Abstract
A global need exists for new and more effective contrast agents for computed tomography and traditional X-ray modalities. Among the few options available nowadays, limitations imposed by industrial production, performance, and efficacy restrict the use and reduce the potential of both imaging techniques. The use of nanomaterials as new contrast agents for X-ray and computed tomography is an innovative and viable way to increase the options and enhance performance. In this study, we evaluated eight nanomaterials: hydroxyapatite doped with zinc (Zn-HA 10%); hydroxyapatite doped with strontium (Sr-HA 10%); hydroxyapatite without thermal treatment (HA 282 STT); thermally treated hydroxyapatite (HA 212 500 °C and HA 01.256 CTT 1000 °C); hydroxyapatite microspheres (HA microspheres); gold nanoparticles (AuNP); and graphene oxide doped with copper (Cu-GO). The results showed that for both imaging modalities; HA microspheres were the best option, followed by hydroxyapatite thermally treated at 1000 °C. The nanomaterials with the worst results were hydroxyapatite doped with zinc (Zn-HA 10%), and hydroxyapatite doped with strontium (Sr-HA 10%). Our data demonstrated the potential of using nanomaterials, especially HA microspheres, and hydroxyapatite with thermal treatment (HA 01.256 CTT 1000 °C) as contrast agents for X-ray and computed tomography.
1. Introduction
Medical imaging plays a key role in the early diagnosis, monitoring of medical treatments, and therapeutic response assessment [1,2]. Medical imaging enables the noninvasive visualization of the inside of the human body and provides anatomical, morphological, and functional information. Ultrasonography, X-ray imaging, computed tomography (CT), magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET) are some of the medical imaging techniques commonly used today. Among these, X-ray imaging and CT are very popular worldwide. X-ray imaging is extensively used because it is fast and probably the most cost-effective medical imaging modality [3]. CT is also an X-ray-based technology with widespread use due to its high 3D spatial resolution of anatomic features, low cost, fast scan time, and good patient compatibility [4,5].
During imaging, X-rays cross through the patient and are attenuated by the tissue. This attenuation depends on the mass attenuation coefficient (μ/ρ; cm2/g) and the density (ρ; g/cm3) of all materials in that path [6]. While some tissues are easily detected, such as lungs and bones, X-ray imaging techniques struggle to distinguish soft tissues [7]. Conventional radiography is unable to visualize low-contrast tissues and structures due to, for instance, inefficient X-ray absorption, high scatter-to-primary X-ray ratios, superimposition and conspicuity [8]. Therefore, X-ray imaging techniques may require the administration of contrast agents to improve the contrast between tissue, fluid, and/or anatomical structures [9]. The contrast agents commonly reported contain elements with a high atomic number (Z) because the attenuation of X-rays is dependent on Z raised to the third power [9,10]. Iodine (Z = 53), tantalum (Z = 73), rhenium (Z = 75), gold (Z = 79), and bismuth (Z = 83) are some examples of high-Z elements that have been evaluated as contrast agents [6,9,11,12,13]. Based on the combination of cost, availability, toxicity, and imaging performance, iodine is the only element approved among them for intravascular administration for CT and X-ray imaging [6]. However, several symptoms, such as headache, itching, nausea, vomiting, fever, skin rash, and musculoskeletal pain, have been reported by some patients as delayed adverse reactions 1 h to 1 week after intravascular iodinated agent injection [14]. In addition, other aspects related to iodine-based contrast agents are also concerns, such as the possibility of anaphylactic shock [15], and the need to repeatedly use large doses to counteract rapid renal excretion to obtain a good contrast [9].
Therefore, many works have been focused on discovering new contrast agents that are more suitable for X-ray/CT scans, mostly using nanoparticles due to their very attractive properties. Nanosized particles have a larger surface-to-volume ratio and longer circulation time than microparticles [16], enabling image acquisition at delayed time points after administration of low doses. In addition, nanoparticles can be easily surface-functionalized and prepared with different shapes and sizes to mediate the targeting ability, distribution, elimination, and toxicity [17,18]. Nanoparticles are able to passively accumulate, for instance, in solid tumors because of the enhanced permeability and retention (EPR) effect, locally generating X-ray images [19,20]. Gold [4,5,20], bismuth [21,22], tantalum [23,24], rhenium [25], silver [26], and other nanoparticles have been reported so far with promising outcomes as X-ray contrast agents. Among the metal core-based nanoparticles, gold has been highlighted due to its high X-ray attenuation coefficient, with a K-edge energy of 80.7 keV and density of 19.3 g/cm3, suitable biocompatibility, and well-known control of surface chemistry, shape, and size during production [4,5,7].
On the other hand, hydroxyapatite is a calcium phosphate ceramic widely used in biomedical applications due to its similar chemical composition to bone and teeth [27]. This ceramic biomaterial is biocompatible, bioactive, and thermodynamically stable in body fluids [28]. The flexible hexagonal structure of hydroxyapatite enables the replacement of the phosphate anions by other anionic complexes as well as the substitution of the calcium cations by many metal cations [29,30]. These favorable properties have expanded the biomedical applications of hydroxyapatite. For medical imaging, silver (Ag+) and gadolinium (Gd3+) ions co-substituted on hydroxyapatite nanoparticles have exhibited potential as bimodal contrast agents for CT and MRI [31]. In addition, doping of hydroxyapatite nanocrystals with europium (Eu3+) and gadolinium (Gd3+) ions resulted in tri-modal contrast in fluorescence imaging, MRI, and X-ray imaging [32].
Furthermore, graphene oxide (GO) is another promising candidate as a contrast agent for CT [26,33,34,35]. GO has a two-dimensional crystalline structure organized in a hexagonal sp2-carbon pattern with several oxygen-containing functionalities (hydroxyl, carboxyl, epoxide) on the surface [36]. These oxygen groups enable easy covalent functionalization to improve biocompatibility and reduce toxicity. GO also has strong thermal and electrical conductive properties as well as long-range π conjugation and the ability to form stable suspensions in water [34,36]. Previous works have reported the excellent potential as contrast agents for CT and X-ray imaging of graphene oxide/Ag nanoparticles [26,33,34], and reduced graphene nanoplatelets doped with iodine and manganese ions for bimodal CT/MRI imaging [35]. In addition, multifunctional stimuli-responsive 2D-based smart nanocomposites, comprising gold nanoparticles and superparamagnetic iron oxides scaffolded within graphene oxide nanosheets, were found to be promising candidates for multimodal CT/MRI image-guided cancer therapy [37].
In this study, we assessed hydroxyapatite nanoparticles (HA NP) produced using varied protocols, graphene oxide doped with copper (Cu-GO), and gold nanoparticles (AuNP) as contrast agents for X-ray and CT imaging.
2. Methodology
2.1. Production and Characterization of Nanoparticles
All the HA NP and Cu-GO nanoparticles were synthesized and characterized using previous works, as described below. The AuNP were acquired from Sigma Aldrich (São Paulo, Brazil) with the following characteristics: nanopowder, <100 nm particle size, and 99.9% trace metals basis. All the reagents and solvents were purchased from Sigma Aldrich (São Paulo, Brazil).
2.1.1. Hydroxyapatite
Hydroxyapatite (HA) powders were precipitated by dropwise addition of a (NH4)2HPO4 aqueous solution containing NH4OH to a Ca(NO3)2 solution at 90 °C and pH of 11, following procedures of Resende et al. [38]. The precipitate was separated by filtration, repeatedly washed with boiling deionized water, and dried at 100 °C for 24 h. The dried powder was manually ground, and <210 µm particles were separated by sieving. HA powders from various syntheses were maintained without thermal treatment or calcined at 500 °C and 1000 °C, respectively. HA microspheres were prepared from a mixture of HA powder and the polymer alginate and extruded into a CaCl2 solution. The microspheres were dried overnight at 25 °C, and sintered and calcined at 1100 °C for 2 h. HA doped with zinc (ZnHA, 5% molar) or strontium (SrHA, 10% molar) was synthesized by adding (NH4)2HPO4 aqueous solution to a Ca(NO3)2 and Zn(NO3)2 or Sr(NO3)2 solution at 90 °C and pH of 9. Calcium and phosphorous concentration, crystalline phases, particle size, and morphology were determined by fluorescence of X-ray (FRX), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM).
2.1.2. Hydroxyapatite Doped with Zinc (Zn-HA 10%)
Hydroxyapatite dopped with zinc (10%) was produced and fully characterized as described by Pedrosa et al. [39] and Martinez-Zelaya et al. [40]. Briefly, Zn-HA was precipitated through the dropwise addition of an aqueous (NH4)2HPO4 solution to a solution containing Zn(NO3)2 at a pH of 9.0, followed by stirring of the suspension for 3 h at 90 °C. The precipitate was then separated by filtration, repeatedly washed with boiling deionized water, and subsequently dried at 100 °C for 24 h. The characterization was performed by FTIR, atomic absorption spectroscopy (AAS), and XRD.
2.1.3. Hydroxyapatite Doped with Strontium (Sr-HA 10%)
Hydroxyapatite doped with strontium (10%) was produced and fully characterized as described by Terra et al. [41]. Briefly, strontium-doped HA (Sr-HA) produced by precipitation with an increasing ion exchange between calcium (Ca) and Sr-HA was precipitated through dropwise addition of an aqueous (NH4)2HPO4 solution to a solution containing Sr(NO3)2 at pH of 9.0, followed by stirring of the suspension for 3 h at 90 °C. The precipitate was then separated by filtration, repeatedly washed with boiling deionized water, and subsequently dried at 100 °C for 24 h. The powder was characterized by XRD, (FTIR), and extended X-ray absorption fine structure (EXAFS) spectroscopy.
2.1.4. Hydroxyapatite (HA) without Thermic Treatment (HA 282 STT) and with Thermic Treatment at 500 °C (HA 212 500 °C) and 1000 °C (HA 01.256 CTT 1000 °C)
These three types of hydroxyapatite were produced and fully characterized as described by Albernaz et al. [42]. Briefly, hydroxyapatite was precipitated by dropwise addition of (NH4)2HPO4 aqueous solution containing NH4OH to a Ca(NO3)2 solution at 37 °C, and pH equal to 11. The precipitate was separated by filtration and repeatedly washed with deionized boiling water. Then, the HA without thermic treatment was dried at 100 °C for 24 h. On the other hand, the thermally treated HA was dried at 500 °C or 1000°C, respectively, for 24 h. Calcium and phosphorous concentrations (Ca/P = 1.66) were determined by X-ray fluorescence spectroscopy. Mineral phase and crystallinity were evaluated by XRD. Phosphate species and OH− groups in apatite structure were identified by FTIR in transmission mode from 400 to 4000 cm−1. Crystalline size (D) along hydroxyapatite (002) and (300) directions was determined by the Debye–Sherrer formula (Equation (1)), where β is full width at half maximum of the diffraction peak (values in radians) and k = 0.9.
2.1.5. Microspheres of Hydroxyapatite (HA Microspheres)
The hydroxyapatite microspheres were produced and fully characterized as described by Martinez-Zelaya et al. [40]. Briefly, HA powder was gently dispersed in a 10 mg/mL aqueous solution of sodium alginate to achieve an alginate/powder ratio of 1:15 (6.7 wt% of alginate). The alginate/powder mixture was extruded dropwise at room temperature into a 0.15 M CaCl2 solution, using a needle with 0.70 mm diameter. Microspheres were formed instantaneously and were allowed to mature in the CaCl2 solution for 24 h for complete gelation. The microsphere was dried overnight at 25 °C and sintered and calcined at 1100 °C for 2 h. The authors characterized the microspheres by synchrotron radiation-based X-ray microtomography (SR-μCT) and SEM. 2.1.6 Graphene oxide doped with copper (Cu-GO).
2.1.6. Graphene Oxide Doped with Copper (Cu-GO)
The graphene oxide doped with copper was produced and fully characterized following the method described by D Ni’maturrohmah et al. [43]. Briefly, 0.5 g of graphene oxide and 0.1 M of NaCl were diluted in 25 mL of deionized water, stirred for 20 min, followed by electrolysis using an electrochemical cell at 3 and 5 volts for 30 min at room temperature using a carbon rod as cathode and a copper plate as anode. Finally, the resulting product was dried at 100 °C for 1 h. The characterization was performed by X-ray powder diffraction and SEM.
2.2. Bimodal Imaging
2.2.1. Computed Tomography
The CT attenuation properties of all the samples were evaluated using a Siemens SOMATOM Emotion scanner (CT 2014), and axial sections were analyzed. The parameters were as follows: mAs fixed: 40, kV: 80, pitch: 1.5 mm, slice: 5 mm (Acq. 16 × 0.6 mm), and reconstruction of 67 images (1.5 mm/1.5 mm), FOV 120 mm, acquisition time 5.18 s, with B60S medium sharp (lung parenchyma) filter reconstruction, lung window, craniocaudal direction, and increment of 1.5 mm.
2.2.2. X-ray Imaging
The X-ray attenuation properties of all the samples were evaluated using a Siemens Polydoros LX 50 X-ray generator. The study was performed by the standard wrist technique (mAs 20, ms 71, and 46 Kv).
3. Results
Zn-HA 10%, Sr-HA 10%, HA 01.256 CTT 1000 °C, HA microspheres, AuNP, and Cu-GO samples exhibited a significant X-ray attenuation (Figure 1). In addition, the CT imaging (Figure 2) results demonstrated that all the samples evaluated are able to attenuate the X-ray and form a contrast.
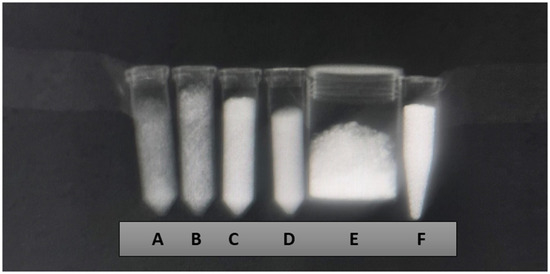
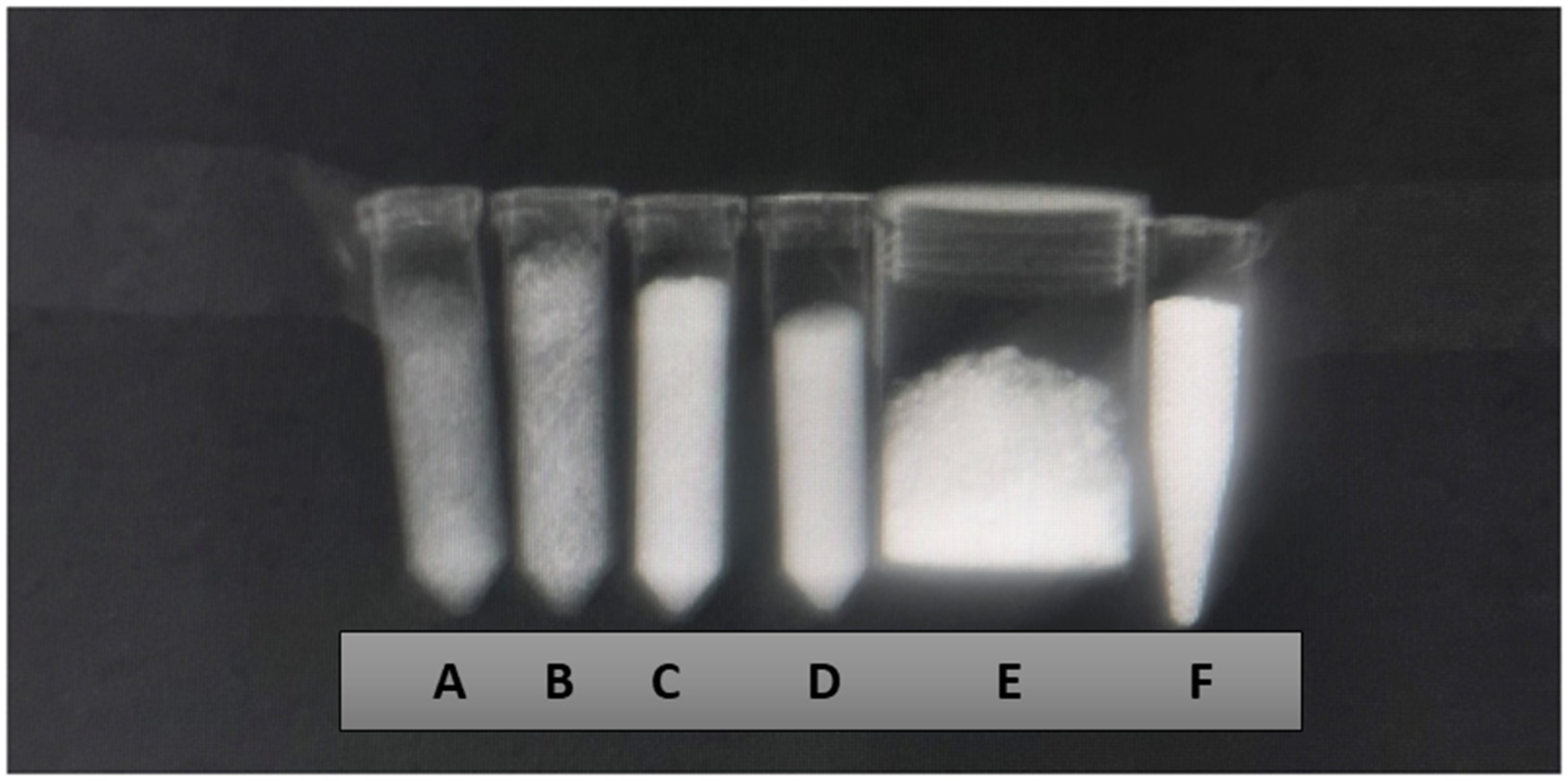
Figure 1.
X-ray imaging using the wrist technique (mAs 20, ms 71, and 46 Kv) of the samples Zn-HA 10% (A), Sr-HA 10% (B), HA 01.256 CTT 1000 °C (C), HA microspheres (D), AuNP (E), and Cu-GO (F).
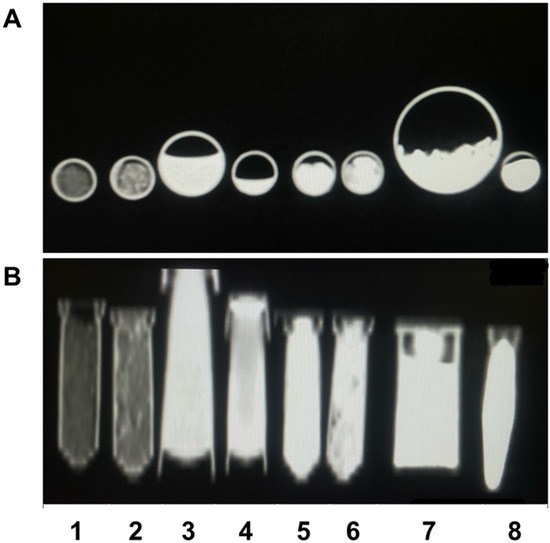
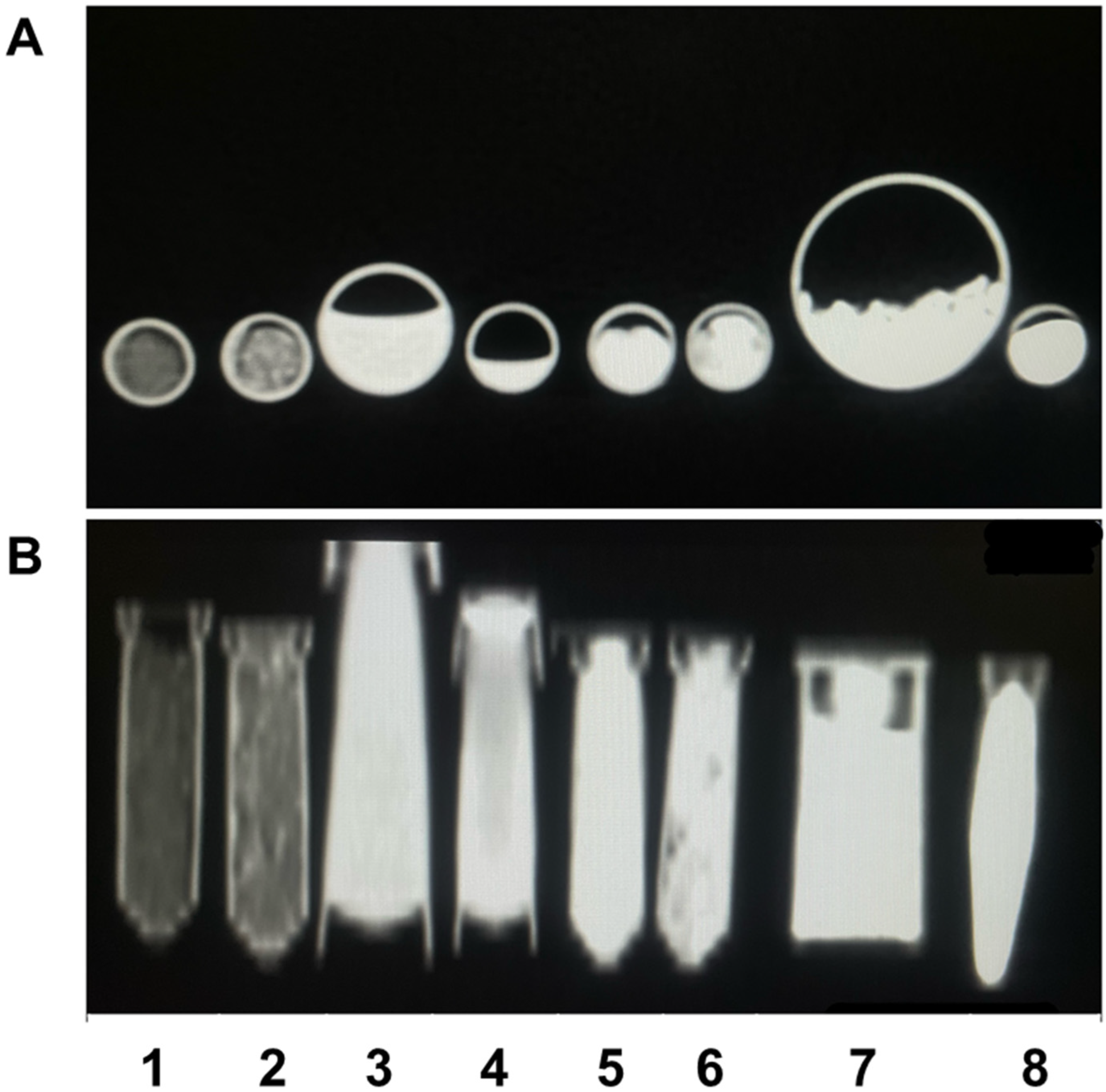
Figure 2.
CT imaging showing axial section (A) and coronal section (B) of the samples Zn-HA 10% (1), Sr-HA 10% (2), HA 282 STT (3), HA 212 500 °C (4), HA 01.256 CTT 1000 °C (5), HA microspheres (6), AuNP (7), and Cu-GO (8).
The X-ray attenuation coefficient (µ) expressed in cm−1 and Hounsfield units for the eight samples are overviewed by Table 1. It possible to observe that the better agent for X-ray contrast is the hydroxyapatite microspheres followed by hydroxyapatite thermally treated at 1000 °C. The worst agent for contrast was hydroxyapatite doped with zinc, followed by hydroxyapatite doped with strontium.

Table 1.
Results of the X-ray attenuation coefficient (µ) expressed in cm−1 and Hounsfield units for Zn-HA 10% (1), Sr-HA 10% (2), HA 282 STT (3), HA 212 500 °C (4), HA 01.256 CTT 1000 °C (5), HA microspheres (6), AuNP (7), and Cu-GO (8) samples.
4. Discussion
The resulting X-ray and CT image is a grayscale map that is directly related to the linear radiation attenuation coefficients within each material or tissue [7,44,45]. The attenuation coefficient is a measure used to estimate the absorption of radiation suffered by the X-ray beam when passing through an object. Thus, materials that absorb more photons have higher attenuation values and are displayed in white in the image, while materials or tissues that absorb fewer photons have lower attenuation values and are represented in black [44,45].
The computer is able to calculate the numerical value that represents the attenuation coefficient for each volume element (voxel). This value corresponds to the average amount of radiation absorption of a given tissue/material, represented by the pixel on the monitor [46,47]. After the calibration performed internally in the tomography, the density of the tomogram is adjusted to reference values such as pure water equal to 0 Hounsfield units (HU) and standardized air to −1000 HU. The relationship between the tissue/material attenuation coefficient and Hounsfield units is known as the Hounsfield Scale [46].
X-ray/CT contrast agents play a crucial role in distinguishing between tissues with low or similar attenuation coefficients. For example, soft tissues such as adipose tissue or soft materials consisting of hydrocarbon structures are not very sensitive to X-rays [47,48]. Thus, to improve image quality, contrast agents can be used. Contrast agents contain molecules that have a high attenuation coefficient and are expected to increase the attenuation of X-rays at the site of interest compared to surrounding tissues, improving image visualization [48,49,50,51].
In the present study, we evaluated hydroxyapatite doped with zinc (Zn-HA 10%) or strontium (Sr-HA 10%), hydroxyapatite without thermal treatment (HA 282 STT) or thermally treated at 500 °C (HA 212 500 °C) and 1000 °C (HA 01.256 CTT 1000 °C), HA microspheres, as well as graphene oxide doped with copper (Cu-GO), and AuNP as contrast agents for X-ray/CT scans. Among them, HA microspheres exhibited the highest contrast (2195 HU) and X-ray linear attenuation coefficient (0.764 cm−1). This result was similar to the contrast effect reported by Madhumathi et al. [31] for a hydroxyapatite sample co-substituted with 0.25 Ag and 0.75 Gd at. % substitution (2199 HU). These authors demonstrated that silver and gadolinium substitution further improved the contrast in CT scans compared with pure hydroxyapatite (1011 HU) [31].
On the other hand, hydroxyapatite doped with zinc (Zn-HA 10%) or strontium (Sr-HA 10%) displayed the lowest contrast and X-ray attenuation (−575 HU/0.102 cm−1 and −438 HU/0.134 cm−1, respectively). These negative HU values indicated the low radiodensity of the samples, and consequently, their lesser X-ray beam absorption, resulting in weaker contrast of the images [52]. In addition, the low contrast of both doped HA samples was expected due to the lower atomic numbers of zinc (Zn = 30) and strontium (Z = 38) than iodine (Z = 53), producing probably even lower HU than iodine [6].
Furthermore, the increase of temperature from 500 °C to 1000 °C enhanced the contrast in the CT between the thermally treated HA samples (HA 212 500°C: 168 HU/0.279 cm−1 vs. HA 01.256 CTT 1000 °C: 596 HU/0.381 cm−1). Conversely, the HA sample without thermal treatment produced higher contrast (HA 282 STT: 203 HU/0.288 cm−1) than the HA sample thermally treated at 500 °C. The HU values of these three HA nanocomposites were even greater than the HU values for both gold nanoparticles (AuNP: 34 HU/0.247 cm−1) and graphene oxide doped with copper (Cu-GO: 6 HU/0.240 cm−1). All the HA nanoparticles were imaged as dry powders, while both Cu-GO and AuNP were used in solution (2 mg/mL). Other works with gold nanoparticles with different shapes and symmetrical to anisotropic morphology have reported their markedly higher contrast (424–3378 HU) depending on the gold concentration (7.67–35.00 mg/mL) [53]. However, gold is an expensive metal, which could limit the application of AuNP as a contrast agent.
According to previous works, reduced graphene nanoplatelets doped with iodine (33.8 mM) and manganese ions displayed a radiodensity of 1980 HU [35]. Moreover, multifunctional stimuli-responsive 2D-based smart nanocomposites, comprising AuNP and superparamagnetic iron oxides scaffolded within GO nanosheets, showed an approximate CT contrast of 60 HU at a gold concentration of 2.5 mg/mL [37]. Regarding graphene oxide doped with copper as a contrast agent, we are unaware of any reports. Our results showed low contrast for GO-Cu (6 HU), but it was higher than the Zn/Sr-doped samples despite its slightly lower atomic number (Z = 29). Hence, the clearer contrast of the image of the sample GO-Cu was probably due to the chemical properties of GO itself. GO has a two-dimensional crystalline structure organized in a hexagonal sp2-carbon pattern with several oxygen-containing functionalities on the surface [36]. This might convert it into an electron-dense material, enhancing the X-ray attenuation.
Therefore, the HA microspheres presented the highest radiodensity (HU value) and attenuation of the X-ray beam. Hence, they represent the most promising candidate as a contrast agent for CT/X-ray imaging.
Finally, is important to notice that an intercomparison of all the nanomaterials evaluated at the same concentration [54] and using an in vivo model is quite recommended.
5. Conclusions
Nanomaterials are an important class of compounds with special features, including small volume, low toxicity (in many cases), and high surface area, among several others. The applicability of nanomaterials in the biomedical field has been increasing exponentially in the last few years, precisely due to the unique characteristics of these materials. Our results demonstrated that most of the nanomaterials evaluated may be used as contrast agents for both conventional X-ray and Computed Tomography. Among them, the most prominent two were hydroxyapatite microspheres (HA microspheres) and hydroxyapatite thermally treated at 1000 °C (HA 01.256 CTT 1000 °C). Conversely, hydroxyapatite doped with zinc (Zn-HA 10%), and hydroxyapatite doped with strontium (Sr-HA 10%) were the worst materials. The graphene oxide doped with copper and gold nanoparticles are also viable options.
Author Contributions
Conceptualization, R.S.-O.; methodology, B.P.P., C.A. and L.d.C.P.; software: A.O.d.S.d.B. and M.S.O.P.; formal analysis, C.A., L.d.C.P., A.O.d.S.d.B. and M.S.O.P.; investigation, C.A., L.d.C.P., A.O.d.S.d.B., M.S.O.P. and L.M.R.A.; data curation, R.S.-O., A.M.R. and L.d.C.P.; writing—original draft preparation, R.S.-O., A.M.R., E.R.-J. and L.M.R.A.; writing—review and editing, R.S.-O.,A.M.R., E.R.-J. and M.S.O.P.; supervision, R.S.-O.; funding acquisition: R.S.-O. and A.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State (FAPERJ) (Cientista do Nosso Estado: E-26/200.815/2021; Rede NanoSaude: E-26/010.000981/2019, Pesquisa na UEZO: E-26/010.002362/2019; Temáticos: E-26/211.269/2021, Infraestrutura e Pesquisa na UEZO e UERJ: E-26//211.207/2021, Bolsa de Pós-doutorado Senior (PDS): E-26/202.320/2021) CNPq (Bolsa de Produtividade 1B: 301069/2018-2) to Ralph Santos-Oliveira. National Nuclear Energy Commission (Bolsa de Pós-doutorado CNEN: 01341.011064/2021-71) to Martha Sahylí Ortega Pijeira.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data will be available under request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Fadhel, M.A.; Al-Shamma, O.; Zhang, J.; Santamaría, J.; Duan, Y.; Oleiwi, S.R. Towards a Better Understanding of Transfer Learning for Medical Imaging: A Case Study. Appl. Sci. 2020, 10, 4523. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Pfeiffer, F.; Rummeny, E. Advanced X-ray Imaging Technology. In Molecular Imaging in Oncology. Recent Results in Cancer Research; Schober, O., Kiessling, F., Debus, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 216, pp. 3–30. [Google Scholar]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 5837276. [Google Scholar] [CrossRef]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in X-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef]
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for new CT contrast agents to maximize the diagnostic potential of emerging spectral CT technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222. [Google Scholar] [CrossRef]
- Hsu, J.C.; Nieves, L.M.; Betzer, O.; Sadan, T.; Noël, P.B.; Popovtzer, R.; Cormode, D.P. Nanoparticle contrast agents for X-ray imaging applications. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1642. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.W. Principles of CT and CT Technology. J. Nucl. Med. Technol. 2007, 35, 115–128. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, J.C.; Esquinas, P.L.; Gill, J.K.; Jessa, S.; Gill, B.; Thakur, Y.; Saatchi, K.; Häfeli, U.O. Comparison of Rhenium and Iodine as Contrast Agents in X-ray Imaging. Contrast Media Mol. Imaging 2021, 2021, 1250360. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M. (Ed.) The Physics of Radiation Therapy, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Lambert, J.W.; Sun, Y.; Stillson, C.; Li, Z.; Kumar, R.; Wang, S.; FitzGerald, P.F.; Bonitatibus, P.J.; Colborn, R.E.; Roberts, J.C.; et al. An Intravascular Tantalum Oxide–based CT Contrast Agent: Preclinical Evaluation Emulating Overweight and Obese Patient Size. Radiology 2018, 289, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, H.; Bakhshandeh, M.; Montazerabadi, A.; Nazari Moghadam, H.; Mazloom Shahri, S.B.; Keshtkar, M. Comparison of Gold Nanoparticles and Iodinated Contrast Media in Radiation Dose Reduction and Contrast Enhancement in Computed Tomography. Iran. J. Radiol. 2020, 17, e92446. [Google Scholar] [CrossRef]
- Fu, J.; Guo, J.; Qin, A.; Yu, X.; Zhang, Q.; Lei, X.; Huang, Y.; Chen, M.; Li, J.; Zhang, Y.; et al. Bismuth chelate as a contrast agent for X-ray computed tomography. J. Nanobiotechnol. 2020, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.W.; Stacul, F.; Thomsen, H.S.; Morcos, S.K. Members of the *Contrast Media Safety Committee of the European Society of Urogenital Radiology (ESUR). Late adverse reactions to intravascular iodinated contrast media. Eur. Radiol. 2003, 13, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, H.; Wang, Y.; Yang, W.; Qiao, S.; Hu, F. Clinical characteristics and management of iodine contrast media-related anaphylactic shock during cardiac catheterization. World Allergy Organ. J. 2020, 13, 100459. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled nanomaterials for biomedical applications: Radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 381. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Nakagawa, T.; Gonda, K.; Kamei, T.; Cong, L.; Hamada, Y.; Kitamura, N.; Tada, H.; Ishida, T.; Aimiya, T.; Furusawa, N.; et al. X-ray computed tomography imaging of a tumor with high sensitivity using gold nanoparticles conjugated to a cancer-specific antibody via polyethylene glycol chains on their surface. Sci. Technol. Adv. Mater. 2016, 17, 387–397. [Google Scholar] [CrossRef]
- Badrigilan, S.; Shaabani, B.; Aghaji, N.G.; Mesbahi, A. Graphene Quantum Dots-Coated Bismuth Nanoparticles for Improved CT Imaging and Photothermal Performance. Int. J. Nanosci. 2020, 19, 1850043. [Google Scholar] [CrossRef]
- Badrigilan, S.; Shaabani, B.; Gharehaghaji, N.; Mesbahi, A. Iron oxide/bismuth oxide nanocomposites coated by graphene quantum dots: “Three-in-one” theranostic agents for simultaneous CT/MR imaging-guided in vitro photothermal therapy. Photodiagn. Photodyn. Ther. 2019, 25, 504–514. [Google Scholar] [CrossRef]
- Freedman, J.D.; Lusic, H.; Snyder, B.D.; Grinstaff, M.W. Tantalum Oxide Nanoparticles for the Imaging of Articular Cartilage Using X-ray Computed Tomography: Visualization of Ex Vivo/In Vivo Murine Tibia and Ex Vivo Human Index Finger Cartilage. Angew. Chemie Int. Ed. 2014, 53, 8406–8410. [Google Scholar] [CrossRef]
- Chakravarty, S.; Hix, J.M.L.; Wiewiora, K.A.; Volk, M.C.; Kenyon, E.; Shuboni-Mulligan, D.D.; Blanco-Fernandez, B.; Kiupel, M.; Thomas, J.; Sempere, L.F.; et al. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale 2020, 12, 7720–7734. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Pan, J.; Zhao, F.; Kan, D.; Cheng, R.; Zhang, X.; Sun, S.-K. Rhenium Sulfide Nanoparticles as a Biosafe Spectral CT Contrast Agent for Gastrointestinal Tract Imaging and Tumor Theranostics in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 33650–33658. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, L.; Liu, J.; Qi, W.; Wu, Q.; Wang, H.; Ali, M.C.; Wu, W.; Qiu, H. Graphene Oxide/Ag Nanoparticles Cooperated with Simvastatin as a High Sensitive X-Ray Computed Tomography Imaging Agent for Diagnosis of Renal Dysfunctions. Adv. Healthc. Mater. 2017, 6, 1700413. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Madhumathi, K.; Kumar, S.; Sanjeed, M.; Muhammed, S.; Nazrudeen, S.; Sharanya, D. Silver and Gadolinium Ions Co-substituted Hydroxyapatite Nanoparticles as Bimodal Contrast Agent for Medical Imaging. Bioceram. Dev. Appl. 2014, 4, 2. [Google Scholar] [CrossRef]
- Ashokan, A.; Menon, D.; Nair, S.; Koyakutty, M. A molecular receptor targeted, hydroxyapatite nanocrystal based multi-modal contrast agent. Biomaterials 2010, 31, 2606–2616. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Zhang, J.; Ma, R.; Gao, J.; Liu, Y.; Zhang, C.; Zhang, Z. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials 2014, 35, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Mukherjee, D.; Maiti, T.K.; Sarkar, N. Protein-Guided Formation of Silver Nanoclusters and Their Assembly with Graphene Oxide as an Improved Bioimaging Agent with Reduced Toxicity. J. Phys. Chem. Lett. 2017, 8, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Sundararaj, J.L.; Schaefer, K.; Button, T.; Sitharaman, B. Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging-X-ray computed tomography contrast agent. J. Mater. Chem. B 2014, 2, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Magne, T.M.; de Oliveira Vieira, T.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.U.D.; Freire, R.M.; Golokhvast, K.; Metrangolo, P.; et al. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostruct. Chem. 2022, 12, 693–727. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Alamzadeh, Z.; Beik, J.; Sarikhani, A.; Mousavi, M.; Irajirad, R.; Khani, T.; Davani, E.S.; Farashahi, A.; Ardakani, T.S.; et al. A 2D nanotheranostic platform based on graphene oxide and phase-change materials for bimodal CT/MR imaging, NIR-activated drug release, and synergistic thermo-chemotherapy. Nanotheranostics 2022, 6, 350–364. [Google Scholar] [CrossRef]
- Resende, R.F.B.; Fernandes, G.V.O.; Santos, S.R.A.; Rossi, A.M.; Lima, I.; Granjeiro, J.M.; Calasans-Maia, M.D. Long-term biocompatibility evaluation of 0.5% zinc containing hydroxyapatite in rabbits. J. Mater. Sci. Mater. Med. 2013, 24, 1455–1463. [Google Scholar] [CrossRef]
- Pedrosa, M.C.G.; dos Anjos, S.A.; Mavropoulos, E.; Bernardo, P.L.; Granjeiro, J.M.; Rossi, A.M.; Dias, M.L. Structure and biological compatibility of polycaprolactone/zinc-hydroxyapatite electrospun nanofibers for tissue regeneration. J. Bioact. Compat. Polym. 2021, 36, 314–333. [Google Scholar] [CrossRef]
- Martinez-Zelaya, V.R.; Zarranz, L.; Herrera, E.Z.; Alves, A.T.; Uzeda, M.J.; Mavropoulos, E.; Rossi, A.L.; Mello, A.; Granjeiro, J.M.; Calasans-Maia, M.D.; et al. In vitro and in vivo evaluations of nanocrystalline Zn-doped carbonated hydroxyapatite/alginate microspheres: Zinc and calcium bioavailability and bone regeneration. Int. J. Nanomed. 2019, 14, 3471–3490. [Google Scholar] [CrossRef] [PubMed]
- Terra, J.; Dourado, E.R.; Eon, J.-G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The structure of strontium-doped hydroxyapatite: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Albernaz, M.D.S.; Ospina, C.A.; Rossi, A.M.; Santos-Oliveira, R. Radiolabelled nanohydroxyapatite with 99mTc: Perspectives to nanoradiopharmaceuticals construction. Artif. Cells Nanomed. Biotechnol. 2014, 42, 88–91. [Google Scholar] [CrossRef]
- Ni’maturrohmah, D.; Maharani, D.; Ruzicka, O.; Gitasari, U.H.; Adhitama, E.; Saraswati, T.E. Copper-Graphene Composite: Electrochemical Synthesis and Structural Characterization. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012002. [Google Scholar] [CrossRef]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, X.; Guo, R.; Ye, Z.; Fu, H.; Fu, N.; Guo, Z.; Zhang, J.; Zhang, J. Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging. Molecules 2021, 26, 7063. [Google Scholar] [CrossRef]
- Shapurian, T.; Damoulis, P.D.; Reiser, G.M.; Griffin, T.J.; Rand, W.M. Quantitative evaluation of bone density using the Hounsfield index. Int. J. Oral Maxillofac. Implants 2006, 21, 290–297. [Google Scholar] [PubMed]
- Koç, M.M.; Aslan, N.; Kao, A.P.; Barber, A.H. Evaluation of X-ray tomography contrast agents: A review of production, protocols, and biological applications. Microsc. Res. Tech. 2019, 82, 812–848. [Google Scholar] [CrossRef]
- Attia, M.F.; Wallyn, J.; Anton, N.; Vandamme, T.F. Inorganic Nanoparticles for X-ray Computed Tomography Imaging. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 391–431. [Google Scholar] [CrossRef]
- Chingo Aimacaña, C.M.; Quinchiguango Perez, D.A.; Rocha Pinto, S.; Debut, A.; Attia, M.F.; Santos-Oliveira, R.; Whitehead, D.C.; Terencio, T.; Alexis, F.; Dahoumane, S.A. Polytetrafluoroethylene-like Nanoparticles as a Promising Contrast Agent for Dual Modal Ultrasound and X-ray Bioimaging. ACS Biomater. Sci. Eng. 2021, 7, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Nguyen, M.-D. Gold Nanoparticle-Based Fluorescent Contrast Agent with Enhanced Sensitivity. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 977, pp. 399–407. [Google Scholar]
- Guo, J.; Xu, L.; Zhang, H.; Yang, Q. Clinical Analysis of Magnetic Nanoparticle Contrast Agent in the Diagnosis of Occult Fracture by Multislice Spiral CT and MRI. J. Nanosci. Nanotechnol. 2020, 20, 6568–6576. [Google Scholar] [CrossRef]
- DenOtter, T.; Schubert, J. Hounsfield Unit; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Silvestri, A.; Zambelli, V.; Ferretti, A.M.; Salerno, D.; Bellani, G.; Polito, L. Design of functionalized gold nanoparticle probes for computed tomography imaging. Contrast Media Mol. Imaging 2016, 11, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, R.; Sprouse, M.L.; Wang, D.; Ricchetti, S.; Hirsch, M.; Ferrante, N.; Butler, E.B.; Demarchi, D.; Grattoni, A.; Filgueira, C.S. Intratumoral Gold Nanoparticle-Enhanced CT Imaging: An in Vivo Investigation of Biodistribution and Retention. In Proceedings of the 2020 IEEE 20th International Conference on Nanotechnology (IEEE-NANO), Montreal, QC, Canada, 28–31 July 2020; Volume 2020, pp. 349–353. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).