Synthetic CT in Carbon Ion Radiotherapy of the Abdominal Site
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Pre-Processing
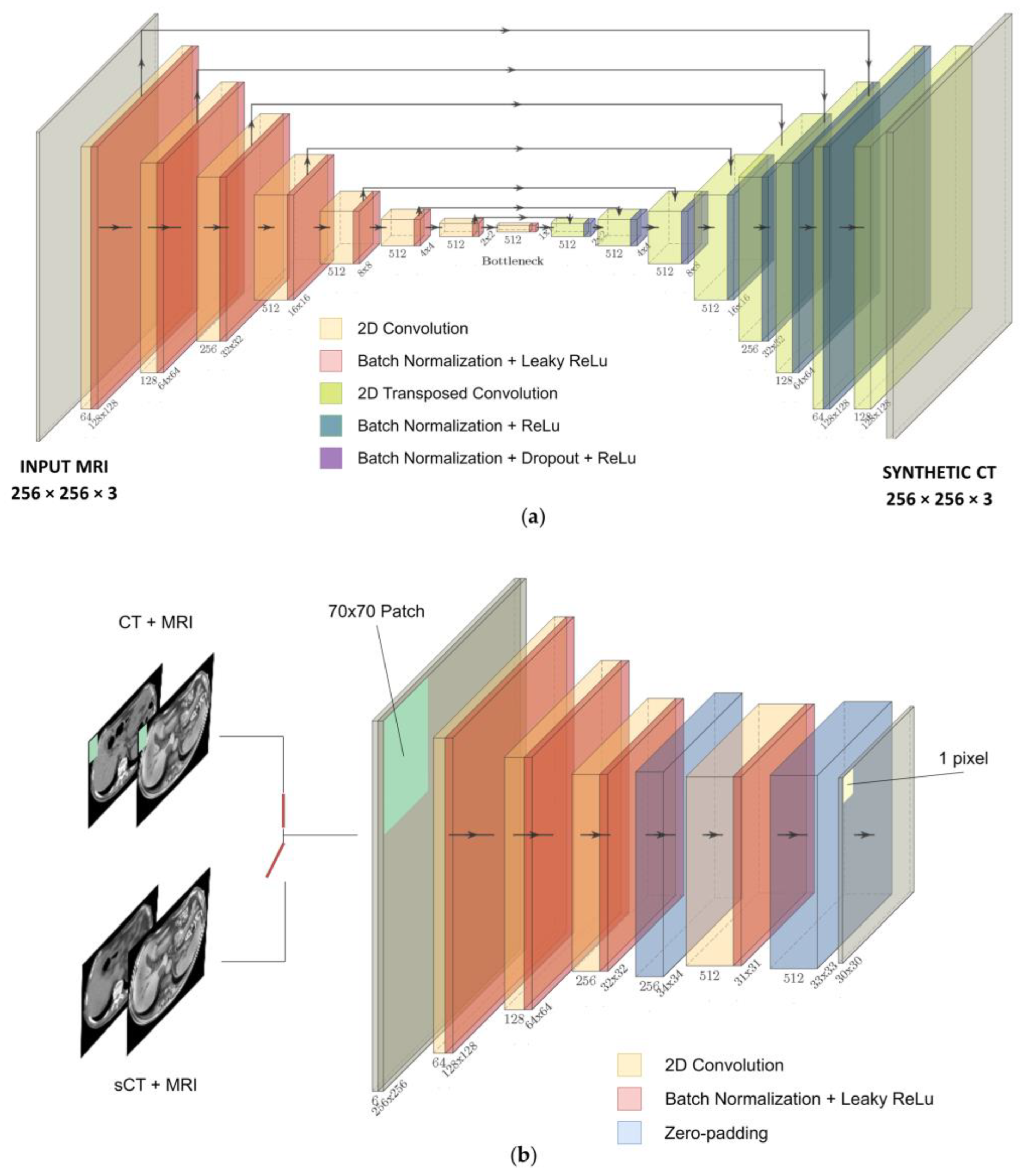
2.3. Neural Network
2.4. Experiments
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-Particle Therapy in Cancer: Clinical Uses and Future Perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef]
- Liermann, J.; Shinoto, M.; Syed, M.; Debus, J.; Herfarth, K.; Naumann, P. Carbon Ion Radiotherapy in Pancreatic Cancer: A Review of Clinical Data. Radiother. Oncol. 2020, 147, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, L.; Bert, C.; Chang, J.; Flampouri, S.; Jee, K.; Lin, L.; Moyers, M.; Mori, S.; Rottmann, J.; et al. AAPM Task Group Report 290: Respiratory Motion Management for Particle Therapy. Med. Phys. 2022, 49, e50–e81. [Google Scholar] [CrossRef]
- Jaffray, D.A. Image-Guided Radiotherapy: From Current Concept to Future Perspectives. Nat. Rev. Clin. Oncol. 2012, 9, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Otazo, R.; Lambin, P.; Pignol, J.P.; Ladd, M.E.; Schlemmer, H.P.; Baumann, M.; Hricak, H. MRI-Guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology. Radiology 2021, 298, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Brighi, C.; Glide-Hurst, C.; Liney, G.; Liu, P.Z.Y.; Lydiard, S.; Paganelli, C.; Pham, T.; Shan, S.; Tree, A.C.; et al. Integrated MRI-Guided Radiotherapy—Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2022, 19, 458–470. [Google Scholar] [CrossRef]
- Kurz, C.; Buizza, G.; Landry, G.; Kamp, F.; Rabe, M.; Paganelli, C.; Baroni, G.; Reiner, M.; Keall, P.J.; van den Berg, C.A.T.; et al. Medical Physics Challenges in Clinical MR-Guided Radiotherapy. Radiat. Oncol. 2020, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Oborn, B.; Moteabbed, M.; Yan, S.; Bortfeld, T.; Knopf, A.; Fuchs, H.; Georg, D.; Seco, J.; Spadea, M.F.; et al. MR-Guided Proton Therapy: A Review and a Preview. Radiat. Oncol. 2020, 15, 129. [Google Scholar] [CrossRef]
- Paganelli, C.; Meschini, G.; Molinelli, S.; Riboldi, M.; Baroni, G. Patient-Specific Validation of Deformable Image Registration in Radiation Therapy: Overview and Caveats. Med. Phys. 2018, 45, e908–e922. [Google Scholar] [CrossRef] [PubMed]
- Meschini, G.; Vai, A.; Paganelli, C.; Molinelli, S.; Maestri, D.; Fontana, G.; Pella, A.; Vitolo, V.; Valvo, F.; Ciocca, M.; et al. Investigating the Use of Virtual 4DCT from 4DMRI in Gated Carbon Ion Radiation Therapy of Abdominal Tumors. Z. Med. Phys. 2022, 32, 98–108. [Google Scholar] [CrossRef]
- Johnstone, E.; Wyatt, J.J.; Henry, A.M.; Short, S.C.; Sebag-Montefiore, D.; Murray, L.; Kelly, C.G.; McCallum, H.M.; Speight, R. Systematic Review of Synthetic Computed Tomography Generation Methodologies for Use in Magnetic Resonance Imaging–Only Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 199–217. [Google Scholar] [CrossRef]
- Han, X. MR-Based Synthetic CT Generation Using a Deep Convolutional Neural Network Method. Med. Phys. 2017, 44, 1408–1419. [Google Scholar] [CrossRef]
- Dinkla, A.M.; Florkow, M.C.; Maspero, M.; Savenije, M.H.F.; Zijlstra, F.; Doornaert, P.A.H.; van Stralen, M.; Philippens, M.E.P.; van den Berg, C.A.T.; Seevinck, P.R. Dosimetric Evaluation of Synthetic CT for Head and Neck Radiotherapy Generated by a Patch-Based Three-Dimensional Convolutional Neural Network. Med. Phys. 2019, 46, 4095–4104. [Google Scholar] [CrossRef]
- Maspero, M.; Savenije, M.H.F.; Dinkla, A.M.; Seevinck, P.R.; Intven, M.P.W.; Jurgenliemk-Schulz, I.M.; Kerkmeijer, L.G.W.; van den Berg, C.A.T. Dose Evaluation of Fast Synthetic-CT Generation Using a Generative Adversarial Network for General Pelvis MR-Only Radiotherapy. Phys. Med. Biol. 2018, 63, 185001. [Google Scholar] [CrossRef]
- Spadea, M.F.; Pileggi, G.; Zaffino, P.; Salome, P.; Catana, C.; Izquierdo-Garcia, D.; Amato, F.; Seco, J. Deep Convolution Neural Network (DCNN) Multiplane Approach to Synthetic CT Generation From MR Images—Application in Brain Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 495–503. [Google Scholar] [CrossRef]
- Kazemifar, S.; Montero, A.M.B.; Souris, K.; Rivas, S.T.; Timmerman, R.; Park, Y.K.; Jiang, S.; Geets, X.; Sterpin, E.; Owrangi, A. Dosimetric Evaluation of Synthetic CT Generated with GANs for MRI-Only Proton Therapy Treatment Planning of Brain Tumors. J. Appl. Clin. Med. Phys. 2020, 21, 76–86. [Google Scholar] [CrossRef]
- Koerkamp, M.L.G.; de Hond, Y.J.M.; Maspero, M.; Kontaxis, C.; Mandija, S.; Vasmel, J.E.; Charaghvandi, R.K.; Philippens, M.E.P.; van Asselen, B.; van den Bongard, H.J.G.D.; et al. Synthetic CT for Single-Fraction Neoadjuvant Partial Breast Irradiation on an MRI-Linac. Phys. Med. Biol. 2021, 66, 085010. [Google Scholar] [CrossRef]
- Spadea, M.F.; Maspero, M.; Zaffino, P.; Seco, J. Deep Learning Based Synthetic-CT Generation in Radiotherapy and PET: A Review. Med. Phys. 2021, 48, 6537–6566. [Google Scholar] [CrossRef]
- Isola, P.; Zhu, J.; Efros, A.A.; Ai, B.; Berkeley, U.C. Image-to-Image Translation with Conditional Adversarial Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Cusumano, D.; Lenkowicz, J.; Votta, C.; Boldrini, L.; Placidi, L.; Catucci, F.; Dinapoli, N.; Antonelli, M.V.; Romano, A.; de Luca, V.; et al. A Deep Learning Approach to Generate Synthetic CT in Low Field MR-Guided Adaptive Radiotherapy for Abdominal and Pelvic Cases. Radiother. Oncol. 2020, 153, 205–212. [Google Scholar] [CrossRef]
- Fu, J.; Singhrao, K.; Cao, M.; Yu, V.; Anand, P.S.; Yang, Y.; Guo, M.; Raldow, A.C.; Ruan, D.; Lewis, J.H. Generation of Abdominal Synthetic CTs from 0.35T MR Images Using Generative Adversarial Networks for MR-Only Liver Radiotherapy. Biomed. Phys. Eng. Express 2020, 6, 015033. [Google Scholar] [CrossRef]
- Xu, L.; Zeng, X.; Zhang, H.; Li, W.; Lei, J.; Huang, Z. BPGAN: Bidirectional CT-to-MRI Prediction Using Multi-Generative Multi-Adversarial Nets with Spectral Normalization and Localization. Neural Netw. 2020, 128, 82–96. [Google Scholar] [CrossRef]
- Xu, K.; Cao, J.; Xia, K.; Yang, H.; Zhu, J.; Wu, C.; Jiang, Y.; Qian, P. Multichannel Residual Conditional GAN-Leveraged Abdominal Pseudo-CT Generation via Dixon MR Images. IEEE Access 2019, 7, 163823–163830. [Google Scholar] [CrossRef]
- Florkow, M.C.; Guerreiro, F.; Zijlstra, F.; Seravalli, E.; Janssens, G.O.; Maduro, J.H.; Knopf, A.C.; Castelein, R.M.; van Stralen, M.; Raaymakers, B.W.; et al. Deep Learning-Enabled MRI-Only Photon and Proton Therapy Treatment Planning for Paediatric Abdominal Tumours. Radiother. Oncol. 2020, 153, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y.; Wang, Y.; Wang, T.; Ren, L.; Lin, L.; McDonald, M.; Curran, W.J.; Liu, T.; Zhou, J.; et al. MRI-Based Treatment Planning for Proton Radiotherapy: Dosimetric Validation of a Deep Learning-Based Liver Synthetic CT Generation Method. Phys. Med. Biol. 2019, 64, 145015. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Xu, K.; Wang, T.; Zheng, Q.; Yang, H.; Baydoun, A.; Zhu, J.; Traughber, B.; Muzic, R.F. Estimating CT from MR Abdominal Images Using Novel Generative Adversarial Networks. J. Grid Comput. 2020, 18, 211–226. [Google Scholar] [CrossRef]
- Olberg, S.; Chun, J.; Choi, B.S.; Park, I.; Kim, H.; Kim, T.; Kim, J.S.; Green, O.; Park, J.C. Abdominal Synthetic CT Reconstruction with Intensity Projection Prior for MRI-Only Adaptive Radiotherapy. Phys. Med. Biol. 2021, 66, 204001. [Google Scholar] [CrossRef]
- Liu, L.; Johansson, A.; Cao, Y.; Dow, J.; Lawrence, T.; Balter, J. Abdominal Synthetic CT Generation from MR Dixon Images Using a U-Net Trained with ‘Semi-Synthetic’ CT Data. Phys. Med. Biol. 2020, 65, 125001. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Hara, R.; Mori, S.; Yanagi, T.; Asakura, H.; Kishimoto, R.; Kato, H.; Yamada, S.; Kandatsu, S.; Kamada, T. Impact of Intrafractional Bowel Gas Movement on Carbon Ion Beam Dose Distribution in Pancreatic Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Rietzel, E.; Schardt, D.; Haberer, T. Range Accuracy in Carbon Ion Treatment Planning Based on CT-Calibration with Real Tissue Samples. Radiat. Oncol. 2007, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Witt, M.; Weber, U.; Kellner, D.; Engenhart-Cabillic, R.; Zink, K. Optimization of the Stopping-Power-Ratio to Hounsfield-Value Calibration Curve in Proton and Heavy Ion Therapy. Z. Med. Phys. 2015, 25, 251–263. [Google Scholar] [CrossRef]
- Knäusl, B.; Kuess, P.; Stock, M.; Georg, D.; Fossati, P.; Georg, P.; Zimmermann, L. Possibilities and Challenges When Using Synthetic Computed Tomography in an Adaptive Carbon-Ion Treatment Workflow. Z. Med. Phys. 2022. [Google Scholar] [CrossRef]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Udupa, J.K. On Standardizing the MR Image Intensity Scale. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 1999, 42, 1072–1081. [Google Scholar]
- Gupta, D.; Kim, M.; Vineberg, K.A.; Balter, J.M. Generation of Synthetic CT Images from MRI for Treatment Planning and Patient Positioning Using a 3-Channel U-Net Trained on Sagittal Images. Front. Oncol. 2019, 9, 964. [Google Scholar] [CrossRef]
- Ulyanov, D.; Vedaldi, A.; Lempitsky, V. Instance Normalization: The Missing Ingredient for Fast Stylization. arXiv 2016, arXiv:1607.08022. [Google Scholar]
- Segars, W.P.; Sturgeon, G.; Mendonca, S.; Grimes, J.; Tsui, B.M.W. 4D XCAT Phantom for Multimodality Imaging Research. Med. Phys. 2010, 37, 4902–4915. [Google Scholar] [CrossRef]
- Paganelli, C.; Summers, P.; Gianoli, C.; Bellomi, M.; Baroni, G.; Riboldi, M. A Tool for Validating MRI-Guided Strategies: A Digital Breathing CT/MRI Phantom of the Abdominal Site. Med. Biol. Eng. Comput. 2017, 55, 2001–2014. [Google Scholar] [CrossRef]
- Pileggi, G.; Speier, C.; Sharp, G.C.; Izquierdo Garcia, D.; Catana, C.; Pursley, J.; Amato, F.; Seco, J.; Spadea, M.F. Proton Range Shift Analysis on Brain Pseudo-CT Generated from T1 and T2 MR. Acta Oncol. 2018, 57, 1521–1531. [Google Scholar] [CrossRef]
- Vitolo, V.; Cobianchi, L.; Brugnatelli, S.; Barcellini, A.; Peloso, A.; Facoetti, A.; Vanoli, A.; Delfanti, S.; Preda, L.; Molinelli, S.; et al. Preoperative Chemotherapy and Carbon Ions Therapy for Treatment of Resectable and Borderline Resectable Pancreatic Adenocarcinoma: A Prospective, Phase II, Multicentre, Single-Arm Study. BMC Cancer 2019, 19, 922. [Google Scholar] [CrossRef]
- Meschini, G.; Vai, A.; Paganelli, C.; Molinelli, S.; Fontana, G.; Pella, A.; Preda, L.; Vitolo, V.; Valvo, F.; Ciocca, M.; et al. Virtual 4DCT from 4DMRI for the Management of Respiratory Motion in Carbon Ion Therapy of Abdominal Tumors. Med. Phys. 2020, 47, 909–916. [Google Scholar] [CrossRef]




| Patient | N of Beams | Prescribed Dose [Gy(RBE)] | Fractions | Position | Tumor Location |
|---|---|---|---|---|---|
| P17 | 1 | 43 | 10 | Prone | Pancreas |
| P20 | 1 | 38.4 | 8 | Prone | Pancreas |
| P21 | 2 | 57.6 | 12 | Supine | Pancreas |
| P27 | 1 | 38.4 | 8 | Prone | Pancreas |
| P31 | 1 | 48 | 10 | Prone | Pancreas |
| MAE_Body [HU] | RMSE [HU] | SSIM | PSNR [dB] | NCC | MAE_Air [HU] | MAE_Bone [HU] | MAE_Soft [HU] | ||
|---|---|---|---|---|---|---|---|---|---|
| Our work | CV | 56.52 (8.31) | 97.24 (17.56) | 0.651 (0.043) | 27.73 (1.23) | 0.857 (0.054) | 46.19 (6.30) | 90.76 (7.86) | 54.79 (8.98) |
| TEST | 57.08 (2.79) | 99.69 (4.90) | 0.67 (0.06) | 27.64 (0.68) | 0.92 (0.02) | 54.42 (11.48) | 86.03 (10.76) | 55.39 (3.41) | |
| MRI-ONLY | 88.22 (9.88) | 181.10 (11.84) | 0.59 (0.08) | 20.99 (1.49) | 0.76 (0.10) | 279.01 (142.46) | 154.87 (22.90) | 75.00 (8.12) | |
| Literature | [20] | 78.71 (18.46) | - | - | - | - | - | 152.71 (30.14) | 53.89 (10.7) |
| [24] | 62(13) | - | - | 30.0 (1.8) | - | 104(38) ** | 167 (22) | 36 (8) * | |
| [25] | 72.48 (18.16) | - | - | 22.65 (3.63) | 0.92 (0.04) | 108.06 (49.45) | 216.81 (63.0) | 58.62 (30.61) | |
| [26] | 55.56 (2.27) | 106.43 (11.45) | - | - | 0.87 (0.03) | - | - | ||
| [27] | - | - | - | - | - | - | - | 90 (29) | |
| [28] | - | - | - | - | - | - | 110.09 (29.23) *** | - | |
| [21] | 89.8 (18.7) | - | - | 27.4 (1.6) | - | - | - | - | |
| [23] | 60.42 (2.27) | - | - | - | 0.88 (0.03) | - | - | - | |
| [22] | 6.30 (0.56) **** | - | 0.90 (0.42) | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrella, G.; Vai, A.; Nakas, A.; Garau, N.; Meschini, G.; Camagni, F.; Molinelli, S.; Barcellini, A.; Pella, A.; Ciocca, M.; et al. Synthetic CT in Carbon Ion Radiotherapy of the Abdominal Site. Bioengineering 2023, 10, 250. https://doi.org/10.3390/bioengineering10020250
Parrella G, Vai A, Nakas A, Garau N, Meschini G, Camagni F, Molinelli S, Barcellini A, Pella A, Ciocca M, et al. Synthetic CT in Carbon Ion Radiotherapy of the Abdominal Site. Bioengineering. 2023; 10(2):250. https://doi.org/10.3390/bioengineering10020250
Chicago/Turabian StyleParrella, Giovanni, Alessandro Vai, Anestis Nakas, Noemi Garau, Giorgia Meschini, Francesca Camagni, Silvia Molinelli, Amelia Barcellini, Andrea Pella, Mario Ciocca, and et al. 2023. "Synthetic CT in Carbon Ion Radiotherapy of the Abdominal Site" Bioengineering 10, no. 2: 250. https://doi.org/10.3390/bioengineering10020250
APA StyleParrella, G., Vai, A., Nakas, A., Garau, N., Meschini, G., Camagni, F., Molinelli, S., Barcellini, A., Pella, A., Ciocca, M., Vitolo, V., Orlandi, E., Paganelli, C., & Baroni, G. (2023). Synthetic CT in Carbon Ion Radiotherapy of the Abdominal Site. Bioengineering, 10(2), 250. https://doi.org/10.3390/bioengineering10020250






