Peptides in Dentistry: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
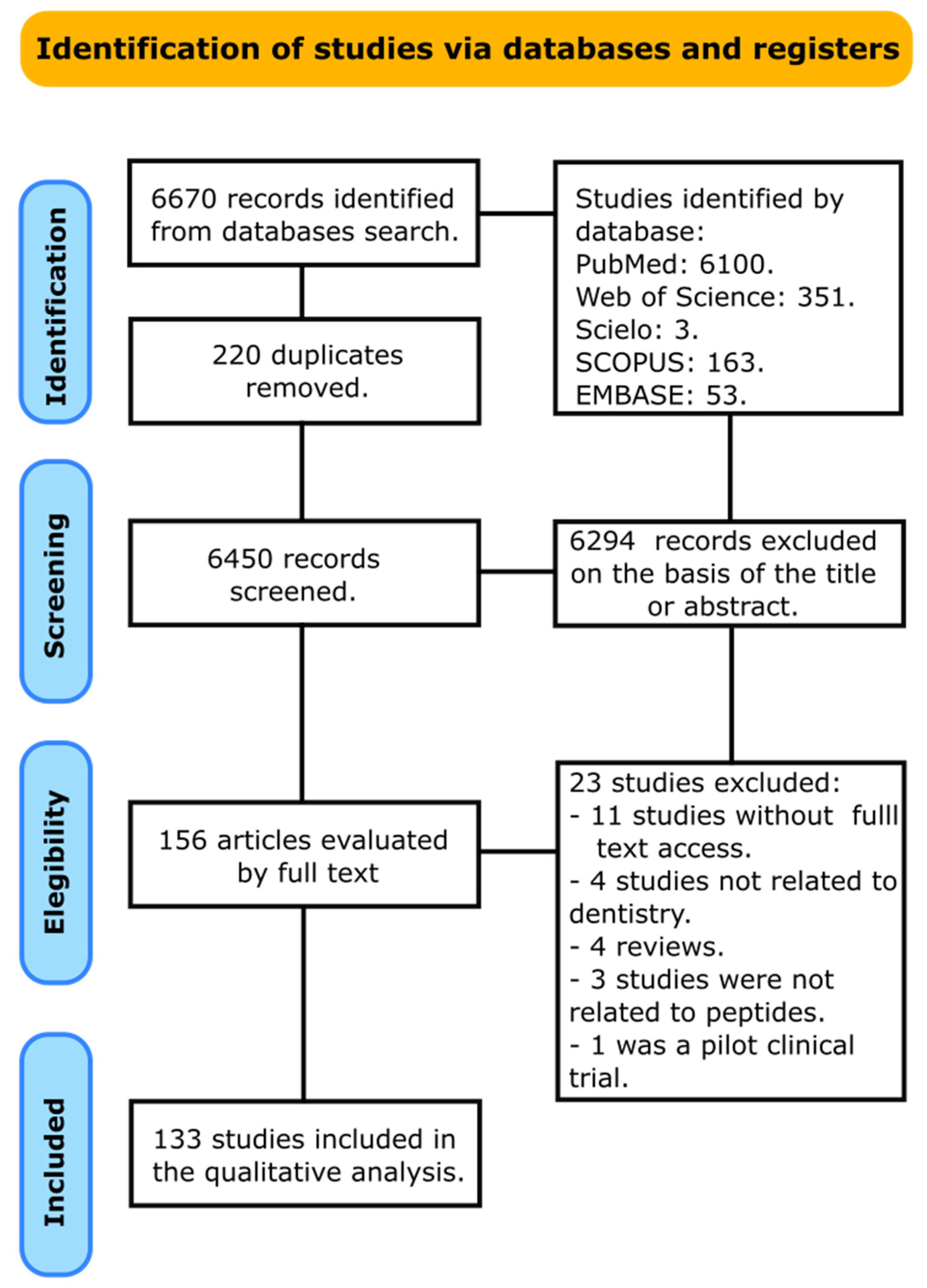
2.1. Information Sources and Search
2.2. Selection Process and Data Collection Process
3. Results
3.1. Characteristics of Studies
3.2. Synthesis of Results and Summary of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Hachem, C.E.; Chedid, J.C.A.; Nehme, W.; Kaloustian, M.K.; Ghosn, N.; Sahnouni, H.; Mancino, D.; Haikel, Y.; Kharouf, N. Physicochemical and Antibacterial Properties of Conventional and Two Premixed Root Canal Filling Materials in Primary Teeth. J. Funct. Biomater. 2022, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Pedullà, E.; La Rosa, G.R.M.; Bukiet, F.; Sauro, S.; Haikel, Y.; Mancino, D. In Vitro Evaluation of Different Irrigation Protocols on Intracanal Smear Layer Removal in Teeth with or without Pre-Endodontic Proximal Wall Restoration. J. Clin. Med. 2020, 9, 3325. [Google Scholar] [CrossRef] [PubMed]
- Malta, C.P.; Barcelos, R.C.S.; Segat, H.J.; Burger, M.E.; Bier, C.A.S.; Morgental, R.D. Toxicity of Bioceramic and Resinous Endodontic Sealers Using an Alternative Animal Model: Artemia Salina. J. Conserv. Dent. 2022, 25, 185. [Google Scholar] [CrossRef]
- Sismanoglu, S.; Ercal, P. The Cytotoxic Effects of Various Endodontic Irrigants on the Viability of Dental Mesenchymal Stem Cells. Aust. Endod. J. 2022, 48, 305–312. [Google Scholar] [CrossRef]
- Bermúdez, M.; Hoz, L.; Montoya, G.; Nidome, M.; Pérez-Soria, A.; Romo, E.; Soto-Barreras, U.; Garnica-Palazuelos, J.; Aguilar-Medina, M.; Ramos-Payán, R. Bioactive Synthetic Peptides for Oral Tissues Regeneration. Front. Mater. 2021, 8, 655495. [Google Scholar] [CrossRef]
- Lien, S.; Lowman, H.B. Therapeutic Peptides. Trends Biotechnol. 2003, 21, 556–562. [Google Scholar] [CrossRef]
- Sato, A.K.; Viswanathan, M.; Kent, R.B.; Wood, C.R. Therapeutic Peptides: Technological Advances Driving Peptides into Development. Curr. Opin. Biotechnol. 2006, 17, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic Therapeutic Peptides: Science and Market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef]
- Scavello, F.; Kharouf, N.; Lavalle, P.; Haikel, Y.; Schneider, F.; Metz-Boutigue, M.-H. The Antimicrobial Peptides Secreted by the Chromaffin Cells of the Adrenal Medulla Link the Neuroendocrine and Immune Systems: From Basic to Clinical Studies. Front. Immunol. 2022, in press. [CrossRef]
- Alkilzy, M.; Santamaria, R.; Schmoeckel, J.; Splieth, C. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv. Dent. Res. 2018, 29, 42–47. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide Therapeutics: Current Status and Future Directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141. [Google Scholar]
- Stürmer, E. Vasopressin, Oxytocin and Synthetic Analogues: The Use of Bioassays. J. Pharm. Biomed. Anal. 1989, 7, 199–210. [Google Scholar] [CrossRef]
- Dang, T.; Süssmuth, R.D. Bioactive Peptide Natural Products as Lead Structures for Medicinal Use. Acc. Chem. Res. 2017, 50, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide Chemistry Toolbox–Transforming Natural Peptides into Peptide Therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, C.; Labriola, A.; Generali, L. Dental Root Surface Treatment with Ethylenediaminetetraacetic Acid Does Not Improve Enamel Matrix Derivative Peptide Treatment within Intrabony Defects: A Retrospective Study. J. Biol. Regul. Homeost. Agents 2019, 33, 1945–1947. [Google Scholar]
- Hossein, B.G.; Sadr, A.; Espigares, J.; Hariri, I.; Nakashima, S.; Hamba, H.; Shafiei, F.; Moztarzadeh, F.; Tagami, J. Study on the Influence of Leucine-Rich Amelogenin Peptide (LRAP) on the Remineralization of Enamel Defects via Micro-Focus X-ray Computed Tomography and Nanoindentation. Biomed. Mater. 2015, 10, 035007. [Google Scholar] [CrossRef]
- Gonçalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; Pessan, J.P.; Romero, G.D.A.; Cannon, M.L.; Danelon, M. Effect of Fluoride, Casein Phosphopeptide-Amorphous Calcium Phosphate and Sodium Trimetaphosphate Combination Treatment on the Remineralization of Caries Lesions: An in Vitro Study. Arch. Oral Biol. 2021, 122, 105001. [Google Scholar] [CrossRef]
- Kim, H.; Lee, W.S.; Jeong, J.; Kim, D.S.; Lee, S.; Kim, S. Effect of Elastin-like Polypeptide Incorporation on the Adhesion Maturation of Mineral Trioxide Aggregates. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2847–2856. [Google Scholar] [CrossRef]
- Li, C.; Yang, P.; Kou, Y.; Zhang, D.; Li, M. The Polypeptide OP3-4 Induced Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells via Protein Kinase B/Glycogen Synthase Kinase 3β/β-Catenin Pathway and Promoted Mandibular Defect Bone Regeneration. Arch. Oral Biol. 2021, 130, 105243. [Google Scholar] [CrossRef]
- Minguela, J.; Müller, D.; Mücklich, F.; Llanes, L.; Ginebra, M.; Roa, J.; Mas-Moruno, C. Peptidic Biofunctionalization of Laser Patterned Dental Zirconia: A Biochemical-Topographical Approach. Mater. Sci. Eng. C 2021, 125, 112096. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.-L.; Mei, M.L.; Chu, C.H. Efficacy of the Dual-Action GA-KR12 Peptide for Remineralising Initial Enamel Caries: An in Vitro Study. Clin. Oral Investig. 2022, 26, 2441–2451. [Google Scholar] [CrossRef]
- Pietruski, J.; Pietruska, M.; Stokowska, W.; Pattarelli, G. Evaluation of Polypeptide Growth Factors in the Process of Dental Implant Osseointegration. Rocz. Akad. Med. Bialymstoku 1995 2001, 46, 19–27. [Google Scholar]
- Wang, R.; Nisar, S.; Vogel, Z.; Liu, H.; Wang, Y. Dentin Collagen Denaturation Status Assessed by Collagen Hybridizing Peptide and Its Effect on Bio-Stabilization of Proanthocyanidins. Dent. Mater. 2022, 38, 748–758. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kojima, T.; Kanekawa, M.; Aihara, N.; Nogimura, A.; Kasai, K. Neuropeptides Stimulate Production of Interleukin-1β, Interleukin-6, and Tumor Necrosis Factor-α in Human Dental Pulp Cells. Inflamm. Res. 2004, 53, 199–204. [Google Scholar] [CrossRef]
- Park, C.; Song, M.; Kim, S.; Min, B. Vitronectin-Derived Peptide Promotes Reparative Dentin Formation. J. Dent. Res. 2022, 101, 1481–1489. [Google Scholar] [CrossRef]
- Yaguchi, A.; Hiramatsu, H.; Ishida, A.; Oshikawa, M.; Ajioka, I.; Muraoka, T. Hydrogel-Stiffening and Non-Cell Adhesive Properties of Amphiphilic Peptides with Central Alkylene Chains. Chem. Eur. J. 2021, 27, 9295–9301. [Google Scholar] [CrossRef]
- Xie, S.; Allington, R.W.; Svec, F.; Fréchet, J.M. Rapid Reversed-Phase Separation of Proteins and Peptides Using Optimized ‘Moulded’Monolithic Poly (Styrene–Co-Divinylbenzene) Columns. J. Chromatogr. A 1999, 865, 169–174. [Google Scholar] [CrossRef]
- Pastor, J.J.; Fernández, I.; Rabanal, F.; Giralt, E. A New Method for the Preparation of Unprotected Peptides on Biocompatible Resins with Application in Combinatorial Chemistry. Org. Lett. 2002, 4, 3831–3833. [Google Scholar] [CrossRef]
- Mizuno, M.; Goto, K.; Miura, T.; Hosaka, D.; Inazu, T. A Novel Peptide Synthesis Using Fluorous Chemistry. Chem. Commun. 2003, 8, 972–973. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral Antimicrobial Peptides: Types and Role in the Oral Cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef]
- Park, S.-C.; Park, Y.; Hahm, K.-S. The Role of Antimicrobial Peptides in Preventing Multidrug-Resistant Bacterial Infections and Biofilm Formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef]
- Li, C.; Bhatt, P.; Johnston, T. Evaluation of a Mucoadhesive Buccal Patch for Delivery of Peptides: In Vitro Screening of Bioadhesion. Drug Dev. Ind. Pharm. 1998, 24, 919–926. [Google Scholar] [CrossRef]
- Kokkonen, H.; Cassinelli, C.; Verhoef, R.; Morra, M.; Schols, H.; Tuukkanen, J. Differentiation of Osteoblasts on Pectin-Coated Titanium. Biomacromolecules 2008, 9, 2369–2376. [Google Scholar] [CrossRef]
- Glimcher, M.; Levine, P. Studies of the Proteins, Peptides and Free Amino Acids of Mature Bovine Enamel. Biochem. J. 1966, 98, 742. [Google Scholar] [CrossRef]
- Takayama, S.; Kato, T.; Imamura, K.; Kita, D.; Ota, K.; Suzuki, E.; Sugito, H.; Saitoh, E.; Taniguchi, M.; Saito, A. Effect of a Mouthrinse Containing Rice Peptide CL (14-25) on Early Dental Plaque Regrowth: A Randomized Crossover Pilot Study. BMC Res. Notes 2015, 8, 531. [Google Scholar] [CrossRef]
- Bagno, A.; Piovan, A.; Dettin, M.; Chiarion, A.; Brun, P.; Gambaretto, R.; Fontana, G.; Di Bello, C.; Palù, G.; Castagliuolo, I. Human Osteoblast-like Cell Adhesion on Titanium Substrates Covalently Functionalized with Synthetic Peptides. Bone 2007, 40, 693–699. [Google Scholar] [CrossRef]
- Artzi, Z.; Weinreb, M.; Tal, H.; Nemcovsky, C.E.; Rohrer, M.D.; Prasad, H.S.; Kozlovsky, A. Experimental Intrabony and Periodontal Defects Treated with Natural Mineral Combined With a Synthetic Cell-Binding Peptide in the Canine: Morphometric Evaluations. J. Periodontol. 2006, 77, 1658–1664. [Google Scholar] [CrossRef]
- Bröseler, F.; Tietmann, C.; Bommer, C.; Drechsel, T.; Heinzel-Gutenbrunner, M.; Jepsen, S. Randomised Clinical Trial Investigating Self-Assembling Peptide P11-4 in the Treatment of Early Caries. Clin. Oral Investig. 2020, 24, 123–132. [Google Scholar] [CrossRef]
- Butz, F.; Bächle, M.; Ofer, M.; Marquardt, K.; Kohal, R.J. Sinus Augmentation with Bovine Hydroxyapatite/Synthetic Peptide in a Sodium Hyaluronate Carrier (PepGen P-15 Putty): A Clinical Investigation of Different Healing Times. Int. J. Oral Maxillofac. Implant. 2011, 26, 1317–1323. [Google Scholar]
- Chung, H.-Y.; Huang, K.-C. Effects of Peptide Concentration on Remineralization of Eroded Enamel. J. Mech. Behav. Biomed. Mater. 2013, 28, 213–221. [Google Scholar] [CrossRef]
- Altankhishig, B.; Polan, M.A.A.; Qiu, Y.; Hasan, M.R.; Saito, T. Dentin Phosphophoryn-Derived Peptide Promotes Odontoblast Differentiation In Vitro and Dentin Regeneration In Vivo. Materials 2021, 14, 874. [Google Scholar] [CrossRef] [PubMed]
- Afami, M.E.; El Karim, I.; About, I.; Coulter, S.M.; Laverty, G.; Lundy, F.T. Ultrashort Peptide Hydrogels Display Antimicrobial Activity and Enhance Angiogenic Growth Factor Release by Dental Pulp Stem/Stromal Cells. Materials 2021, 14, 2237. [Google Scholar] [CrossRef]
- Babaji, P.; Melkundi, M.; Bhagwat, P.; Mehta, V. An in Vitro Evaluation of Remineralizing Capacity of Self-Assembling Peptide (SAP) P11-4 and Casein Phosphopeptides-Amorphous Calcium Phosphate (CPP-ACP) on Artificial Enamel. Pesqui. Bras. Odontopediatr. Clín. Integr. 2019, 19, e4504. [Google Scholar] [CrossRef]
- Dettin, M.; Conconi, M.T.; Gambaretto, R.; Pasquato, A.; Folin, M.; Di Bello, C.; Parnigotto, P.P. Novel Osteoblast-adhesive Peptides for Dental/Orthopedic Biomaterials. J. Biomed. Mater. Res. 2002, 60, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Cirera, A.; Manzanares, M.C.; Sevilla, P.; Ortiz-Hernandez, M.; Galindo-Moreno, P.; Gil, J. Biofunctionalization with a TGFβ-1 Inhibitor Peptide in the Osseointegration of Synthetic Bone Grafts: An in Vivo Study in Beagle Dogs. Materials 2019, 12, 3168. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized Nanofiber Segments Coupled with Calcium-Binding BMP-2 Peptides for Alveolar Bone Regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Liu, S.; Wu, R.; Aparicio, C.; Wu, J. In Vivo Osseointegration of Dental Implants with an Antimicrobial Peptide Coating. J. Mater. Sci. Mater. Med. 2017, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Aref, N.S.; Alrasheed, M.K. Casein Phosphopeptide Amorphous Calcium Phosphate and Universal Adhesive Resin as a Complementary Approach for Management of White Spot Lesions: An In-Vitro Study. Prog. Orthod. 2022, 23, 10. [Google Scholar] [CrossRef]
- Aruna, G. Estimation of N-Terminal Telopeptides of Type I Collagen in Periodontal Health, Disease and after Nonsurgical Periodontal Therapy in Gingival Crevicular Fluid: A Clinico-Biochemical Study. Indian J. Dent. Res. 2015, 26, 152. [Google Scholar] [CrossRef]
- Brunton, P.; Davies, R.; Burke, J.; Smith, A.; Aggeli, A.; Brookes, S.; Kirkham, J. Treatment of Early Caries Lesions Using Biomimetic Self-Assembling Peptides—A Clinical Safety Trial. Br. Dent. J. 2013, 215, E6. [Google Scholar] [CrossRef]
- Fang, D.; Yuran, S.; Reches, M.; Catunda, R.; Levin, L.; Febbraio, M. A Peptide Coating Preventing the Attachment of Porphyromonas Gingivalis on the Surfaces of Dental Implants. J. Periodontal Res. 2020, 55, 503–510. [Google Scholar] [CrossRef]
- Goldberg, A.; Advincula, M.; Komabayashi, T.; Patel, P.; Mather, P.; Goberman, D.; Kazemi, R. Polypeptide-Catalyzed Biosilicification of Dentin Surfaces. J. Dent. Res. 2009, 88, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Donos, N. Differential Effect of Amelogenin Peptides on Osteogenic Differentiation in Vitro: Identification of Possible New Drugs for Bone Repair and Regeneration. Tissue Eng. Part A 2012, 18, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial Properties of HLf1–11 Peptide onto Titanium Surfaces: A Comparison Study between Silanization and Surface Initiated Polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Skora, P.; Hirschfeld, J.; Olk, G.; Hildebrandt, L.; Jepsen, S. The Guardians of the Periodontium—Sequential and Differential Expression of Antimicrobial Peptides during Gingival Inflammation. Results from In Vivo and In Vitro Studies. J. Clin. Periodontol. 2019, 46, 276–285. [Google Scholar] [CrossRef]
- Dommisch, H.; Staufenbiel, I.; Schulze, K.; Stiesch, M.; Winkel, A.; Fimmers, R.; Dommisch, J.; Jepsen, S.; Miosge, N.; Adam, K. Expression of Antimicrobial Peptides and Interleukin-8 during Early Stages of Inflammation: An Experimental Gingivitis Study. J. Periodontal Res. 2015, 50, 836–845. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Chen, X.; Gutierrez-Gonzalez, C.F.; Fernandez, A.; Lopez-Esteban, S.; Aparicio, C. Peptide-Functionalized Zirconia and New Zirconia/Titanium Biocermets for Dental Applications. J. Dent. 2015, 43, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Glindmann, S.; Salcedo, J.; Weber, H.-P.; Park, C.-J.; Sarmiento, H.L. The Effect of Osteopontin and an Osteopontin-Derived Synthetic Peptide Coating on Osseointegration of Implants in a Canine Model. Int. J. Periodontics Restor. Dent. 2016, 36, e88–e94. [Google Scholar] [CrossRef]
- Goeke, J.E.; Kist, S.; Schubert, S.; Hickel, R.; Huth, K.C.; Kollmuss, M. Sensitivity of Caries Pathogens to Antimicrobial Peptides Related to Caries Risk. Clin. Oral Investig. 2018, 22, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A Customized Self-Assembling Peptide Hydrogel for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.; Brookes, S.; Aggeli, A. Self-Assembling Peptide Scaffolds Promote Enamel Remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Kämmerer, P.; Heller, M.; Brieger, J.; Klein, M.; Al-Nawas, B.; Gabriel, M. Immobilisation of Linear and Cyclic RGD-Peptides on Titanium Surfaces and Their Impact on Endothelial Cell Adhesion and Proliferation. Eur. Cell Mater. 2011, 21, 364–372. [Google Scholar] [CrossRef]
- Golland, L.; Schmidlin, P.R.; Schätzle, M. The Potential of Self-Assembling Peptides for Enhancement of in Vitro Remineralisation of White Spot Lesions as Measured by Quantitative Laser Fluorescence. Oral Health Prev. Dent. 2017, 15, 147–152. [Google Scholar] [PubMed]
- Hsu, C.; Chung, H.; Yang, J.-M.; Shi, W.; Wu, B. Influence of 8DSS Peptide on Nano-Mechanical Behavior of Human Enamel. J. Dent. Res. 2011, 90, 88–92. [Google Scholar] [CrossRef]
- Kwak, S.; Litman, A.; Margolis, H.; Yamakoshi, Y.; Simmer, J. Biomimetic Enamel Regeneration Mediated by Leucine-Rich Amelogenin Peptide. J. Dent. Res. 2017, 96, 524–530. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and in Vivo Evaluation of a Novel Histatin-5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Ekat, K.; Kilian, D.; Hettich, T.; Germershaus, O.; Lang, H.; Peters, K.; Kreikemeyer, B. A Versatile Biocompatible Antibiotic Delivery System Based on Self-Assembling Peptides with Antimicrobial and Regenerative Potential. Adv. Healthc. Mater. 2019, 8, 1900167. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Yoshinari, M.; Matsuzaka, K.; Shiba, K.; Inoue, T. Identification of Peptide Motif That Binds to the Surface of Zirconia. Dent. Mater. J. 2011, 30, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef]
- Gonçalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; dos Passos Silva, M.; Cannon, M.L.; Danelon, M. Combined Effect of Casein Phosphopeptide-Amorphous Calcium Phosphate and Sodium Trimetaphosphate on the Prevention of Enamel Demineralization and Dental Caries: An in Vitro Study. Clin. Oral Investig. 2021, 25, 2811–2820. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.-Y.; Jung, S.Y.; Kang, H.K.; Min, B.-M.; Yeo, I.-S.L. A Laminin-Derived Functional Peptide, PPFEGCIWN, Promotes Bone Formation on Sandblasted, Large-Grit, Acid-Etched Titanium Implant Surfaces. Int. J. Oral Maxillofac. Implant. 2019, 34, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Kohgo, T.; Yamada, Y.; Ito, K.; Yajima, A.; Yoshimi, R.; Okabe, K.; Baba, S.; Ueda, M. Bone Regeneration with Self-Assembling Peptide Nanofiber Scaffolds in Tissue Engineering for Osseointegration of Dental Implants. Int. J. Periodontics Restor. Dent. 2011, 31, e9–e16. [Google Scholar]
- Gungormus, M.; Oren, E.E.; Horst, J.A.; Fong, H.; Hnilova, M.; Somerman, M.J.; Snead, M.L.; Samudrala, R.; Tamerler, C.; Sarikaya, M. Cementomimetics—Constructing a Cementum-like Biomineralized Microlayer via Amelogenin-Derived Peptides. Int. J. Oral Sci. 2012, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kakegawa, A.; Oida, S.; Gomi, K.; Nagano, T.; Yamakoshi, Y.; Fukui, T.; Kanazashi, M.; Arai, T.; Fukae, M. Cytodifferentiation Activity of Synthetic Human Enamel Sheath Protein Peptides. J. Periodontal Res. 2010, 45, 643–649. [Google Scholar] [CrossRef]
- Kramer, P.R.; JanikKeith, A.; Cai, Z.; Ma, S.; Watanabe, I. Integrin Mediated Attachment of Periodontal Ligament to Titanium Surfaces. Dent. Mater. 2009, 25, 877–883. [Google Scholar] [CrossRef]
- Hua, J.; Yamarthy, R.; Felsenstein, S.; Scott, R.W.; Markowitz, K.; Diamond, G. Activity of Antimicrobial Peptide Mimetics in the Oral Cavity: I. Activity against Biofilms of Candida Albicans. Mol. Oral Microbiol. 2010, 25, 418–425. [Google Scholar] [CrossRef]
- Kohlgraf, K.G.; Ackermann, A.; Lu, X.; Burnell, K.; Bélanger, M.; Cavanaugh, J.E.; Xie, H.; Progulske-Fox, A.; Brogden, K.A. Defensins Attenuate Cytokine Responses yet Enhance Antibody Responses to Porphyromonas Gingivalis Adhesins in Mice. Future Microbiol. 2010, 5, 115–125. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Abdolhosseini, M.; Li, Y.; Chen, X.; Gorr, S.-U.; Aparicio, C. Bio-Inspired Stable Antimicrobial Peptide Coatings for Dental Applications. Acta Biomater. 2013, 9, 8224–8231. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Argyris, P.P.; Skoe, E.P.; Siqueira, J.M.; Chen, X.; Zhang, L.; Hinrichs, J.E.; Costalonga, M.; Aparicio, C. Peptide Coatings Enhance Keratinocyte Attachment towards Improving the Peri-Implant Mucosal Seal. Biomater. Sci. 2018, 6, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Knaup, T.; Korbmacher-Steiner, H.; Jablonski-Momeni, A. Effect of the Caries-Protective Self-Assembling Peptide P11-4 on Shear Bond Strength of Metal Brackets. J. Orofac. Orthop. Kieferorthopädie 2021, 82, 329–336. [Google Scholar] [CrossRef]
- Kihara, H.; Kim, D.M.; Nagai, M.; Nojiri, T.; Nagai, S.; Chen, C.-Y.; Lee, C.; Hatakeyama, W.; Kondo, H.; Da Silva, J. Epithelial Cell Adhesion Efficacy of a Novel Peptide Identified by Panning on a Smooth Titanium Surface. Int. J. Oral Sci. 2018, 10, 21. [Google Scholar] [CrossRef]
- Jablonski-Momeni, A.; Nothelfer, R.; Morawietz, M.; Kiesow, A.; Korbmacher-Steiner, H. Impact of Self-Assembling Peptides in Remineralisation of Artificial Early Enamel Lesions Adjacent to Orthodontic Brackets. Sci. Rep. 2020, 10, 15132. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.; Hassanein, H.; Elkassas, D.; Hamza, H. Comparative Evaluation of Remineralizing Efficacy of Biomimetic Self-Assembling Peptide on Artificially Induced Enamel Lesions: An in Vitro Study. J. Conserv. Dent. 2018, 21, 536. [Google Scholar] [CrossRef]
- Mao, B.; Xie, Y.; Yang, H.; Yu, C.; Ma, P.; You, Z.; Tsauo, C.; Chen, Y.; Cheng, L.; Han, Q. Casein Phosphopeptide-Amorphous Calcium Phosphate Modified Glass Ionomer Cement Attenuates Demineralization and Modulates Biofilm Composition in Dental Caries. Dent. Mater. J. 2021, 40, 84–93. [Google Scholar] [CrossRef]
- Makihira, S.; Nikawa, H.; Shuto, T.; Nishimura, M.; Mine, Y.; Tsuji, K.; Okamoto, K.; Sakai, Y.; Sakai, M.; Imari, N. Evaluation of Trabecular Bone Formation in a Canine Model Surrounding a Dental Implant Fixture Immobilized with an Antimicrobial Peptide Derived from Histatin. J. Mater. Sci. Mater. Med. 2011, 22, 2765–2772. [Google Scholar] [CrossRef]
- Li, Q.-L.; Ning, T.-Y.; Cao, Y.; Zhang, W.; Mei, M.L.; Chu, C.H. A Novel Self-Assembled Oligopeptide Amphiphile for Biomimetic Mineralization of Enamel. BMC Biotechnol. 2014, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, S.; Duan, S.; Xuliang, D.; Sun, Y.; Zhang, X.; Xu, X.; Guan, B.; Wang, C.; Hu, M. Modification of Titanium Substrates with Chimeric Peptides Comprising Antimicrobial and Titanium-Binding Motifs Connected by Linkers to Inhibit Biofilm Formation. ACS Appl. Mater. Interfaces 2016, 8, 5124–5136. [Google Scholar] [CrossRef]
- Min, S.-K.; Kang, H.K.; Jang, D.H.; Jung, S.Y.; Kim, O.B.; Min, B.-M.; Yeo, I.-S. Titanium Surface Coating with a Laminin-Derived Functional Peptide Promotes Bone Cell Adhesion. BioMed Res. Int. 2013, 2013, 638348. [Google Scholar] [CrossRef]
- Moore, A.; Perez, S.; Hartgerink, J.; D’Souza, R.; Colombo, J. Ex Vivo Modeling of Multidomain Peptide Hydrogels with Intact Dental Pulp. J. Dent. Res. 2015, 94, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Muruve, N.; Feng, Y.; Platnich, J.; Hassett, D.; Irvin, R.; Muruve, D.; Cheng, F. A Peptide-Based Biological Coating for Enhanced Corrosion Resistance of Titanium Alloy Biomaterials in Chloride-Containing Fluids. J. Biomater. Appl. 2017, 31, 1225–1234. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Gao, W.; Patel, S.D.; Siddiqui, Z.; Weiner, S.; Shimizu, E.; Sarkar, B.; Kumar, V.A. Self-Assembly of a Dentinogenic Peptide Hydrogel. ACS Omega 2018, 3, 5980–5987. [Google Scholar] [CrossRef] [PubMed]
- Mardas, N.; Stavropoulos, A.; Karring, T. Calvarial Bone Regeneration by a Combination of Natural Anorganic Bovine-derived Hydroxyapatite Matrix Coupled with a Synthetic Cell-binding Peptide (PepGenTM): An Experimental Study in Rats. Clin. Oral Implant. Res. 2008, 19, 1010–1015. [Google Scholar] [CrossRef]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O. Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS ONE 2015, 10, e0145143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, L.; Lin, X.; Zou, L.; Li, Y.; Ge, X.; Fu, W.; Zhang, Z.; Xiao, K.; Lv, H. Functionalized Self-Assembled Peptide RAD/Dentonin Hydrogel Scaffold Promotes Dental Pulp Regeneration. Biomed. Mater. 2021, 17, 015009. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Chen, X.; Jiang, W.; Jiang, X.; Zeng, Y.; Li, X.; Feng, Z.; Luo, J.; Zhang, L. Antimicrobial Peptide GH12 as Root Canal Irrigant Inhibits Biofilm and Virulence of Enterococcus Faecalis. Int. Endod. J. 2020, 53, 948–961. [Google Scholar] [CrossRef]
- Mancino, D.; Kharouf, N.; Scavello, F.; Hellé, S.; Salloum-Yared, F.; Mutschler, A.; Mathieu, E.; Lavalle, P.; Metz-Boutigue, M.-H.; Haïkel, Y. The Catestatin-Derived Peptides Are New Actors to Fight the Development of Oral Candidosis. Int. J. Mol. Sci. 2022, 23, 2066. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Mauger, M.T.; Niu, L.; Barnes, J.B.; Kao, S.; Bergeron, B.E.; Ling, J.; Tay, F.R. Potential Applications of Antimicrobial Peptides and Their Mimics in Combating Caries and Pulpal Infections. Acta Biomater. 2017, 49, 16–35. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Y.; Han, S.; Li, D.; Tu, H.; Li, W.; Zhou, X.; Zhang, L. Potential of an Amelogenin Based Peptide in Promoting Reminerlization of Initial Enamel Caries. Arch. Oral Biol. 2015, 60, 1482–1487. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Park, J.-B.; Min, D.-S.; Lee, S.-J.; Rhyu, H.K.; Jo, I.-H.; Chung, C.-P.; Park, Y.-J. Assembly of Collagen-Binding Peptide with Collagen as a Bioactive Scaffold for Osteogenesis in Vitro and in Vivo. Biomaterials 2007, 28, 4257–4267. [Google Scholar] [CrossRef]
- Liang, K.; Xiao, S.; Liu, H.; Shi, W.; Li, J.; Gao, Y.; He, L.; Zhou, X.; Li, J. 8DSS Peptide Induced Effective Dentinal Tubule Occlusion in Vitro. Dent. Mater. 2018, 34, 629–640. [Google Scholar] [CrossRef]
- Lee, S.J.; Won, J.-E.; Han, C.; Yin, X.Y.; Kim, H.K.; Nah, H.; Kwon, I.K.; Min, B.-H.; Kim, C.-H.; Shin, Y.S. Development of a Three-Dimensionally Printed Scaffold Grafted with Bone Forming Peptide-1 for Enhanced Bone Regeneration with in Vitro and in Vivo Evaluations. J. Colloid Interface Sci. 2019, 539, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Na, D.H.; Faraj, J.; Capan, Y.; Leung, K.P.; DeLuca, P.P. Chewing Gum of Antimicrobial Decapeptide (KSL) as a Sustained Antiplaque Agent: Preformulation Study. J. Control. Release 2005, 107, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, G.d.A.P.; Fraga, M.A.A.; de Souza Araújo, I.J.; Pacheco, R.R.; Correr, A.B.; Puppin-Rontani, R.M. Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel. J. Funct. Biomater. 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Lallier, T.E.; Palaiologou, A.A.; Yukna, R.A.; Layman, D.L. The Putative Collagen-binding Peptide P-15 Promotes Fibroblast Attachment to Root Shavings but Not Hydroxyapatite. J. Periodontol. 2003, 74, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Lin, C.; Zhang, Q.; Gong, L.; Wang, Y.; Zhang, X. Antibacterial Properties of Small-Size Peptide Derived from Penetratin against Oral Streptococci. Materials 2021, 14, 2730. [Google Scholar] [CrossRef]
- Matsugishi, A.; Aoki-Nonaka, Y.; Yokoji-Takeuchi, M.; Yamada-Hara, M.; Mikami, Y.; Hayatsu, M.; Terao, Y.; Domon, H.; Taniguchi, M.; Takahashi, N. Rice Peptide with Amino Acid Substitution Inhibits Biofilm Formation by Porphyromonas Gingivalis and Fusobacterium Nucleatum. Arch. Oral Biol. 2021, 121, 104956. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Jiang, S.; Dai, X.; Wang, G.; Wang, Y.; Yang, Z.; Gao, J.; Zou, H. An Amelogenin-Based Peptide Hydrogel Promoted the Odontogenic Differentiation of Human Dental Pulp Cells. Regen. Biomater. 2022, 9, rbac039. [Google Scholar]
- Mishra, P.R.N.; Kolte, A.P.; Kolte, R.A.; Pajnigara, N.G.; Shah, K.K. Comparative Evaluation of Open Flap Debridement Alone and in Combination with Anorganic Bone Matrix/Cell-Binding Peptide in the Treatment of Human Infrabony Defects: A Randomized Clinical Trial. J. Indian Soc. Periodontol. 2019, 23, 42. [Google Scholar]
- Padovano, J.; Ravindran, S.; Snee, P.; Ramachandran, A.; Bedran-Russo, A.; George, A. DMP1-Derived Peptides Promote Remineralization of Human Dentin. J. Dent. Res. 2015, 94, 608–614. [Google Scholar] [PubMed]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The Effect of BMP-Mimetic Peptide Tethering Bioinks on the Differentiation of Dental Pulp Stem Cells (DPSCs) in 3D Bioprinted Dental Constructs. Biofabrication 2020, 12, 035029. [Google Scholar]
- Pellissari, C.V.G.; Jorge, J.H.; Marin, L.M.; Sabino-Silva, R.; Siqueira, W.L. Statherin-Derived Peptides as Antifungal Strategy against Candida Albicans. Arch. Oral Biol. 2021, 125, 105106. [Google Scholar] [CrossRef]
- Petzold, C.; Monjo, M.; Rubert, M.; Reinholt, F.P.; Gomez-Florit, M.; Ramis, J.M.; Ellingsen, J.E.; Lyngstadaas, S.P. Effect of Proline-Rich Synthetic Peptide--Coated Titanium Implants on Bone Healing in a Rabbit Model. Oral Craniofac. Tissue Eng. 2012, 2, 35–43. [Google Scholar] [CrossRef]
- Picker, A.; Nicoleau, L.; Nonat, A.; Labbez, C.; Cölfen, H. Identification of Binding Peptides on Calcium Silicate Hydrate: A Novel View on Cement Additives. Adv. Mater. 2014, 26, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Pihl, M.; Galli, S.; Jimbo, R.; Andersson, M. Osseointegration and Antibacterial Effect of an Antimicrobial Peptide Releasing Mesoporous Titania Implant. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1787–1795. [Google Scholar] [PubMed]
- Ren, Q.; Li, Z.; Ding, L.; Wang, X.; Niu, Y.; Qin, X.; Zhou, X.; Zhang, L. Anti-Biofilm and Remineralization Effects of Chitosan Hydrogel Containing Amelogenin-Derived Peptide on Initial Caries Lesions. Regen. Biomater. 2018, 5, 69–76. [Google Scholar]
- Santarpia, R.P., III; Pollock, J.J.; Renner, R.P.; Gwinnett, A.J. In Vivo Antifungal Efficacy of Salivary Histidine-Rich Polypeptides: Preliminary Findings in a Denture Stomatitis Model System. J. Prosthet. Dent. 1991, 66, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.; Zobrist, K.; Attin, T.; Wegehaupt, F. In Vitro Re-Hardening of Artificial Enamel Caries Lesions Using Enamel Matrix Proteins or Self-Assembling Peptides. J. Appl. Oral Sci. 2016, 24, 31–36. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Koepple, M.; Moest, T.; Neumann, K.; Weisel, T.; Schlegel, K.A. In Vivo Evaluation of Biofunctionalized Implant Surfaces with a Synthetic Peptide (P-15) and Its Impact on Osseointegration. A Preclinical Animal Study. Clin. Oral Implant. Res. 2016, 27, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Owen, G.R.; Hamilton, D.W.; de Wild, M.; Textor, M.; Brunette, D.M.; Tosatti, S.G. Biomimetic Modification of Titanium Dental Implant Model Surfaces Using the RGDSP-Peptide Sequence: A Cell Morphology Study. Biomaterials 2006, 27, 4003–4015. [Google Scholar] [CrossRef] [PubMed]
- Schuster, L.; Ardjomandi, N.; Munz, M.; Umrath, F.; Klein, C.; Rupp, F.; Reinert, S.; Alexander, D. Establishment of Collagen: Hydroxyapatite/BMP-2 Mimetic Peptide Composites. Materials 2020, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Secchi, A.G.; Grigoriou, V.; Shapiro, I.M.; Cavalcanti-Adam, E.A.; Composto, R.J.; Ducheyne, P.; Adams, C.S. RGDS Peptides Immobilized on Titanium Alloy Stimulate Bone Cell Attachment, Differentiation and Confer Resistance to Apoptosis. J. Biomed. Mater. Res. Part Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Segvich, S.J.; Smith, H.C.; Kohn, D.H. The Adsorption of Preferential Binding Peptides to Apatite-Based Materials. Biomaterials 2009, 30, 1287–1298. [Google Scholar] [CrossRef]
- Sfeir, C.; Fang, P.-A.; Jayaraman, T.; Raman, A.; Xiaoyuan, Z.; Beniash, E. Synthesis of Bone-like Nanocomposites Using Multiphosphorylated Peptides. Acta Biomater. 2014, 10, 2241–2249. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Wang, Y.; Zhang, J.; Zhao, S.; Yang, G. Biological and Immunotoxicity Evaluation of Antimicrobial Peptide-Loaded Coatings Using a Layer-by-Layer Process on Titanium. Sci. Rep. 2015, 5, 16336. [Google Scholar] [CrossRef]
- Shinkai, K.; Taira, Y.; Suzuki, M.; Kato, C.; Yamauchi, J.; Suzuki, S.; Katoh, Y. Dentin Bond Strength of an Experimental Adhesive System Containing Calcium Chloride, Synthetic Peptides Derived from Dentin Matrix Protein 1 (PA and PB), and Hydroxyapatite for Direct Pulp Capping and as a Bonding Agent. Odontology 2010, 98, 110–116. [Google Scholar] [CrossRef]
- Shinkai, K.; Taira, Y.; Suzuki, M.; Kato, C.; Yamauchi, J.; Suzuki, S.; Katoh, Y. Effect of the Concentrations of Calcium Chloride and Synthetic Peptides in Primers on Dentin Bond Strength of an Experimental Adhesive System. Dent. Mater. J. 2010, 29, 738–746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shuturminska, K.; Tarakina, N.V.; Azevedo, H.S.; Bushby, A.J.; Mata, A.; Anderson, P.; Al-Jawad, M. Elastin-like Protein, with Statherin Derived Peptide, Controls Fluorapatite Formation and Morphology. Front. Physiol. 2017, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yao, S.; Gu, L.; Huang, Z.; Mai, S. Antibacterial Effect and Bond Strength of a Modified Dental Adhesive Containing the Peptide Nisin. Peptides 2018, 99, 189–194. [Google Scholar] [CrossRef]
- Suaid, F.A.; Macedo, G.O.; Novaes, A.B., Jr.; Borges, G.J.; Souza, S.L.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F. The Bone Formation Capabilities of the Anorganic Bone Matrix–Synthetic Cell-Binding Peptide 15 Grafts in an Animal Periodontal Model: A Histologic and Histomorphometric Study in Dogs. J. Periodontol. 2010, 81, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Maeno, M.; Lee, C.; Nagai, S.; Kim, D.M.; Da Silva, J.; Nagai, M.; Kondo, H. Establishment of Epithelial Attachment on Titanium Surface Coated with Platelet Activating Peptide. PLoS ONE 2016, 11, e0164693. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, X.; Tan, X.; Si, Y.; Wang, X.; Chen, F.; Zheng, S. Salivary Peptidome Profiling for Diagnosis of Severe Early Childhood Caries. J. Transl. Med. 2016, 14, 240. [Google Scholar] [CrossRef]
- Takahashi, N.; Sato, T. Dipeptide Utilization by the Periodontal Pathogens Porphyromonas Gingivalis, Prevotella Intermedia, Prevotella Nigrescens and Fusobacterium Nucleatum. Oral Microbiol. Immunol. 2002, 17, 50–54. [Google Scholar] [CrossRef]
- Tanhaieian, A.; Pourgonabadi, S.; Akbari, M.; Mohammadipour, H.-S. The Effective and Safe Method for Preventing and Treating Bacteria-Induced Dental Diseases by Herbal Plants and a Recombinant Peptide. J. Clin. Exp. Dent. 2020, 12, e523. [Google Scholar] [CrossRef]
- Üstün, N.; Aktören, O. Analysis of Efficacy of the Self-assembling Peptide-based Remineralization Agent on Artificial Enamel Lesions. Microsc. Res. Tech. 2019, 82, 1065–1072. [Google Scholar] [CrossRef]
- Wang, B.; Wu, B.; Jia, Y.; Jiang, Y.; Yuan, Y.; Man, Y.; Xiang, L. Neural Peptide Promotes the Angiogenesis and Osteogenesis around Oral Implants. Cell. Signal. 2021, 79, 109873. [Google Scholar] [CrossRef]
- Wang, D.; Shen, Y.; Hancock, R.E.; Ma, J.; Haapasalo, M. Antimicrobial Effect of Peptide DJK-5 Used Alone or Mixed with EDTA on Mono-and Multispecies Biofilms in Dentin Canals. J. Endod. 2018, 44, 1709–1713. [Google Scholar] [CrossRef]
- Warnke, P.H.; Voss, E.; Russo, P.A.; Stephens, S.; Kleine, M.; Terheyden, H.; Liu, Q. Antimicrobial Peptide Coating of Dental Implants: Biocompatibility Assessment of Recombinant Human Beta Defensin-2 for Human Cells. Int. J. Oral Maxillofac. Implant. 2013, 28, 982–988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Werner, S.; Huck, O.; Frisch, B.; Vautier, D.; Elkaim, R.; Voegel, J.-C.; Brunel, G.; Tenenbaum, H. The Effect of Microstructured Surfaces and Laminin-Derived Peptide Coatings on Soft Tissue Interactions with Titanium Dental Implants. Biomaterials 2009, 30, 2291–2301. [Google Scholar] [CrossRef]
- Winfred, S.B.; Meiyazagan, G.; Panda, J.J.; Nagendrababu, V.; Deivanayagam, K.; Chauhan, V.S.; Venkatraman, G. Antimicrobial Activity of Cationic Peptides in Endodontic Procedures. Eur. J. Dent. 2014, 8, 254–260. [Google Scholar] [CrossRef]
- Wu, J.; Mao, S.; Xu, L.; Qiu, D.; Wang, S.; Dong, Y. Odontogenic Differentiation Induced by TGF-Β1 Binding Peptide–Modified Bioglass. J. Dent. Res. 2022, 101, 1190–1197. [Google Scholar] [CrossRef]
- Xie, S.-X.; Boone, K.; VanOosten, S.K.; Yuca, E.; Song, L.; Ge, X.; Ye, Q.; Spencer, P.; Tamerler, C. Peptide Mediated Antimicrobial Dental Adhesive System. Appl. Sci. 2019, 9, 557. [Google Scholar] [CrossRef]
- Xie, S.-X.; Song, L.; Yuca, E.; Boone, K.; Sarikaya, R.; VanOosten, S.K.; Misra, A.; Ye, Q.; Spencer, P.; Tamerler, C. Antimicrobial Peptide–Polymer Conjugates for Dentistry. ACS Appl. Polym. Mater. 2020, 2, 1134–1144. [Google Scholar] [CrossRef]
- Yamamoto, N.; Maeda, H.; Tomokiyo, A.; Fujii, S.; Wada, N.; Monnouchi, S.; Kono, K.; Koori, K.; Teramatsu, Y.; Akamine, A. Expression and Effects of Glial Cell Line-derived Neurotrophic Factor on Periodontal Ligament Cells. J. Clin. Periodontol. 2012, 39, 556–564. [Google Scholar] [CrossRef]
- Yamashita, M.; Lazarov, M.; Jones, A.A.; Mealey, B.L.; Mellonig, J.T.; Cochran, D.L. Periodontal Regeneration Using an Anabolic Peptide with Two Carriers in Baboons. J. Periodontol. 2010, 81, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, P.; Wang, H.; Cai, S.; Liao, Y.; Mo, Z.; Xu, X.; Ding, C.; Zhao, C.; Li, J. Antibacterial and Anti-Biofouling Coating on Hydroxyapatite Surface Based on Peptide-Modified Tannic Acid. Colloids Surf. B Biointerfaces 2017, 160, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Z.; Xiao, H.; Wang, N.; Li, Y.; Xu, X.; Chen, Z.; Tan, H.; Li, J. A Universal and Ultrastable Mineralization Coating Bioinspired from Biofilms. Adv. Funct. Mater. 2018, 28, 1802730. [Google Scholar] [CrossRef]
- Yang, X.; Yang, B.; He, L.; Li, R.; Liao, Y.; Zhang, S.; Yang, Y.; Xu, X.; Zhang, D.; Tan, H. Bioinspired Peptide-Decorated Tannic Acid for in Situ Remineralization of Tooth Enamel: In Vitro and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2017, 3, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, D.; Liu, G.; Wang, J.; Luo, Z.; Peng, X.; Zeng, X.; Wang, X.; Tan, H.; Li, J. Bioinspired from Mussel and Salivary Acquired Pellicle: A Universal Dual-Functional Polypeptide Coating for Implant Materials. Mater. Today Chem. 2019, 14, 100205. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, L.; Haapasalo, M.; Wei, W.; Zhang, D.; Ma, J.; Shen, Y. A Novel Hydroxyapatite-Binding Antimicrobial Peptide against Oral Biofilms. Clin. Oral Investig. 2019, 23, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, B.; Li, M.; Wang, Y.; Yang, X.; Li, J. Salivary Acquired Pellicle-Inspired DpSpSEEKC Peptide for the Restoration of Demineralized Tooth Enamel. Biomed. Mater. 2017, 12, 025007. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, M.; Yang, Y.; Zheng, M.; Liu, X.; Tan, J. Biofunctionalization of Zirconia with Cell-Adhesion Peptides via Polydopamine Crosslinking for Soft Tissue Engineering: Effects on the Biological Behaviors of Human Gingival Fibroblasts and Oral Bacteria. RSC Adv. 2020, 10, 6200–6212. [Google Scholar] [CrossRef]
- Yazici, H.; Fong, H.; Wilson, B.; Oren, E.; Amos, F.; Zhang, H.; Evans, J.; Snead, M.; Sarikaya, M.; Tamerler, C. Biological Response on a Titanium Implant-Grade Surface Functionalized with Modular Peptides. Acta Biomater. 2013, 9, 5341–5352. [Google Scholar] [CrossRef]
- Ye, Q.; Spencer, P.; Yuca, E.; Tamerler, C. Engineered Peptide Repairs Defective Adhesive–Dentin Interface. Macromol. Mater. Eng. 2017, 302, 1600487. [Google Scholar] [CrossRef]
- Ye, W.; Yeghiasarian, L.; Cutler, C.W.; Bergeron, B.E.; Sidow, S.; Xu, H.H.; Niu, L.; Ma, J.; Tay, F.R. Comparison of the Use of D-Enantiomeric and l-Enantiomeric Antimicrobial Peptides Incorporated in a Calcium-Chelating Irrigant against Enterococcus Faecalis Root Canal Wall Biofilms. J. Dent. 2019, 91, 103231. [Google Scholar] [CrossRef]
- Yonehara, N.; Shibutani, T.; Tsai, H.-Y.; Inoki, R. Effects of Opioids and Opioid Peptide on the Release of Substance P-like Material Induced by Tooth Pulp Stimulation in the Trigeminal Nucleus Caudalis of the Rabbit. Eur. J. Pharmacol. 1986, 129, 209–216. [Google Scholar] [CrossRef]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Inoue, T.; Oda, Y.; Okuda, K.; Shimono, M. Adsorption Behavior of Antimicrobial Peptide Histatin 5 on PMMA. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 77, 47–54. [Google Scholar] [CrossRef]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Shiba, K. Prevention of Biofilm Formation on Titanium Surfaces Modified with Conjugated Molecules Comprised of Antimicrobial and Titanium-Binding Peptides. Biofouling 2010, 26, 103–110. [Google Scholar] [CrossRef]
- Yuca, E.; Xie, S.-X.; Song, L.; Boone, K.; Kamathewatta, N.; Woolfolk, S.K.; Elrod, P.; Spencer, P.; Tamerler, C. Reconfigurable Dual Peptide Tethered Polymer System Offers a Synergistic Solution for Next Generation Dental Adhesives. Int. J. Mol. Sci. 2021, 22, 6552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, S.; Li, J.; Bu, X.; Dong, X.; Chen, N.; Li, F.; Zhu, J.; Sang, L.; Zeng, Y. Dual-Sensitive Antibacterial Peptide Nanoparticles Prevent Dental Caries. Theranostics 2022, 12, 4818. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Hancock, R.E.; De La Fuente-Núñez, C.; Haapasalo, M. Treatment of Oral Biofilms by a D-Enantiomeric Peptide. PLoS ONE 2016, 11, e0166997. [Google Scholar] [CrossRef]
- Zhao, M.; Qu, Y.; Liu, J.; Mai, S.; Gu, L. A Universal Adhesive Incorporating Antimicrobial Peptide Nisin: Effects on Streptococcus Mutans and Saliva-Derived Multispecies Biofilms. Odontology 2020, 108, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, Y.; Wei, W.; Mao, J. GEPIs-HA Hybrid: A Novel Biomaterial for Tooth Repair. Med. Hypotheses 2008, 71, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lai, Y.; Huang, W.; Huang, S.; Xu, Z.; Chen, J.; Wu, D. Biofunctionalization of Microgroove Titanium Surfaces with an Antimicrobial Peptide to Enhance Their Bactericidal Activity and Cytocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 552–560. [Google Scholar] [CrossRef]
- Gungormus, M.; Tulumbaci, F. Peptide-Assisted Pre-Bonding Remineralization of Dentin to Improve Bonding. J. Mech. Behav. Biomed. Mater. 2021, 113, 104119. [Google Scholar] [CrossRef]
- Gug, H.R.; Park, Y.-H.; Park, S.-J.; Jang, J.Y.; Lee, J.-H.; Lee, D.-S.; Shon, W.-J.; Park, J.-C. Novel Strategy for Dental Caries by Physiologic Dentin Regeneration with CPNE7 Peptide. Arch. Oral Biol. 2022, 143, 105531. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- González-Cabezas, C. The Chemistry of Caries: Remineralization and Demineralization Events with Direct Clinical Relevance. Dent. Clin. 2010, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Innes, N.; Schwendicke, F. Restorative Thresholds for Carious Lesions: Systematic Review and Meta-Analysis. J. Dent. Res. 2017, 96, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Dirks, O.B. Posteruptive Changes in Dental Enamel. J. Dent. Res. 1966, 45, 503–511. [Google Scholar] [CrossRef]
- Da Pontte, A.C.A.; Damião, A.O.M.C.; Rosa, A.M.; Da Silva, A.N.; Fachin, A.V.; Cortecazzi, A., Jr.; Marinho, A.L.D.; Prudente, A.C.L.; Pulgas, A.T.; Machado, A.D.; et al. Consensus Guidelines for the Management of Inflammatory Bowel Disease. Arq. Gastroenterol. 2010, 47, 313–325. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Caries Remineralisation and Arresting Effect in Children by Professionally Applied Fluoride Treatment–a Systematic Review. BMC Oral Health 2016, 16, 12. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Montagner, A.F.; Soares, F.Z.M.; de Oliveira Rocha, R. Are Topical Fluorides Effective for Treating Incipient Carious Lesions? A Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2016, 147, 84–91. [Google Scholar] [CrossRef]
- Lussi, A.; Hellwig, E.; Klimek, J. Fluorides—Mode of Action and Recommendations for Use. Schweiz. Monatsschr. Zahnmed. 2012, 122, 1030. [Google Scholar]
- Alkilzy, M.; Tarabaih, A.; Santamaria, R.; Splieth, C. Self-Assembling Peptide P11-4 and Fluoride for Regenerating Enamel. J. Dent. Res. 2018, 97, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Bell, M.; Boden, N.; Carrick, L.M.; Strong, A.E. Self-Assembling Peptide Polyelectrolyte Β-Sheet Complexes Form Nematic Hydrogels. Angew. Chem. 2003, 115, 5761–5764. [Google Scholar] [CrossRef]
- Aggeli, A.; Bell, M.; Boden, N.; Keen, J.; Knowles, P.; McLeish, T.; Pitkeathly, M.; Radford, S. Responsive Gels Formed by the Spontaneous Self-Assembly of Peptides into Polymeric β-Sheet Tapes. Nature 1997, 386, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Aggeli, A.; Beevers, A.; Boden, N.; Carrick, L.; Fishwick, C.; McLeish, T.; Nyrkova, I.; Semenov, A. Self-Assembling β-Sheet Tape Forming Peptides. Supramol. Chem. 2006, 18, 435–443. [Google Scholar] [CrossRef]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Recombinant Self-Assembling Peptides as Biomaterials for Tissue Engineering. Biomaterials 2010, 31, 9395–9405. [Google Scholar] [CrossRef]
- Dabdoub, S.; Tsigarida, A.; Kumar, P. Patient-Specific Analysis of Periodontal and Peri-Implant Microbiomes. J. Dent. Res. 2013, 92, 168S–175S. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, M.; Aparicio, C.; Manero, J.; Gil, F. Low Elastic Modulus Metals for Joint Prosthesis: Tantalum and Nickel–Titanium Foams. J. Eur. Ceram. Soc. 2007, 27, 3391–3398. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegrated Implants in the Treatment of the Edentulous Jaw. Experience from a 10-Year Period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Schwartz-Arad, D.; Kidron, N.; Dolev, E. A Long-term Study of Implants Supporting Overdentures as a Model for Implant Success. J. Periodontol. 2005, 76, 1431–1435. [Google Scholar] [CrossRef]
- Lindquist, L.; Carlsson, G.; Jemt, T. A Prospective 15-year Follow-up Study of Mandibular Fixed Prostheses Supported by Osseointegrated Implants. Clinical Results and Marginal Bone Loss. Clin. Oral Implant. Res. 1996, 7, 329–336. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Adatrow, P.; Haggard, W.O.; Norowski, P.A. Emerging Antibacterial Biomaterial Strategies for the Prevention of Peri-Implant Inflammatory Diseases. Int. J. Oral Maxillofac. Implant. 2011, 26, 553–560. [Google Scholar]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface Treatments of Titanium Dental Implants for Rapid Osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D. Chemical and Biological Functionalization of Titanium for Dental Implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Aparicio, C.; Nazarpour, S.; Chaker, M. Thin Films and Coatings in Biology; Biological and Medical Physics–Biomedical Engineering Series; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Neu, H.C. The Crisis in Antibiotic Resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseini, M.; Nandula, S.R.; Song, J.; Hirt, H.; Gorr, S.-U. Lysine Substitutions Convert a Bacterial-Agglutinating Peptide into a Bactericidal Peptide That Retains Anti-Lipopolysaccharide Activity and Low Hemolytic Activity. Peptides 2012, 35, 231–238. [Google Scholar] [CrossRef]
- Hirt, H.; Gorr, S.-U. Antimicrobial Peptide GL13K Is Effective in Reducing Biofilms of Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4903–4910. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of Biomimetic Habitats for Tissue Engineering with P-15, a Synthetic Peptide Analogue of Collagen. Tissue Eng. 1999, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Steindorff, M.M.; Lehl, H.; Winkel, A.; Stiesch, M. Innovative Approaches to Regenerate Teeth by Tissue Engineering. Arch. Oral Biol. 2014, 59, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Cheng, L.; Jiang, Y.; Melo, M.A.S.; Weir, M.D.; Oates, T.W.; Zhou, X.; Xu, H.H. Novel Dental Composite with Capability to Suppress Cariogenic Species and Promote Non-Cariogenic Species in Oral Biofilms. Mater. Sci. Eng. C 2019, 94, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Azenha, G.R.; Araujo, G.; Puppin Rontani, R. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate and Lysozyme, Lactoferrin, and Lactoperoxidase in Reducing Streptococcus Mutans. Gen. Dent. 2017, 65, 47–50. [Google Scholar]
- Divyapriya, G.; Yavagal, P.C.; Veeresh, D. Casein Phosphopeptide-Amorphous Calcium Phosphate in Dentistry: An Update. Int. J. Oral Health Sci. 2016, 6, 18. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef]
- Elizalde-Hernández, A.; Hardan, L.; Bourgi, R.; Isolan, C.P.; Moreira, A.G.; Zamarripa-Calderón, J.E.; Piva, E.; Cuevas-Suárez, C.E.; Devoto, W.; Saad, A.; et al. Effect of Different Desensitizers on Shear Bond Strength of Self-Adhesive Resin Cements to Dentin. Bioengineering 2022, 9, 372. [Google Scholar] [CrossRef]
- Rodríguez, F.; Glawe, D.D.; Naik, R.R.; Hallinan, K.P.; Stone, M.O. Study of the Chemical and Physical Influences upon in Vitro Peptide-Mediated Silica Formation. Biomacromolecules 2004, 5, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.C.; Schneider, J.P. Self-Assembling Materials for Therapeutic Delivery. Acta Biomater. 2009, 5, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tang, C.; Elsawy, M.A.; Smith, A.M.; Miller, A.F.; Saiani, A. Controlling Self-Assembling Peptide Hydrogel Properties through Network Topology. Biomacromolecules 2017, 18, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Song, L.; Parthasarathy, R.; Boone, K.; Misra, A.; Tamerler, C. Threats to Adhesive/Dentin Interfacial Integrity and next Generation Bio-enabled Multifunctional Adhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2673–2683. [Google Scholar] [CrossRef]
- Hardan, L.; Lukomska-Szymanska, M.; Zarow, M.; Cuevas-Suárez, C.E.; Bourgi, R.; Jakubowicz, N.; Sokolowski, K.; D’Arcangelo, C. One-Year Clinical Aging of Low Stress Bulk-Fill Flowable Composite in Class II Restorations: A Case Report and Literature Review. Coatings. 2021, 11, 504. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Zarow, M.; Kharouf, N.; Mancino, D.; Villares, C.F.; Skaba, D.; Lukomska-Szymanska, M. The Bond Strength and Anti-bacterial Activity of the Universal Dentin Bonding System: A Systematic Review and Meta- Analysis. Microorganisms 2021, 9, 1230. [Google Scholar] [CrossRef]
- Bourgi, R.; Daood, U.; Bijle, M.N.; Fawzy, A.; Ghaleb, M.; Hardan, L. Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry. Polymers 2021, 13, 704. [Google Scholar] [CrossRef]
| (Peptide) OR (Polypeptides) OR (Polypeptide) AND (Materials, Dental) OR (Dental Material) OR (Material, Dental) |
| Study and Year | Type of Study | Peptide Used | Application | Main Results |
|---|---|---|---|---|
| Bagno, 2007 [41] | In vitro | Two adhesive peptides: an RGD-containing peptide and a peptide recorded on human vitronectin | Implant osseointegration | It was observed that there was a capacity of the peptides to promote enhanced cell adhesion |
| Artzi, 2006 [42] | Experimental study | A synthetic peptide (P-15) | Guided tissue regeneration and guided bone regeneration techniques | The use of a synthetic peptide showed increased osteoconductive and biocompatible features |
| Bröseler, 2020 [43] | Randomized clinical trial | Self-assembling peptide (SAP) P11-4 | Early buccal carious lesions | Self-assembling peptide regenerated enamel caries lesions |
| Butz, 2011 [44] | Prospective in vivo study | Synthetic Peptide in a Sodium Hyaluronate Carrier (PepGen P-15 Putty) | Sinus grafting | The peptide evaluated was successful for maxillary sinus augmentation |
| Chung, 2013 [45] | In vitro | Asparagine–serine–serine (NSS) peptide. | Remineralization of eroded enamel. | Peptide increased the nanohardness and elastic modulus of eroded enamel |
| Altankhishig, 2021 [46] | In vitro and in vivo | Peptide | Vital pulp therapy | The dentin phosphophoryn-derived arginine-glycine-aspartic acid-containing peptide showed adequate properties as a bioactive material for dentin regeneration |
| Afami, 2021 [47] | In vitro | Ultrashort peptide hydrogel, (naphthalene-2-ly)-acetyl-diphenylalanine-dilysine-OH (NapFFεKεK-OH) | Antimicrobial activity and angiogenic growth factor release by dental pulp stem/stromal cells | Peptide-containing hydrogels have potential in tissue engineering for pulp regeneration |
| Babaji, 2019 [48] | In vitro | SAP P11-4 and casein phosphopeptides-amorphous calcium phosphate (CPP-ACP) | Enamel remineralization | The peptide was more effective and efficient when compared to CPP-ACP |
| Dettin, 2002 [49] | In vitro | Novel osteoblast-adhesive peptides | Osteoblast adhesion | The novel peptide promotes proteoglycan-mediated osteoblast adhesion efficiently |
| Cirera, 2019 [50] | In vivo | TGF-β1 inhibitor peptide: P144 | Osseointegration of synthetic bone grafts | The healing period of osseointegrated biomaterials can be shortened when peptide biofunctionalization is used |
| Boda, 2020 [51] | In vitro | Mineralized nanofiber segments combined with calcium-binding bone morphogenetic protein 2 (BMP-2)-mimicking peptides | Alveolar bone regeneration | Mineralized nanofibers functionalized with peptides have the potential to regenerate craniofacial bone defects |
| Chen, 2017 [52] | In vivo | GL13K-peptide | Osseointegration of implants | This study showed that titanium dental implants with an antimicrobial GL13K peptide coating enables in vivo implant osseointegration |
| Aref, 2022 [53] | In vitro | CPP-ACP | White spot lesion | CPP-ACP could be a promising approach to manage WSLs efficiently, with subsequent universal adhesive resin infiltration |
| Aruna, 2015 [54] | Clinical | Gingival crevicular fluid (GCF) N-terminal telopeptides of type I collagen (NTx) | Periodontal therapy | Cross-linked NTx can be successfully estimated in the GCF of chronic periodontitis subjects |
| Brunton, 2013 [55] | A clinical trial | Biomimetic SAP: P11-4 | Early caries lesions | Treatment of early caries lesions with P11-4 is safe, and a single application of this peptide is associated with significant enamel regeneration |
| Fang, 2020 [56] | In vitro | Two hexapeptide coatings | Dental implants | The novel hexapeptide coating can inhibit the attachment of Porphyromonas gingivalis and prevent the formation of dental biofilm |
| Goldberg, 2009 [57] | In vitro | Polypeptide | Occluding dentin tubules | Peptide catalysts that mediate mineral formation can retain functionality on dentin, suggesting a wide range of preventive and treatment strategies |
| Amin, 2012 [58] | In vitro | Amelogenin Peptides | Osteogenic differentiation | Amelogenin-derived peptide could be a useful tool for limiting pathological bone cell growth |
| Godoy-Gallardo, 2015 [59] | In vitro | hLf1-11 Peptide | Antibacterial properties on titanium surfaces | A greater amount of peptide attached to the surfaces functionalized via atom transfer radical polymerization than those functionalized via silane |
| Dommisch, 2019 [60] | In vivo and in vitro | Antimicrobial peptides | Gingival inflammation | The study delivers evidence on the role of antimicrobial peptides as guardians of a healthy periodontium |
| Dommisch, 2015 [61] | Experimental study | Antimicrobial peptides | Gingivitis | Differential temporal expression for antimicrobial peptides could guarantee continuous antimicrobial activity alongside changes in the bacterial composition of the growing dental biofilm |
| Fernandez-Garcia, 2015 [62] | In vitro | Peptide-functionalized zirconia | Implant | Surface bioactivation of zirconia-containing constituents for dental implant applications will allow their perfected clinical implementation by incorporating signaling oligopeptides to accelerate osseointegration, improve mucosal sealing, and/or incorporate antimicrobial properties to avoid peri-implant infections |
| Fiorellini, 2016 [63] | In vitro | Osteopontin-derived synthetic peptide: OC-1016 | Osseointegration of implants | OC-1016 was capable of meaningfully accelerating the initial stage of osseointegration and bone healing around implants |
| Goeke, 2018 [64] | Clinical | Antimicrobial peptides | Caries risk | The incidence of low-susceptible strains to antimicrobial peptides appears to relate to individual caries status |
| Galler, 2012 [65] | In vitro | SAP hydrogel | Dental pulp tissue engineering | The use of this innovative biomaterial was considered a highly favorable candidate for upcoming treatment hypotheses in regenerative endodontics |
| Kirkham, 2007 [66] | In situ | SAP scaffolds | Enamel remineralization | SAP might be useful for dental tissue engineering |
| Kämmerer, 2011 [67] | In vitro | RGD peptides | Dental implants | Modifications of titanium surfaces with c-RGD peptides are an encouraging way to endorse endothelial cell growth |
| Golland, 2017 [68] | In vitro | SAP | Remineralization of white spot lesions | The application of SAP on demineralized bovine enamel indicated an irregular crystal or a lack of remineralization |
| Hsu, 2010 [69] | In vitro | Aspartate-serine-serine (8DSS) pep- tides | Nucleation of calcium phosphate carbonate from free ions | 8DSS peptides reduced the surface roughness of demineralized enamel and promoted the uniform deposition of nano-crystalline calcium phosphate carbonate over demineralized enamel surfaces |
| Kwak, 2017 [70] | In vitro | Leucine-rich amelogenin peptide (LRAP) | Enamel regeneration | LRAP has the power to enhance the linear growth of mature enamel crystals |
| Kong, 2015 [71] | In vivo | Histatin-5 (Hst-5) | Oral Candidiasis | Hst-5 was able to clear existing lesions |
| Koch, 2019 [72] | In vitro | SAP: P11-4 and P11-28/29 | Periodontal therapy | SAP hydrogels were effective for periodontal therapy |
| Hashimoto, 2011 [73] | In vitro | Peptide motif | Zirconia | A peptide motif was successful in binding zirconia |
| Kind, 2017 [74] | In vitro | SAP: P11-4 | Remineralization of carious lesions | The application of P11-4 might facilitate the subsurface regeneration of the enamel lesion |
| Gonçalves, 2020 [75] | In vitro | Casein phosphopeptide-amorphous calcium phosphate (MI Paste Plus) | Enamel demineralization and dental caries | MI Paste Plus might be effective in improving oral health |
| Kim, 2019 [76] | In vitro and in vivo | A laminin-derived functional peptide | Implant | Peptide DN3 promotes bone healing |
| Kohgo, 2011 [77] | In vitro | SAP | Implant | SAP could be useful for bone regeneration around dental implants |
| Gungormus, 2012 [78] | Ex vivo | Amelogenin-derived peptides | Periodontal tissues | Amelogenin-derived peptide 5 promoted the regeneration of periodontal tissues |
| Kakegawa, 2010 [79] | In vitro | Enamel sheath protein peptides | Construction of the enamel sheath during tooth development | A specific peptide sequence encourages the cytodifferentiation and mineralization activity of human periodontal ligaments |
| Kramer, 2009 [80] | In vitro | Integrin blocking peptide | Titanium surfaces | Antibody and peptide treatment reduced the number of fibroblast cells involved on the implant surfaces |
| Hua, 2010 [81] | In vitro | Antimicrobial peptide | Oral cavity | The antimicrobial peptide was demonstrated as an anti-Candida agent |
| Hua, 2010 [81] | In vitro | Antimicrobial peptide | Oral cavity | The antimicrobial peptide exhibits potent activity against both A. actinomycetemcomitans and P. gingivalis biofilms |
| Kohlgraf, 2010 [82] | In vitro | Human neutrophil peptide α-defensins (HNPs) | Cytokine responses | The ability of HNPs to attenuate proinflammatory cytokines was dependent upon both the defensin and antigen of P. gingivalis |
| Holmberg, 2013 [83] | In vitro | Antimicrobial peptide: GL13K | Dental and orthopedic implants | The antimicrobial activity and cytocompatibility of GL13K-biofunctionalized titanium make it a promising candidate for sustained inhibition of bacterial biofilm growth |
| Koidou, 2019 [84] | In vitro | Bioinspired peptide coatings | Peri-implant mucosal Seal | Peptide coatings were considered a promising candidate for inducing a peri-mucosal seal around dental implants |
| Knaup, 2021 [85] | In vitro | SAP: P11-4 | Metal brackets | The application of the caries-protective SAP P11-4 before the bonding of brackets did not influence the shear bond strength |
| Kihara, 2018 [86] | In vitro | Novel synthetic peptide (A10) | Titanium surface | The novel peptide has a useful presentation that might enhance advanced clinical outcomes by means of titanium implants and abutments by preventing or reducing peri-implant disease |
| Jablonski-Momeni, 2020 [87] | In vitro | SAP P11-4 | Early enamel lesions adjacent to orthodontic brackets | The application of p11-4 with fluoride varnish was demonstrated to be superior for the remineralization of enamel adjacent to brackets when compared to the use of fluorides alone |
| Kamal, 2018 [88] | In vitro | SAP P11-4 | Artificially induced enamel lesions | SAP confers a higher remineralizing efficacy |
| Mao, 2021 [89] | In vitro | CPP-ACP | Dental caries | The use of 5% CPP-ACP reduced 39% of bacterial biofilm |
| Makihira, 2011 [90] | In vivo | Antimicrobial peptide derived from histatin: JH8194 | Dental implant | JH8194 might deliver a viable biological modification of titanium surfaces to amplify trabecular bone formation around dental implants |
| Li, 2014 [91] | In vitro | Synthetic and self-assembled oligopeptide amphiphile (OPA) | Mineralization of enamel | OPA was successful in the biomimetic mineralization of demineralized enamel |
| Liu, 2016 [92] | Experimental | Chimeric peptides comprising antimicrobial and titanium-binding motifs | Biofilm formation | Chimeric peptides provide a promising alternative to inhibit the formation of biofilms on titanium surfaces with the power to prevent peri-implantitis |
| Min, 2013 [93] | In vitro | Laminin-derived functional peptide, Ln2-P3 | Implant | An Ln2-P3-coated implant surface enhances bone cell adhesion |
| Moore, 2015 [94] | Ex vivo | Multidomain peptide hydrogels | Dental pulp | Multidomain peptide hydrogels offered centrally and peripherally within whole dental pulp tissue are demonstrated to be biocompatible and preserve the architecture of the local tissue |
| Muruve, 2017 [95] | In vitro | PEGylated metal-binding peptide (D-K122-4-PEG) | Titanium surface | D-K122-4-PEG promotes resistance to corrosion |
| Nguyen, 2018 [96] | In vitro | Dentinogenic peptide | Dental pulp stem cells | The SAP promised guided dentinogenesis |
| Mardas, 2007 [97] | An experimental study in rats | PepGen | Bone regeneration | The anorganic bovine-derived hydroxyapatite matrix coupled with a synthetic cell-binding peptide failed to promote new bone formation |
| Mateescu, 2015 [98] | In vitro | Antimicrobial peptide Cateslytin | Peri-implant diseases | The new peptide could be ideal in the prevention of peri-implant diseases |
| Liu, 2021 [99] | In vitro | RADA16-I: (SAP) | Pulp regeneration | The novel SAP could be ideal in endodontic tissue engineering |
| Li, 2020 [100] | In vitro | GH12: antimicrobial peptide | Root canal irrigant | GH12 suppressed E. faecali in dentinal tubules |
| Mancino, 2022 [101] | In vitro | Catestatin-derived peptides | Oral candidiasis | The catestatin-derived peptides were considered for the treatment of oral candidiasis |
| Mai, 2016 [102] | In vitro | Antimicrobial peptides | Caries and pulpal infections | Antimicrobial peptide mimics offer opportunities for new therapeutics in regenerative endodontics and root canal treatments |
| Lv, 2015 [103] | In vitro | Amelogenin based peptide | Remineralization of initial enamel caries | The amelogenin-based peptide enhances enamel caries remineralization |
| Lee, 2007 [104] | In vitro and in vivo | Collagen-binding peptide | Osteogenesis | Collagen-binding peptide induced biomineralization of bone |
| Liang, 2018 [105] | In vitro | 8DSS peptide | Dentinal tubule occlusion | 8DSS peptide induced strong dentinal tubule occlusion and can be used in dentin hypersensitivity |
| Lee, 2018 [106] | In vitro and in vivo | Bone formation peptide-1 (BFP1) | Bone regeneration | BFP1 was considered promising for bone repair |
| Na, 2005 [107] | Preformulation study | Antimicrobial decapeptide (KSL) | Antiplaque agent | KSL served as a novel antiplaque agent in the oral cavity |
| Magalhães, 2022 [108] | In vitro | Self-assembly peptide: P11-4 | Bleached enamel | The use of P11-4 after bleaching results in the fastest recovery to baseline enamel properties |
| Lallier, 2003 [109] | In vitro | Collagen-binding peptide P-15 | Periodontal treatment | P-15 promoted fibroblast attachment to root surfaces |
| Li, 2021 [110] | In vitro | Small-size peptide: RR9 | Oral streptococci | RR9 might be considered a possible antimicrobial agent for periodontal disease |
| Matsugishi, 2021 [111] | In vitro | Rice peptide | Biofilm formation | Rice peptide hindered the biofilm formation of F. nucleatum and P. gingivalis |
| Li, 2022 [112] | In vitro | Amelogenin-based peptide hydrogel | Human dental pulp cells | The amelogenin peptide hydrogel enhanced mineralization and encouraged odontogenic differentiation |
| Mishra, 2019 [113] | A randomized clinical trial | Anorganic bone matrix/cell-binding peptide (ABM/P-15) | Human infrabony periodontal defects | The combination of ABM/P-15 was established to be a favorable material for periodontal regeneration |
| Padovano, 2015 [114] | In vitro | DMP1-derived peptides | Remineralization of human dentin | DMP1-derived peptides could be useful in modulating mineral deposition |
| Park, 2020 [115] | In vitro | BMP-mimetic peptide | Dental pulp stem cells | BMP-mimetic peptide accelerated human dental pulp stem cells |
| Pellissari, 2021 [116] | In vitro | Statherin-derived peptides | Biofilm development | The natural peptides from statherin are able to decrease biofilm proliferation and Candida albicans colonization |
| Petzold, 2012 [117] | In vivo | Proline-rich synthetic peptide | Titanium implants | Proline-rich peptides have a probable biocompatible capacity for endorsing osseointegration by lessening bone resorption |
| Picker, 2014 [118] | In vitro | Binding peptides | Calcium silicate hydrate | A new strong calcium silicate hydrate-binding additive influenced the physical properties of cement |
| Pihl, 2021 [119] | In vivo | Antimicrobial peptide: RRP9W4N | Titania implant | RRP9W4N was demonstrated to be successful in the control of infection in osseointegrating implants |
| Ren, 2018 [120] | In vitro | Chitosan hydrogel containing amelogenin-derived peptide | Initial caries lesions | Chitosan hydrogel containing amelogenin-derived peptide was demonstrated to be effective in controlling caries and promoting the remineralization of the initial enamel carious lesion |
| Santarpia, 1991 [121] | In vivo | Histidine-rich polypeptides | Denture stomatitis | Histidine-rich polypeptides were effective in the treatment of denture stomatitis |
| Schmidlin, 2015 [122] | In vitro | SAP | Mineralization of artificial caries lesions | SAP improved the hardness profile of deep demineralized artificial lesions |
| Schmitt, 2016 [123] | In vivo | Synthetic peptide (P-15) | Osseointegration | There is no advantage in the early phase of osseointegration for dental implants with P-15-containing surfaces |
| Schuler, 2006 [124] | In vitro | RGDSP-peptide sequence | Titanium dental implant | There is no communication between RGD-peptide surface density and surface topography for osteoblasts |
| Schuster, 2020 [125] | In vitro | Hydroxyapatite/BMP-2 mimetic peptide | Bone tissue engineering | Biofunctionalization of collagen-hydroxyapatite composites with BMP-2 simulated peptides was considered cost-effective and fast for prolonged and improved jaw periosteal cell proliferation |
| Secchi, 2007 [126] | In vitro | Arginine-glycine-aspartic acid (RGDS) peptides | Implant | The modification of the titanium surface with RGDS peptides promoted osseointegration |
| Segvich, 2009 [127] | In vitro | Binding peptide sequences | Bone regeneration | The binding peptide sequences can be used in dentin and bone tissue engineering |
| Sfeir, 2014 [128] | In vitro | Multiphosphorylated peptides | Mineralized collagen fibrils of bone and dentin | Using phosphopeptides, there is progress in biomimetic nanostructured materials for mineralized tissue regeneration and repair |
| Shi, 2015 [129] | In vitro | Antimicrobial peptide-loaded coatings | Dental implant | The antimicrobial peptide-loaded coatings were demonstrated to be a potential approach for preventing peri-implantitis |
| Shinkai, 2010 [130] | In vitro | Synthetic peptides derived from dentin matrix protein 1 (pA and pB) | Direct pulp capping and bonding agent | The primer containing synthetic peptides derived from dentin matrix protein 1 negatively affected the bond strength to dentin |
| Shinkai, 2010 [131] | In vitro | Synthetic peptides (pA and pB) | Bonding agent | A significant difference was seen in bond strength among CaCl2 concentrations in Primer-I (comprising 10 wt.% CaCl2) and pA/pB concentrations in Primer-II comprising 10 wt.% pA/pB, and there is a noteworthy interaction between these two factors |
| Shuturminska, 2017 [132] | In vitro | Statherin-derived peptide | Enamel biomineralization | The use of statherin-derived peptide was considered effective in enamel therapy |
| Su, 2017 [133] | In vitro | Peptide nisin | Dental adhesive | The cured nisin included in the dental adhesive showed a noteworthy inhibitory effect on the growth of S. mutans |
| Suaid, 2010 [134] | Histologic and histomorphometric study | Anorganic bone matrix–synthetic cell-binding peptide 15 | Periodontal class III furcation defects | The use of anorganic bone matrix–synthetic cell-binding peptide 15 was effective in bone formation |
| Sugawara, 2016 [135] | In vitro | Platelet-activating peptide | Titanium surface | An epithelial basement membrane was formed on the titanium surface when platelet activating peptide was used |
| Sun, 2016 [136] | Clinical | Peptidome | Early childhood caries | The magnetic bead-founded matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was considered an effective technique for screening distinctive peptides from the saliva of junior patients with early childhood caries |
| Takahashi, 2002 [137] | In vitro | Dipeptide: aspartylaspartate and glutamylglutamate | Periodontal pathogens | Dipeptides can be employed as growth substrates for P. intermedia, P. gingivalis, F. nucleatum, and P. nigrescens |
| Tanhaieian, 2020 [138] | In vitro | Recombinant peptide | Dental diseases | The recombinant peptide was demonstrated effective as an antimicrobial agent against E. faecalis and oral streptococci |
| Üstuün, 2019 [139] | In vitro | SAP: P11-4 | Artificial enamel lesions | P11-4 was demonstrated to have the best remineralization efficacy |
| Wag, 2020 [140] | In vivo | Neural peptide | Angiogenesis and osteogenesis around oral implants | Alpha-calcitonin gene-related peptide up-regulated the expression of Hippo-YAP and downstream genes in order to encourage osteogenesis and angiogenesis around the implants |
| Wang, 2015 [141] | In vitro | Peptide DJK-5 | Dentin canals | The peptide DJK-5 showed an imperative antibacterial property against mono- and multispecies biofilms in dentin canals |
| Warnke, 2013 [142] | In vitro | Human beta-defensins (HBDs), small cationic antimicrobial peptides | Dental implants | HBD-2 is not only biocompatible with but further encourages the proliferation of human mesenchymal stem cells |
| Wener, 2009 [143] | In vitro | Laminin-derived peptide | Dental implants | Laminin-derived peptide improved and enhanced the integration of soft tissue on titanium implants used in dentistry |
| Winfred, 2014 [144] | In vitro | Cationic peptides | Endodontic procedures | Cationic peptides prevented the spread of endodontic infections |
| Wu, 2022 [145] | In vitro | TGF-β1 binding peptide–modified bioglass | Endodontic therapy | TGF-β1 binding peptide–modified bioglass was effective for regeneration in endodontic therapy |
| Xue Xie, 2019 [146] | In vitro | Antimicrobial peptide | Dental adhesive system | Antimicrobial peptide-hydrophilic adhesive delivers an advanced adhesive/dentin interface |
| Xue Xie, 2020 [147] | In vitro | Antimicrobial peptide | Dental adhesive system | Peptide-conjugated dentin adhesives were effective in secondary caries treatment and improved the durability of dental composites |
| Yakufu, 2020 [147] | In vitro | Osteogenic growth peptide (OGP) | Osteogenesis activity | OGP was promising in dental and orthopedic applications |
| Yamamoto, 2012 [148] | In vivo | Peptide including Arg-Gly-Asp (RGD) sequence | Periodontal ligament cells | Glial cell line-derived neurotrophic factor, which was hindered by pre-treatment with the peptide-embracing Arg-Gly-Asp (RGD) sequence, enhanced the appearance of bone sialoprotein and fibronectin on human periodontal ligament cells |
| Yamashita, 2010 [149] | In vitro | Anabolic peptide | Periodontal regeneration | Anabolic peptide has a positive influence on bone cells |
| Yang, 2017 [150] | In vitro | Peptide-modified tannic acid | Hydroxyapatite surface | Peptide-modified tannic acid inhibited the adhesion of bacteria |
| Yang, 2018 [151] | In vitro | Salivary acquired pellicle (SAPe)-inspired peptide DDDEEK | Biofilms | SAPe-inspired peptide DDDEEK has a great advantage in the field of implant materials |
| Yang, 2017 [152] | In vitro and in vivo | Bioinspired peptide-decorated tannic acid | Remineralization of tooth enamel | Bioinspired peptide-decorated tannic acid has a good influence on the remineralization of tooth enamel |
| Yang, 2019 [153] | In vitro | Dual-functional polypeptide | Implant materials | Dual-functional polypeptide has a potential application in the treatment of hard tissue-related diseases |
| Yang, 2019 (b) [154] | In vitro and in vivo | Immunomodulatory peptide 1018 | Plaque biofilms | Immunomodulatory peptide 1018 was effective as an anti-biofilm agent |
| Yang, 2017 (b) [155] | In vitro | DpSpSEEKC peptide | Demineralized tooth enamel | DpSpSEEKC restored demineralized tooth enamel |
| Yang, 2020 [156] | In vitro | Cell-adhesion peptides via polydopamine crosslinking | Zirconia abutment surfaces | Cell-adhesion peptides improved soft tissue integration around zirconia abutments via polydopamine crosslinking |
| Yazici, 2013 [157] | In vitro | Modular peptides | Titanium implant | Modular peptides on titanium surfaces improved the bioactivity of fibroblast and osteoblast cells on implant-grade materials |
| Ye, 2017 [158] | In vitro | Peptide-based approach | Adhesive-dentin interface | The peptide-based remineralization approach was effective in designing integrated tissue-biomaterial interfaces |
| Ye, 2019 [159] | In vitro | D-enantiomeric and L-enantiomeric antimicrobial peptides | Root canal wall biofilms | D-enantiomeric peptides exhibited more antimicrobial potent activity than L-enantiomeric peptides against E. faecalis biofilms on the canal space |
| Yonehara, 1986 [160] | In vivo | Opioids and opioid peptides | Tooth pulp stimulation | There is an interaction between substance P and enkephalin systems in the superficial layer of the brain-stem trigeminal sensory nuclear complex for the regulation of dental pain transmission. In addition, the native application of naloxone (5 × 10−7 M) partly antagonized the inhibitory effects of locally applied morphine and the opioid peptide |
| Yoshinari, 2005 [161] | In vitro | Antimicrobial peptide histatin 5 | Poly (methyl methacrylate) denture base | C. albicans colonization on histatin-adsorbed PMMA was knowingly less than the control |
| Yoshinari, 2010 [162] | In vitro | Antimicrobial and titanium-binding peptides | Titanium surfaces | Antimicrobial and titanium-binding peptides were encouraging for the reduction of biofilm formation on titanium surfaces |
| Yuca, 2021 [163] | In vitro | Dual-peptide tethered polymer system | Dental adhesives | The adhesive system formed of co-tethered peptides revealed both localized calcium phosphate remineralization and strong metabolic inhibition of S. mutans |
| Zhang, 2022 [164] | In-vitro | Dual-sensitive antibacterial peptide | Dental caries | This peptide prevented damage from bacteria and, thus, from dental caries |
| Zhang, 2016 [165] | In vitro | D-Enantiomeric peptide | Oral biofilms | D-enantiomeric peptide was effective against oral biofilms |
| Zhao, 2020 [166] | In vitro | Antimicrobial peptide nisin | Dental adhesive | 3% (w/v) nisin-incorporated Single Bond Universal substantially inhibited the development of both saliva-derived multispecies biofilms and monospecific S. mutans biofilms without hindering the bonding performance |
| Zhou, 2008 [167] | In vitro | Genetically engineered peptides for inorganics | Tooth repair | Genetically engineered peptides for inorganics were effective in tooth repair |
| Zhou, 2015 [168] | In vitro | Antimicrobial peptide | Titanium surfaces | Antimicrobial peptide provided a promising bifunctional surface |
| Gungormus, 2021 [169] | In vitro | Peptide-assisted pre-bonding | Remineralization of dentin | Pre-bonding remineralization of dentin using peptide during 10 min notably enhanced the stiffness of dentin and the resistance to hydrolysis. In addition, it can reduce shrinkage due to drying |
| Koidou, 2018 [84] | In vitro | Laminin 332- and ameloblastin-derived peptides (Lam, Ambn) | Peri-implant mucosal seal | Laminin 332- and ameloblastin-derived peptides were demonstrated to be effective in producing a peri-mucosal seal around dental implants |
| Gug, 2022 [170] | In vivo | CPNE7-derived functional peptide | Dentin regeneration of dental caries | CPNE7-derived functional peptide repaired caries by dentin regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardan, L.; Chedid, J.C.A.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Tosco, V.; Monjarás-Ávila, A.J.; Jabra, M.; Salloum-Yared, F.; Kharouf, N.; et al. Peptides in Dentistry: A Scoping Review. Bioengineering 2023, 10, 214. https://doi.org/10.3390/bioengineering10020214
Hardan L, Chedid JCA, Bourgi R, Cuevas-Suárez CE, Lukomska-Szymanska M, Tosco V, Monjarás-Ávila AJ, Jabra M, Salloum-Yared F, Kharouf N, et al. Peptides in Dentistry: A Scoping Review. Bioengineering. 2023; 10(2):214. https://doi.org/10.3390/bioengineering10020214
Chicago/Turabian StyleHardan, Louis, Jean Claude Abou Chedid, Rim Bourgi, Carlos Enrique Cuevas-Suárez, Monika Lukomska-Szymanska, Vincenzo Tosco, Ana Josefina Monjarás-Ávila, Massa Jabra, Fouad Salloum-Yared, Naji Kharouf, and et al. 2023. "Peptides in Dentistry: A Scoping Review" Bioengineering 10, no. 2: 214. https://doi.org/10.3390/bioengineering10020214
APA StyleHardan, L., Chedid, J. C. A., Bourgi, R., Cuevas-Suárez, C. E., Lukomska-Szymanska, M., Tosco, V., Monjarás-Ávila, A. J., Jabra, M., Salloum-Yared, F., Kharouf, N., Mancino, D., & Haikel, Y. (2023). Peptides in Dentistry: A Scoping Review. Bioengineering, 10(2), 214. https://doi.org/10.3390/bioengineering10020214















