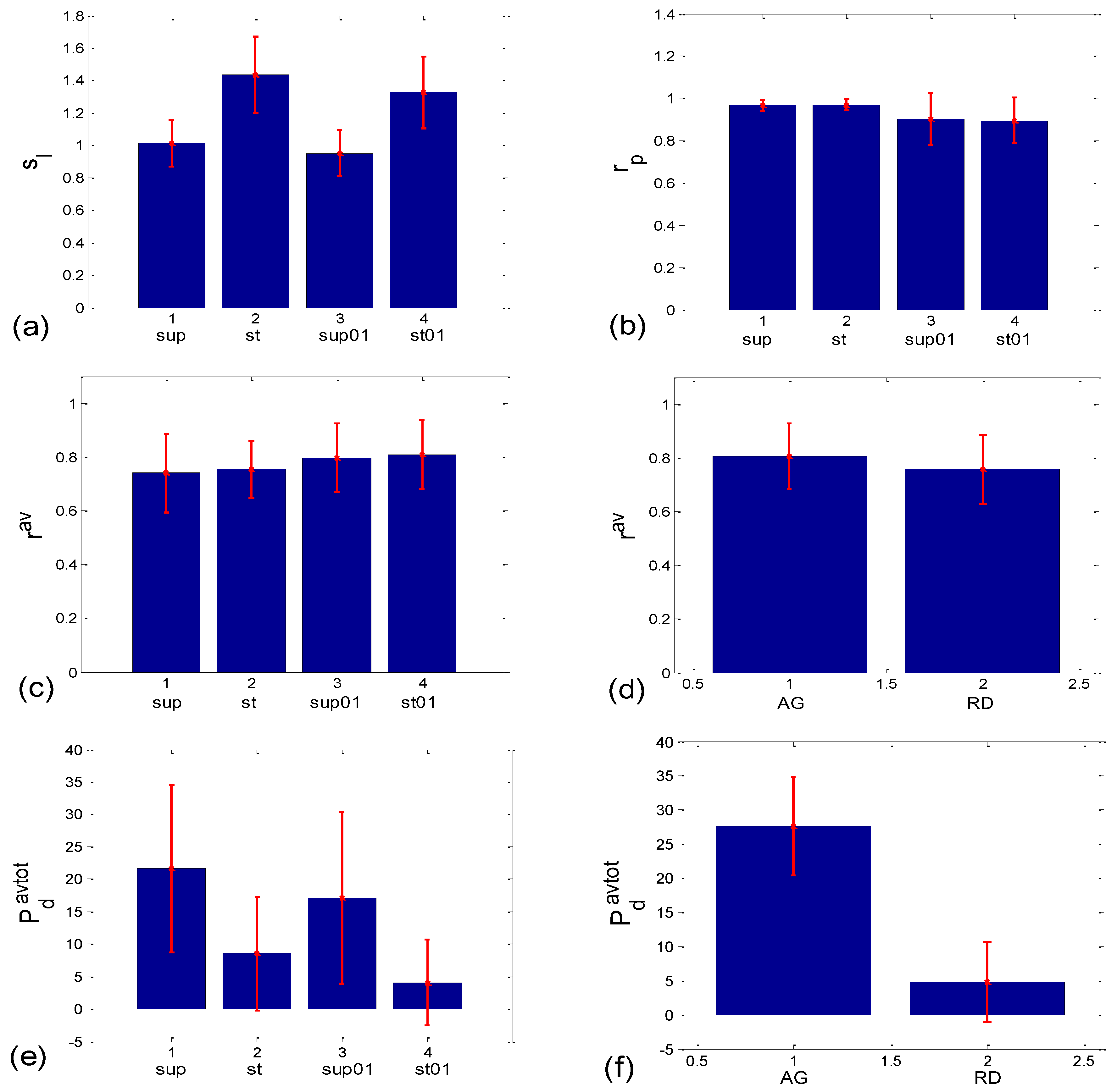
3.1.2. Statistics of Slopes and Pearson’s Coefficient
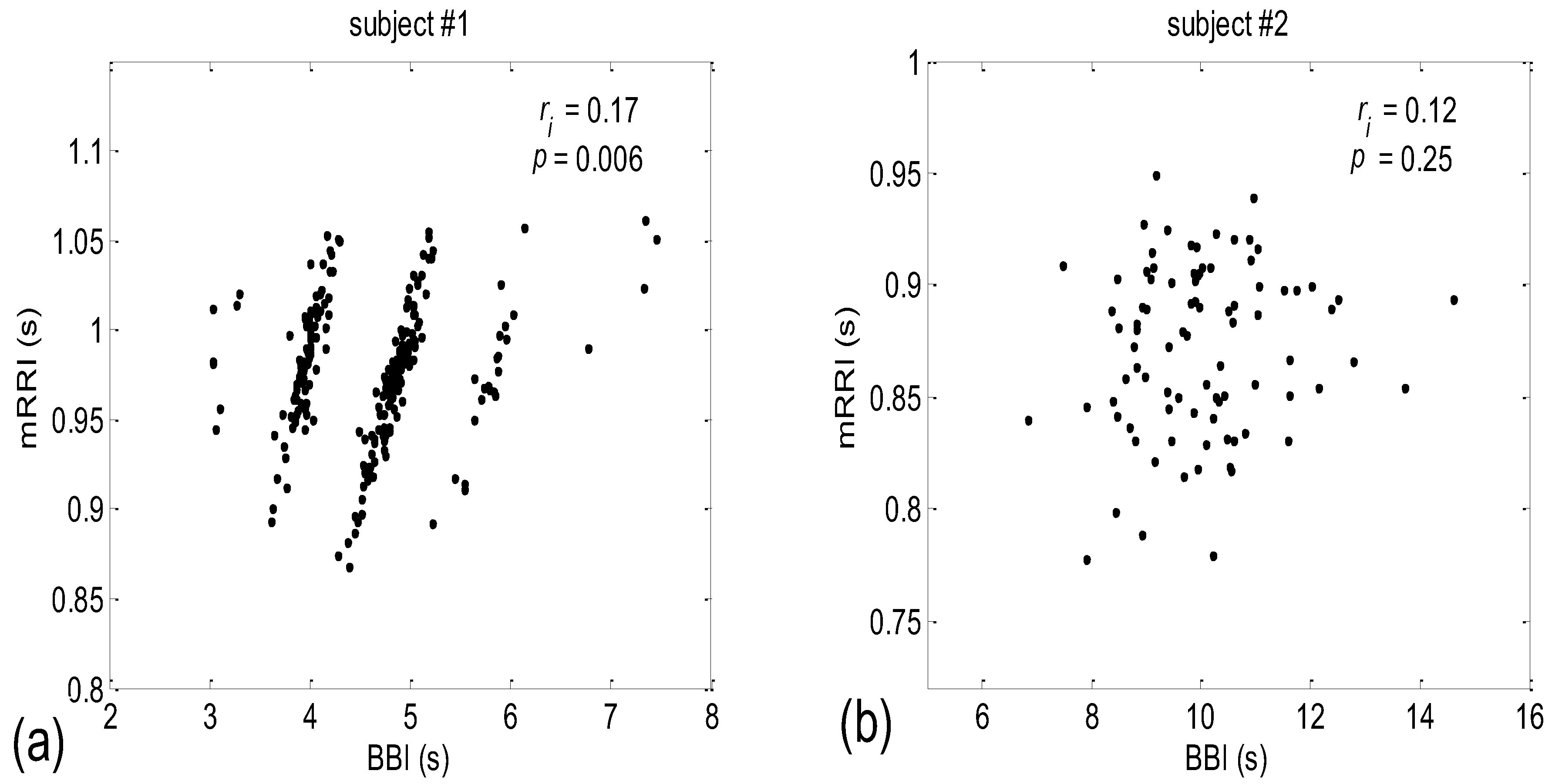
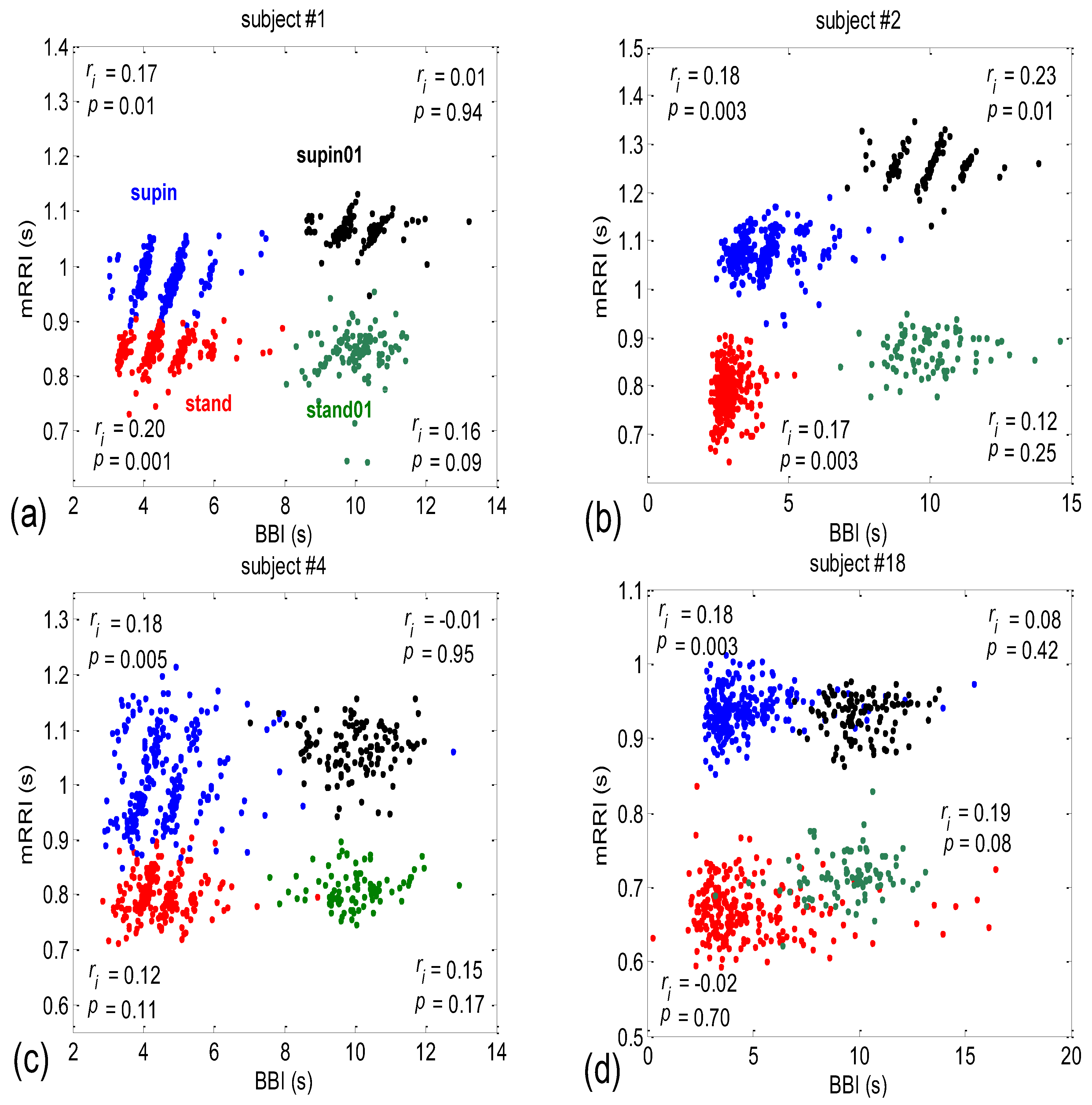
3.2. Correlations between BBI and mRRI
- Subjects where the scatter plots had visible clusters of high within-cluster correlations of BBI vs. mRRI data in all four experimental conditions (Figure 7a).
- Subjects showing scatter plots displaying high within-cluster correlations only when in a supine position, regardless of the breathing regime (Figure 7b).
- Subjects with scatter plots displaying high within-cluster correlations, but only in the regime of spontaneous breathing, regardless of the body posture (Figure 7c).
- Subjects showing scatter plots without any clustering of the data, regardless of the body posture or breathing regime (Figure 7d).
- Subjects that had scatter plots displaying high within-cluster correlations, seen only in one of the four experimental conditions or other combinations of clustering cases (not shown).
3.2.1. Dynamic Behavior of (BBI and mRRI) Points
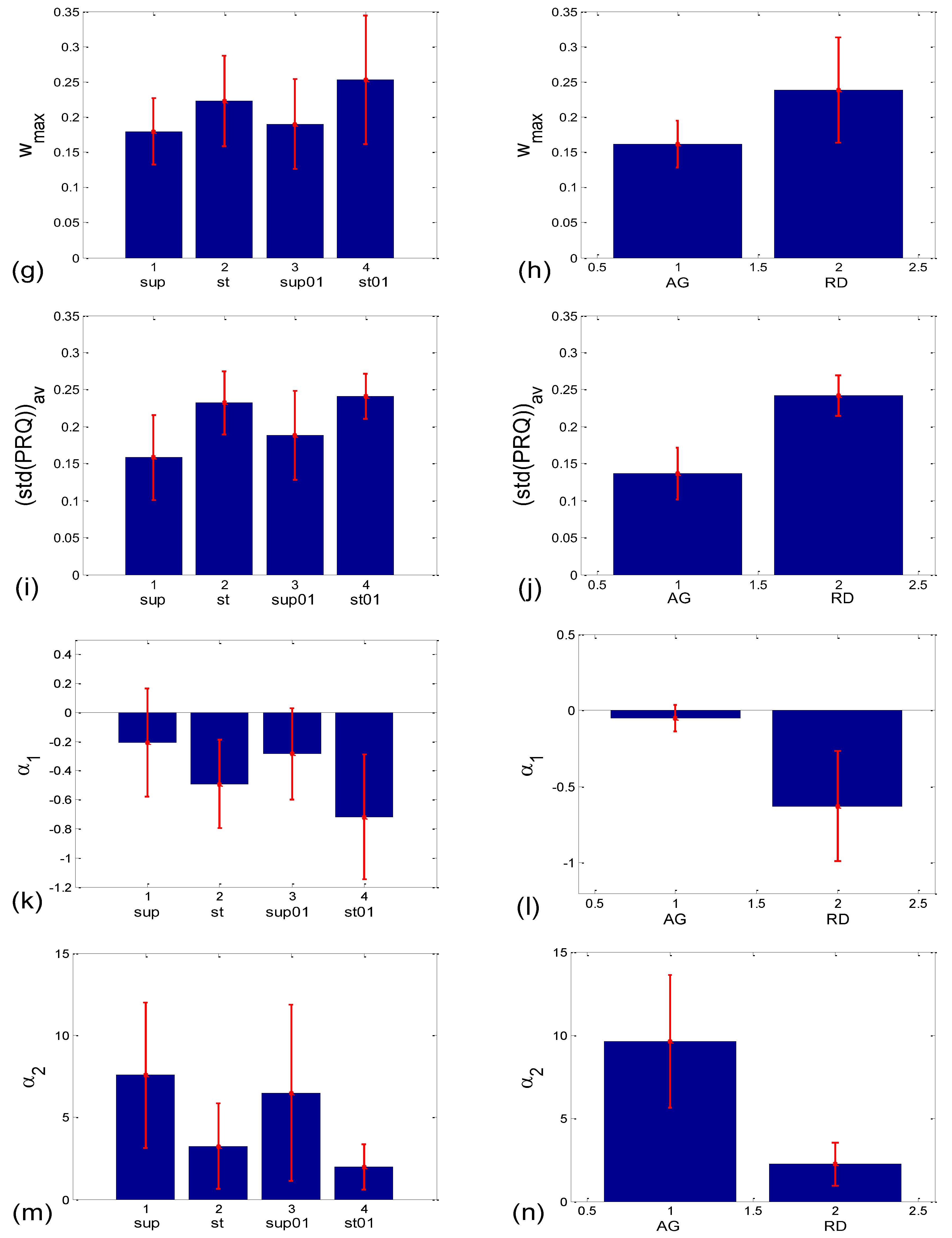
3.2.2. Statistics of Aggregation Measures
3.3. Cardio-Respiratory Synchronization
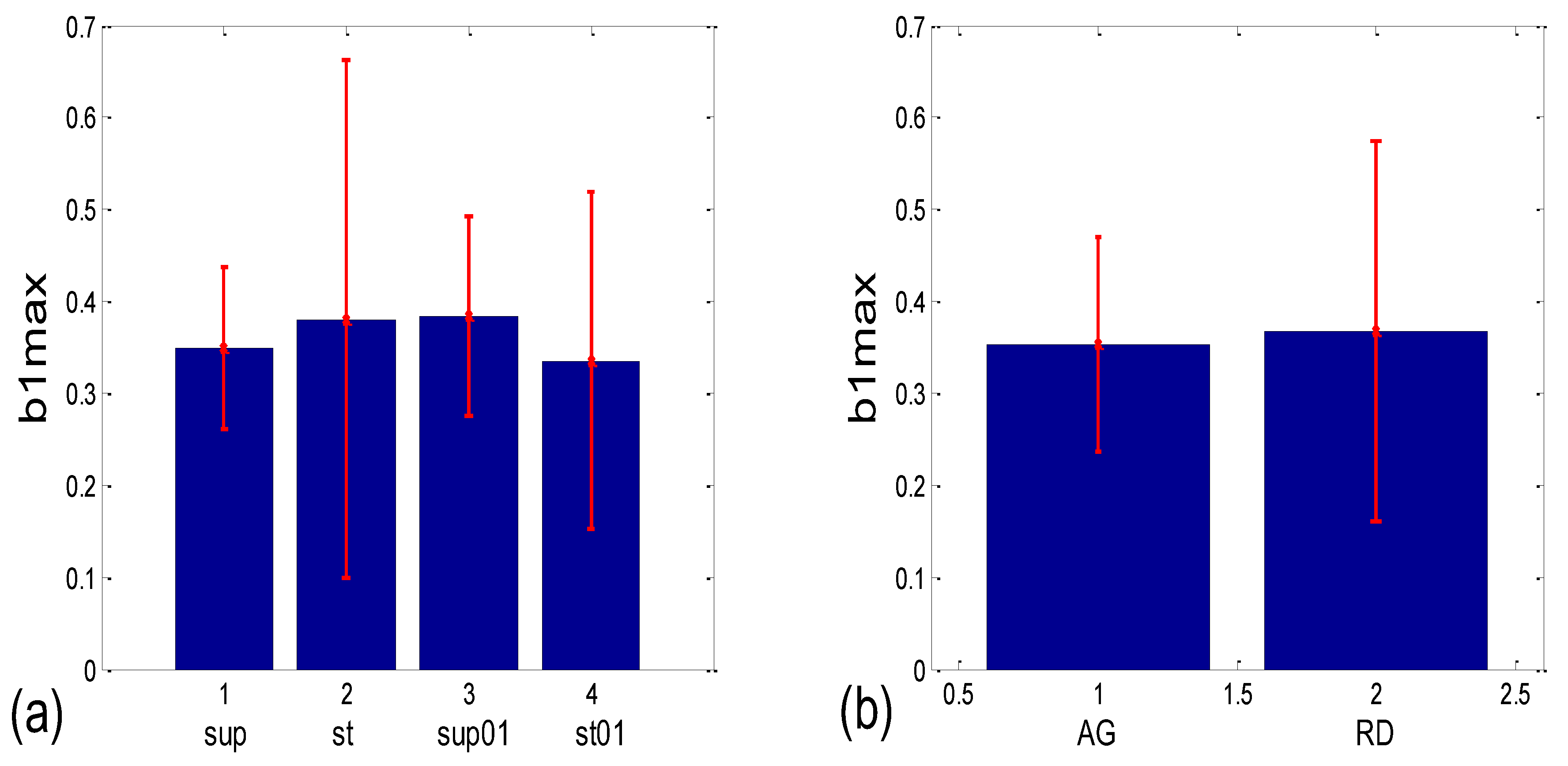
3.3.1. b1 Averaging
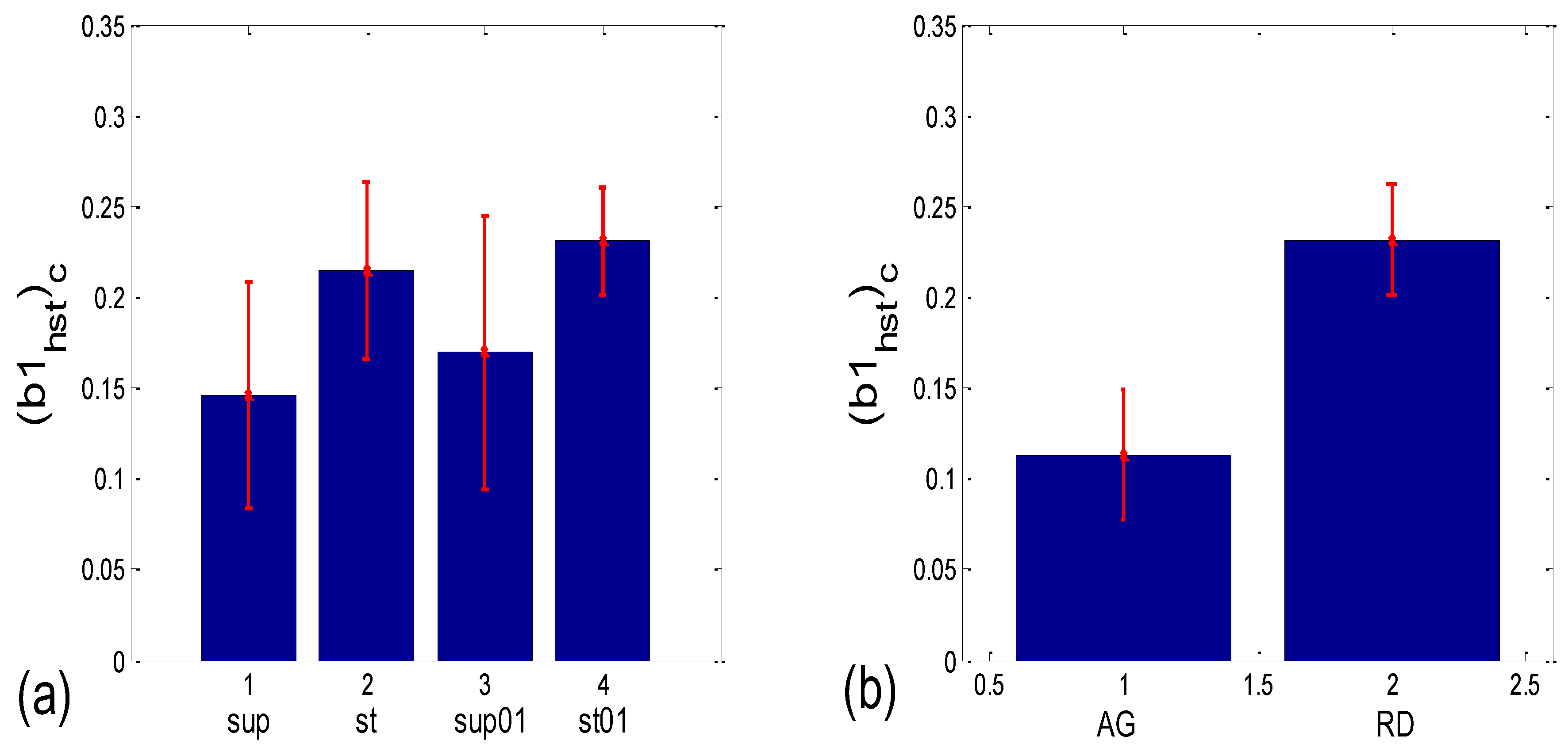
3.3.2. b1 Locking and Circular Correction
| Corr Type | Measure | Friedman ANOVA | Post hoc W Sup-St | Post hoc W Sup- Sup01 | Post hoc W Sup- St01 | Post hoc W St- Sup01 | Post hoc W St-St01 | Post hoc W Sup01- St01 | MWU AG vs. RD |
|---|---|---|---|---|---|---|---|---|---|
| BBI vs. PRQ | sl | 0.000 | 0.000 | 0.021 | 0.000 | 0.000 | 0.002 | 0.000 | |
| rp | 0.000 | 0.351 | 0.023 | 0.001 | 0.015 | 0.001 | 0.970 | ||
| BBI vs. mRRI | rav | 0.323 | 0.085 | ||||||
| Pdavtot | 0.000 | 0.002 | 0.067 | 0.000 | 0.028 | 0.037 | 0.000 | 0.000 | |
| wmax | 0.005 | 0.027 | 0.644 | 0.001 | 0.171 | 0.380 | 0.011 | 0.000 | |
| (std(PRQ))av | 0.000 | 0.000 | 0.010 | 0.000 | 0.021 | 0.332 | 0.004 | 0.000 | |
| α1 | 0.000 | 0.010 | 0.044 | 0.000 | 0.086 | 0.145 | 0.000 | 0.000 | |
| α2 | 0.000 | 0.001 | 0.117 | 0.000 | 0.044 | 0.052 | 0.000 | 0.000 | |
| b1 synch. | (b1a)g * | 0.001 | 0.204 | 0.191 | 0.052 | 0.145 | 0.279 | 0.019 | 0.069 |
| (b1hst)c | 0.004 | 0.001 | 0.048 | 0.000 | 0.062 | 0.117 | 0.010 | 0.000 |
4. Discussion
4.1. Correlation between BBI and PRQ
4.2. Correlations between BBI and mRRI
4.3. CR Synchronization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schäfer, C.; Rosenblum, M.G.; Abel, H.H.; Kurths, J. Synchronization in the human cardiorespiratory system. Phys. Rev. E 1999, 60, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Rosenblum, M.G.; Kurths, J.; Abel, H.H. Heartbeat synchronized with ventilation. Nature 1998, 392, 239–240. [Google Scholar] [CrossRef]
- Kotani, K.; Takamasu, K.; Ashkenazy, Y.; Stanley, H.E.; Yamamoto, Y. Model for cardiorespiratory synchronization in humans. Phys. Rev. E 2002, 65, 051923. [Google Scholar] [CrossRef] [PubMed]
- Cysarz, D.; Büssing, A. Cardiorespiratory synchronization during Zen meditation. Eur. J. Appl. Physiol. 2005, 95, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Kantelhardt, J.W.; Penzel, T.; Havlin, S. Experimental evidence for phase synchronization transitions in the human cardiorespiratory system. Phys. Rev. Lett. 2007, 98, 054102. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskii, V.M.; Polischuk, L.V. Cardiorespiratory synchronism in estimation of regulatory and adaptive organism status. J. Integr. Neurosci. 2016, 15, 19–35. [Google Scholar] [CrossRef]
- Sobiech, T.; Buchner, T.; Krzesiński, P.; Gielerak, G. Cardiorespiratory coupling in young healthy subjects. Phys. Meas. 2017, 38, 2186. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, J. Increased cardiorespiratory synchronization evoked by a breath controller based on heartbeat detection. Biomed. Eng. Online 2019, 18, 61. [Google Scholar] [CrossRef]
- Moser, M.; Voica, M.; Kenner, T.; Lehofer, M.; Egner, S.; Hildebrandt, G. Phase- and Frequency Coordination of Cardiac and Respiratory Function. Biol. Rhythm Res. 1995, 26, 100–111. [Google Scholar] [CrossRef]
- Friedrich, E.; Hak, F.; Kleinrath, U.; Lühr, B.; Matthiessen, P.F.; Weinzirl, J.; Cysarz, D. Impact of Colored Light on Cardiorespiratory Coordination. Evid. Based Complement. Altern. Med. 2013, 2013, 810876. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, U. The Pulse-Respiration Quotient: A Powerful but Untapped Parameter for Modern Studies about Human Physiology and Pathophysiology. Front. Phys. 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Zohdi, H.; Wolf, U. The Resting-State Pulse-Respiration Quotient of Humans: Lognormally Distributed and Centered around a Value of Four. Phys. Res. 2019, 68, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, H.; Scholkmann, F.; Wolf, W. Frontal Cerebral Oxygenation Asymmetry: Intersubject Variability and Dependence on Systemic Physiology, Season, and Time of Day. Neurophotonics 2020, 7, 025006. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Frühwirth, M.; Lackner, H.K.; Goswami, N.; Köstenberger, M.; Likar, R.; Moser, M. Cardiorespiratory Interaction and Autonomic Sleep Quality Improve during Sleep in Beds Made from Pinus cembra (Stone Pine) Solid Wood. Int. J. Environ. Res. Public Health 2021, 18, 9749. [Google Scholar] [CrossRef]
- Ren, Y.; Syrnyk, B.; Avadhanam, N. Improving video-based heart rate and respiratory rate estimation via pulse-respiration quotient. In Proceedings of the 1st Workshop on Healthcare AI and COVID-19, ICML 2022, Baltimore, MD, USA, 22 July 2022; pp. 136–145. [Google Scholar]
- Matić, Z.A.; Kalauzi, A.; Moser, M.; Platiša, M.M.; Lazarević, M.; Bojić, T. Pulse respiration quotient as a measure sensitive to changes in dynamic behavior of cardiorespiratory coupling such as body posture and breathing regime. Front. Physiol. 2022, 13, 946613. [Google Scholar] [CrossRef]
- Matić, Z.; Platiša, M.M.; Kalauzi, A.; Bojić, T. Slow 0.1 Hz breathing and body posture induced perturbations of RRI and respiratory signal complexity and cardiorespiratory coupling. Front. Physiol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Kuhnhold, A.; Schumann, A.Y.; Bartsch, R.P.; Ubrich, R.; Barthel, P.; Schmidt, G.; Kantelhardt, J.W. Quantifying cardio-respiratory phase synchronization—A comparison of five methods using ECGs of post-infarction patients. Physiol. Meas. 2017, 38, 925. [Google Scholar] [CrossRef]
- Rosenblum, M.G.; Pikovsky, A.S.; Kurths, J. Phase synchronization of chaotic oscillators. Phys. Rev. Lett. 1996, 76, 1804. [Google Scholar] [CrossRef]
- Rosenblum, M.G.; Kurths, J. Analysing synchronization phenomena from bivariate data by means of the Hilbert transform. In Nonlinear Analysis of Physiological Data; Kantz, H., Kurths, J., Mayer-Kress, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 91–99. [Google Scholar]
- Rosenblum, M.G.; Cimponeriu, L.; Bezerianos, A.; Patzak, A.; Mrowka, R. Identification of coupling direction: Application to cardiorespiratory interaction. Phys. Rev. E 2002, 65, 041909. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Wu, H.; Chai, X.; Wang, W.; Peng, C.K. Effects of slow and regular breathing exercise on cardiopulmonary coupling and blood pressure. Med. Biol. Eng. Comput. 2017, 55, 327–341. [Google Scholar] [CrossRef]
- Kalauzi, A.; Vuckovic, A.; Bojić, T. EEG alpha phase shifts during transition from wakefulness to drowsiness. Int. J. Psychophysiol. 2012, 86, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Berne, R.M.; Levy, M.N. Principles of Physiology; Mosby-Year Book, Inc.: St. Louis, MO, USA, 1996. [Google Scholar]
- Goldberger, A.L. Complex systems. Proc. Am. Thorac. Soc. 2006, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology (Guyton Physiology), 14th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Levy, M.N.; Martin, P.J. Autonomic control of cardiac conduction and automaticity. In Nervous Control of the Heart; Shepherd, J.T., Vatner, S.F., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1996; pp. 201–223. [Google Scholar]
- Koeppen, B.M.; Stanton, B.A. Berne and Levy Physiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gellhorn, E. The emotions and the ergotropic and trophotropic systems. Psychol. Forsch. 1970, 34, 48–66. [Google Scholar] [CrossRef]
- Feldman, J.L.; Ellenberger, H.H. Central coordination of respiratory and cardiovascular control in mammals. Annu. Rev. Physiol. 1988, 50, 593–606. [Google Scholar] [CrossRef]
- Porges, S.W. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Fruehwirth, M.; Penter, R.; Winker, R. Why Life Oscillates—From a topographical towards a functional chronobiology. Cancer Causes Control 2006, 17, 591–599. [Google Scholar] [CrossRef]
- Cotuk, H.B.; Müller, G.; Pelz, H.; Duru, A.D.; Perlitz, V. Synergetic brainstem consensualization at the 0.15 Hz intermediary rhythm is the genuine marker of the trophotropic state. In Selbstorganisation–ein Paradigma für die Humanwissenschaften; Springer: Wiesbaden, Germany, 2020; pp. 389–402. [Google Scholar]
- Bojić, T.; Matić, Z.; Platiša, M.; Kalauzi, A. Fractal angle modality patterns in RRI and respiration signal of healthy subjects responding to orthostasis and slow breathing. In Proceedings of the 12th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Štrbske Pleso, Slovakia, 9–12 October 2022. [Google Scholar] [CrossRef]
- Orr, W.C.; Stahl, M.L.; Whitsett, T.; Langevin, E. Physiological sleep patterns and cardiac arrhythmias. Am. Heart J. 1979, 97, 128–129. [Google Scholar] [CrossRef]
- Verrier, R.L.; Harper, R.M. Cardiovascular physiology: Central and autonomic regulation. In Principles and Practice of Sleep Medicine; Kryger, M.H., Roth, T., Dement, W.C., Eds.; W.B. Sounders Company: New York, NY, USA, 2000; pp. 179–191. [Google Scholar]
- Bojić, T. Mechanisms of Neural Control and Effects of Acoustic Stimulation on Cardiovascular System During the Wake-Sleep Cycle. Ph.D. Dissertation, Alma Mater Università di Bologna, Bologna, Italy, 2003. [Google Scholar]
- Bojić, T. Insomnia as a risk factor for cardiocerebrovascular disorders. Engrami 2014, 36, 31–34. (In Serbian) [Google Scholar] [CrossRef]
- Bojić, T. Editorial: Neurocardiovascular diseases: New aspects of the old issues. Front. Neurosci. 2018, 12, 1032. [Google Scholar] [CrossRef]
- Bojić, T.; Matić, Z.; Stojković, M.; Platiša, M.M.; Kalauzi, A.; Lazarević, M.; Moser, M. Cardiorespiratory Coupling is influenced by Body Position and Slow Paced 0.1Hz Breathing in a State Specific Manner. In Proceedings of the FENS 2020 Virtual Forum. Federation of European Neuroscience Societies (FENS) and British Neuroscience Association (BNA), Glasgow, UK, 11–15 July 2020; Available online: https://cslide.ctimeetingtech.com/fens2020/attendee/eposter/poster/5958?r=snm%7E571 (accessed on 23 January 2023).
- Porta, A.; Bassani, T.; Bari, V.; Tobaldini, E.; Takahashi, A.C.; Catai, A.M.; Montano, N. Model-based assessment of baroreflex and cardiopulmonary couplings during graded head-up tilt. Comput. Biol. Med. 2012, 42, 298–305. [Google Scholar] [CrossRef]
- Boyette, L.C.; Manna, B. Physiology, Myocardial Oxygen Demand. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Porta, A.; Faes, L. Wiener–Granger causality in network physiology with applications to cardiovascular control and neuroscience. Proc. IEEE 2015, 104, 282–309. [Google Scholar] [CrossRef]
- Radovanović, N.N.; Pavlović, S.U.; Milašinović, G.; Kirćanski, B.; Platiša, M.M. Bidirectional Cardio-Respiratory Interactions in Heart Failure. Front. Physiol. 2018, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.; Pikovsky, A. Nonlinear phase coupling functions: A numerical study. Philos. Trans. A Math. Phys. Eng. Sci. 2019, 377, 20190093. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Adochiei, F.-C.; Edu, I.-R.; Schroeder, R.; Costin, H.; Bär, K.-J.; Voss, A. Cardiovascular and cardiorespiratory coupling analyses: A review. Phil. Trans. R. Soc. A 2013, 371, 20120191. [Google Scholar] [CrossRef]
- Porta, A.; Baselli, G.; Lombardi, F.; Montano, N.; Malliani, A.; Cerutti, S. Conditional entropy approach for the evaluation of the coupling strength. Biol. Cybern. 1999, 81, 119–129. [Google Scholar] [CrossRef]
- Lotrič, M.B.; Stefanovska, A. Synchronization and modulation in the human cardiorespiratory system. Phys. A Stat. Mech. Its Appl. 2000, 283, 451–461. [Google Scholar] [CrossRef]
- Porta, A.; Maestri, R.; Bari, V.; De Maria, B.; Cairo, B.; Vaini, E.; La Rovere, M.T.; Pinna, G.D. Paced breathing increases the redundancy of cardiorespiratory control in healthy individuals and chronic heart failure patients. Entropy 2018, 20, 949. [Google Scholar] [CrossRef]
- Porta, A.; Castiglioni, P.; Di Rienzo, M.; Bassani, T.; Bari, V.; Faes, L.; Nollo, G.; Cividjan, A.; Quintin, L. Cardiovascular control and time domain Granger causality: Insights from selective autonomic blockade. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20120161. [Google Scholar] [CrossRef]
- Cairo, B.; de Abreu, R.M.; Bari, V.; Gelpi, F.; De Maria, B.; Rehder-Santos, P.; Sakaguchi, C.A.; da Silva, C.D.; De Favari Signini, É.; Catai, A.M.; et al. Optimizing phase variability threshold for automated synchrogram analysis of cardiorespiratory interactions in amateur cyclists. Philos. Trans. A Math. Phys. Eng. Sci. 2021, 379, 20200251. [Google Scholar] [CrossRef]
- Acampa, M.; Voss, A.; Bojić, T. Editorial: Cardiorespiratory Coupling-Novel Insights for Integrative Biomedicine. Front. Neurosci. 2021, 15, 671900. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalauzi, A.; Matić, Z.; Platiša, M.M.; Bojić, T. Two Operational Modes of Cardio-Respiratory Coupling Revealed by Pulse-Respiration Quotient. Bioengineering 2023, 10, 180. https://doi.org/10.3390/bioengineering10020180
Kalauzi A, Matić Z, Platiša MM, Bojić T. Two Operational Modes of Cardio-Respiratory Coupling Revealed by Pulse-Respiration Quotient. Bioengineering. 2023; 10(2):180. https://doi.org/10.3390/bioengineering10020180
Chicago/Turabian StyleKalauzi, Aleksandar, Zoran Matić, Mirjana M. Platiša, and Tijana Bojić. 2023. "Two Operational Modes of Cardio-Respiratory Coupling Revealed by Pulse-Respiration Quotient" Bioengineering 10, no. 2: 180. https://doi.org/10.3390/bioengineering10020180
APA StyleKalauzi, A., Matić, Z., Platiša, M. M., & Bojić, T. (2023). Two Operational Modes of Cardio-Respiratory Coupling Revealed by Pulse-Respiration Quotient. Bioengineering, 10(2), 180. https://doi.org/10.3390/bioengineering10020180








