Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview
Abstract
:1. Introduction
2. Neuropathology of Depression and Anxiety
2.1. Depression
2.2. Anxiety
3. Preclinical Models in Depression and Anxiety
3.1. Vogel’s Conflict Test (VCT)
3.2. Elevated Plus Maze (EPM)
3.3. Open Field Test (OFT, OPF)
3.4. Light–Dark Box (LBD) Test
3.5. Single Prolonged Stress (SPS)
3.6. Olfactory Bulbectomy (OB)
3.7. Forced Swim Test (FST) or Porsolt Swim Test (PST)
3.8. Flinder’s Sensitive Line (FSL)
3.9. Tail Suspension Test (TST)
4. Neuropeptides
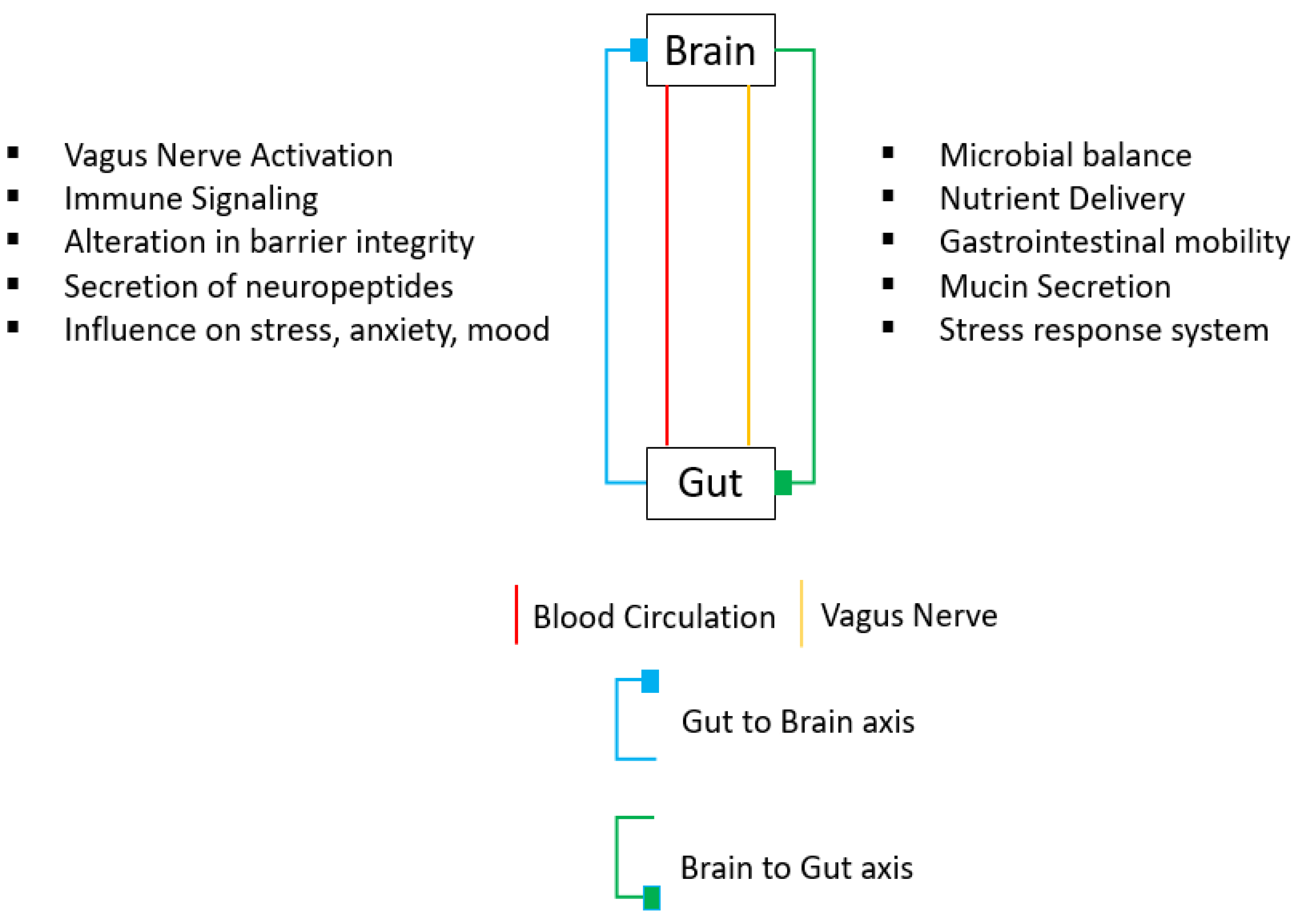
4.1. Gut–Brain Peptides
4.1.1. Neuropeptide Y (NPY)
- NPY in Depression
- NPY in Anxiety
4.1.2. Substance P (SP)
- SP in Depression
- SP in Anxiety
4.1.3. Neurotensin (NT)
- NT in Depression
- NT in Anxiety
4.1.4. Galanin (GAL)
- GAL in Depression
- GAL in Anxiety
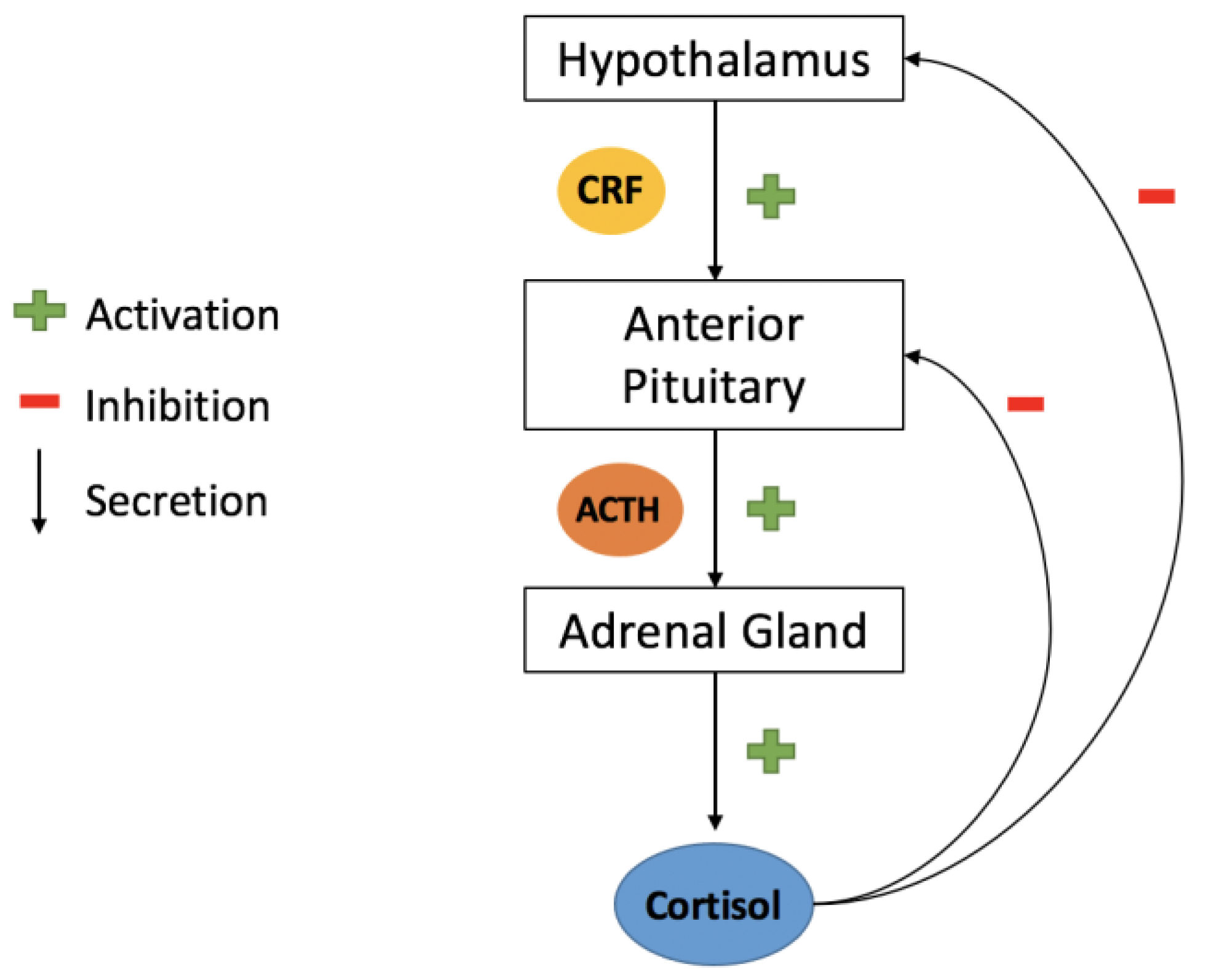
4.2. Hypothalamic Releasing Hormones (HRH)
4.2.1. Corticotrophin-Releasing Factor (CRF)
- CRF in Depression
- CRF in Anxiety
4.2.2. Hypocretin/Orexin
- Orexin in Depression
- Orexin in Anxiety
4.2.3. Melanin-Concentrating Hormone (MCH)
- MCH in Depression
- MCH in Anxiety
4.2.4. Oxytocin (OT)
- OT in Depression
- OT in Anxiety
4.3. Opioid Neuropeptides
4.3.1. Enkephalin (ENK)
- ENK in Depression
- ENK in Anxiety
4.3.2. Endorphins
- Endorphins in Depression
- Endorphins in Anxiety
4.4. Pituitary Hormones
4.4.1. Arginine-Vasopressin (AVP)
- AVP in Depression
- AVP in Anxiety
4.4.2. Adrenocorticotropic Hormone (ACTH)
- ACTH in Depression
- ACTH in Anxiety
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Connell, M.E.; Boat, T.; Warner, K.E. (Eds.) National Research Council (US) and Institute of Medicine (US) Committee on the Prevention of Mental Disorders and Substance Abuse among Children, Youth, and Young Adults: Research Advances and Promising Interventions, Preventing Mental, Emotional, and Behavioral Disorders among Young People: Progress and Possibilities; National Academies Press (US): Washington, DC, USA, 2009. Available online: http://www.ncbi.nlm.nih.gov/books/NBK32775/ (accessed on 31 January 2022).
- Yu, Z.; Lin, Y.-T.; Chen, J.-C. Knockout of NPFFR2 Prevents LPS-Induced Depressive-Like Responses in Mice. Int. J. Mol. Sci. 2021, 22, 7611. [Google Scholar] [CrossRef]
- De Sousa Tomaz, V.; Chaves Filho, A.J.M.; Cordeiro, R.C.; Jucá, P.M.; Soares, M.V.R.; Barroso, P.N.; Cristino, L.M.F.; Jiang, W.; Teixeira, A.L.; de Lucena, D.F.; et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J. Affect. Disord. 2020, 268, 188–200. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Sachdeva, M.; Mehta, V.; Sharma, N.; Singh, S.; Bungau, S. Exploring Sonic Hedgehog Cell Signaling in Neurogenesis: Its Potential Role in Depressive Behavior. Neurochem. Res. 2021, 46, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.J.; Price, R.B.; Charney, D.S. Recent advances in the neurobiology of anxiety disorders: Implications for novel therapeutics. Am. J. Med. Genet. Part C Semin. Med. Genet. 2008, 148C, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Beesdo, K.; Pine, D.S.; Lieb, R.; Wittchen, H.-U. Incidence and Risk Patterns of Anxiety and Depressive Disorders and Categorization of Generalized Anxiety Disorder. Arch. Gen. Psychiatry 2010, 67, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotzinger, S.; Lovejoy, D.A.; Tan, L.A. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 2010, 31, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Tabikh, M.; Chahla, C.; Okdeh, N.; Kovacic, H.; Sabatier, J.-M.; Fajloun, Z. Parkinson disease: Protective role and function of neuropeptides. Peptides 2022, 151, 170713. [Google Scholar] [CrossRef]
- Koolschijn, P.C.M.; van Haren, N.E.; Lensvelt-Mulders, G.J.; Pol, H.H.; Kahn, R.S. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009, 30, 3719–3735. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Laird, A.R.; Maller, J.; Daskalakis, Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008, 29, 683–695. [Google Scholar] [CrossRef]
- Murphy, F.C.; Nimmo-Smith, I.; Lawrence, A.D. Functional neuroanatomy of emotions: A meta-analysis. Cogn. Affect. Behav. Neurosci. 2003, 3, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.K.; Khalid, S.; Loukas, M.; Tubbs, R.S. Neuroanatomy of Anxiety: A Brief Review. Cureus 2018, 10, e2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-B.; Zhang, Y.-Q.; Xue, R.-R.; Yang, Z.-Z.; Zhang, X.-F. Corticotropin-releasing hormone 1 receptor antagonism attenuates chronic unpredictable mild stress-induced depressive-like behaviors in rats. Neuroreport 2020, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Swiergiel, A.H. Effects of Acute and Chronic Stressors and CRF in Rat and Mouse Tests for Depression. Ann. N. Y. Acad. Sci. 2008, 1148, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Serefko, A.; Szopa, A.; Rojek, K.; Poleszak, E.; Skalicka-Woźniak, K.; Dudka, J. Inhibition of the CRF1 receptor influences the activity of antidepressant drugs in the forced swim test in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 769–774. [Google Scholar] [CrossRef]
- Kosari-Nasab, M.; Sadeghi, T.; Bashiri, H.; Shokouhi, G.; Salari, A.-A. The blockade of corticotropin-releasing factor 1 receptor attenuates anxiety-related symptoms and hypothalamus–pituitary–adrenal axis reactivity in mice with mild traumatic brain injury. Behav. Pharmacol. 2019, 30, 220–228. [Google Scholar] [CrossRef]
- Śmiałowska, M.; Zięba, B.; Domin, H. A role of noradrenergic receptors in anxiolytic-like effect of high CRF in the rat frontal cortex. Neuropeptides 2021, 88, 102162. [Google Scholar] [CrossRef]
- Takahashi, A. ASubchapter 7A—Enkephalin. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 55–57. [Google Scholar] [CrossRef]
- Kuteeva, E.; Wardi, T.; Lundström, L.; Sollenberg, U.; Langel, Ü.; Hökfelt, T.; Ögren, S.O. Differential Role of Galanin Receptors in the Regulation of Depression-Like Behavior and Monoamine/Stress-Related Genes at the Cell Body Level. Neuropsychopharmacology 2008, 33, 2573–2585. [Google Scholar] [CrossRef]
- De Souza, M.M.; Silote, G.P.; Herbst, L.S.; Funck, V.R.; Joca, S.R.L.; Beijamini, V. The antidepressant-like effect of galanin in the dorsal raphe nucleus of rats involves GAL 2 receptors. Neurosci. Lett. 2018, 681, 26–30. [Google Scholar] [CrossRef]
- García-Durán, L.; Flores-Burgess, A.; Cantero-García, N.; Puigcerver, A.; Narváez, J.; Fuxe, K.; Santín, L.; Millón, C.; Díaz-Cabiale, Z. Galanin(1–15) Potentiates the Antidepressant-like Effects Induced by Escitalopram in a Rat Model of Depression. Int. J. Mol. Sci. 2021, 22, 10848. [Google Scholar] [CrossRef]
- Saar, I.; Runesson, J.; Järv, J.; Kurrikoff, K.; Langel, Ü. Novel Galanin Receptor Subtype Specific Ligand in Depression Like Behavior. Neurochem. Res. 2012, 38, 398–404. [Google Scholar] [CrossRef]
- Narváez, M.; Escuela, D.O.B.; Millón, C.; Gago, B.; Flores, A.; Santin, L.J.; Fuxe, K.; Narvaez, J.A.; Cabiale, M.Z.D. Galanin receptor 2-neuropeptide Y Y1 receptor interactions in the dentate gyrus are related with antidepressant-like effects. Anat. Embryol. 2015, 221, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.; Souza, M.; Campanha, T.; Muller, C.; Bittencourt, A.; Bortoli, V.; Schenberg, L.; Beijamini, V. Galanin subtype 1 and subtype 2 receptors mediate opposite anxiety-like effects in the rat dorsal raphe nucleus. Behav. Brain Res. 2016, 314, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Funck, V.; Fracalossi, M.; Vidigal, A.; Beijamini, V. Dorsal hippocampal galanin modulates anxiety-like behaviours in rats. Brain Res. 2018, 1687, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lagos, P.; Urbanavicius, J.; Scorza, M.C.; Miraballes, R.; Torterolo, P. Depressive-like profile induced by MCH microinjections into the dorsal raphe nucleus evaluated in the forced swim test. Behav. Brain Res. 2011, 218, 259–266. [Google Scholar] [CrossRef]
- Urbanavicius, J.; Lagos, P.; Torterolo, P.; Scorza, C. Prodepressive effect induced by microinjections of MCH into the dorsal raphe: Time course, dose dependence, effects on anxiety-related behaviors, and reversion by nortriptyline. Behav. Pharmacol. 2014, 25, 316–324. [Google Scholar] [CrossRef]
- Ye, H.; Cui, X.-Y.; Ding, H.; Cui, S.-Y.; Hu, X.; Liu, Y.-T.; Zhao, H.-L.; Zhang, Y.-H. Melanin-Concentrating Hormone (MCH) and MCH-R1 in the Locus Coeruleus May Be Involved in the Regulation of Depressive-Like Behavior. Int. J. Neuropsychopharmacol. 2018, 21, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-Y.; Liu, Q.F.; Hua, C.; Jeong, H.J.; Jang, J.-H.; Jeon, S.; Park, S.J.A.H.-J. Intranasal Administration of Melanin-Concentrating Hormone Reduces Stress-Induced Anxiety- and Depressive-Like Behaviors in Rodents. Exp. Neurobiol. 2020, 29, 453–469. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Zhang, N.; Huang, J.; Ming, X.; Guo, R.; Hu, Y.; Ji, P.; Guo, F. Melanin-concentrating hormone promotes anxiety and intestinal dysfunction via basolateral amygdala in mice. Front. Pharmacol. 2022, 13, 906057. [Google Scholar] [CrossRef]
- Lee, C.; Parks, G.S.; Civelli, O. Anxiolytic Effects of the MCH1R Antagonist TPI 1361-17. J. Mol. Neurosci. 2010, 43, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Ollmann, T.; Péczely, L.; László, K.; Kovács, A.; Gálosi, R.; Kertes, E.; Kállai, V.; Zagorácz, O.; Karádi, Z.; Lénárd, L. Anxiolytic effect of neurotensin microinjection into the ventral pallidum. Behav. Brain Res. 2015, 294, 208–214. [Google Scholar] [CrossRef]
- Li, B.; Chang, L.-L.; Xi, K. Neurotensin 1 receptor in the prelimbic cortex regulates anxiety-like behavior in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 104, 110011. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Matsuura, T.; Xue, M.; Chen, Q.-Y.; Liu, R.-H.; Lu, J.-S.; Shi, W.; Fan, K.; Zhou, Z.; Miao, Z.; et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 2021, 36, 109411. [Google Scholar] [CrossRef] [PubMed]
- Mikrouli, E.; Wörtwein, G.; Soylu, R.; Mathé, A.A.; Petersén, Å. Increased numbers of orexin/hypocretin neurons in a genetic rat depression model. Neuropeptides 2011, 45, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-J.; Zhang, X.-Y.; Chen, Z.; Wang, J.-J.; Zhu, J.-N. Orexin prevents depressive-like behavior by promoting stress resilience. Mol. Psychiatry 2018, 24, 282–293. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wei, C.; Wang, H.; Sui, N.; Kirouac, G.J. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology 2010, 212, 251–265. [Google Scholar] [CrossRef]
- Nakamachi, T.; Shibata, H.; Sakashita, A.; Iinuma, N.; Wada, K.; Konno, N.; Matsuda, K. Orexin A enhances locomotor activity and induces anxiogenic-like action in the goldfish, Carassius auratus. Horm. Behav. 2014, 66, 317–323. [Google Scholar] [CrossRef]
- Avolio, E.; Alò, R.; Carelli, A.; Canonaco, M. Amygdalar orexinergic–GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav. Brain Res. 2011, 218, 288–295. [Google Scholar] [CrossRef]
- Millan, M.J.; Brocco, M. The Vogel conflict test: Procedural aspects, γ-aminobutyric acid, glutamate and monoamines. Eur. J. Pharmacol. 2003, 463, 67–96. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. J. Vis. Exp. 2008, 22, e1088. [Google Scholar] [CrossRef] [Green Version]
- Nordquist, R.E.; Meijer, E.; van der Staay, F.J.; Arndt, S.S. Pigs as Model Species to Investigate Effects of Early Life Events on Later Behavioral and Neurological Functions. In Animal Models for the Study of Human Disease, 2nd ed.; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1003–1030. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Lisieski, M.J.; Eagle, A.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Single-Prolonged Stress: A Review of Two Decades of Progress in a Rodent Model of Post-traumatic Stress Disorder. Front. Psychiatry 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, M. The Olfactory Bulbectomised Mouse. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests, Volume II; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 267–286. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The Mouse Forced Swim Test. J. Vis. Exp. 2012, 59, e3638. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Wegener, G. The Flinders Sensitive Line Rat Model of Depression—25 Years and Still Producing. Pharmacol. Rev. 2013, 65, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. J. Vis. Exp. 2011, 59, e3769. [Google Scholar] [CrossRef] [Green Version]
- Burbach, J.P.H. What Are Neuropeptides? In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 789, pp. 1–36. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [Green Version]
- Kask, A.; Harro, J.; von Hörsten, S.; Redrobe, J.P.; Dumont, Y.; Quirion, R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002, 26, 259–283. [Google Scholar] [CrossRef]
- Hirsch, D.; Zukowska, Z. NPY and Stress 30 Years Later: The Peripheral View. Cell. Mol. Neurobiol. 2012, 32, 645–659. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.C.; Beck-Sickinger, A.; Cox, H.; Doods, H.N.; Herzog, H.; Larhammar, D.; Quirion, R.; Schwartz, T.; Westfall, T., XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998, 50, 143–150. [Google Scholar]
- Stanley, B.G.; Leibowitz, S.F. Neuropeptide Y injected in the paraventricular hypothalamus: A powerful stimulant of feeding behavior. Proc. Natl. Acad. Sci. USA 1985, 82, 3940–3943. [Google Scholar] [CrossRef] [Green Version]
- Kalra, S.P.; Clark, J.T.; Sahu, A.; Dube, M.G.; Kalra, P.S. Control of feeding and sexual behaviors by neuropeptide Y: Physiological implications. Synapse 1988, 2, 254–257. [Google Scholar] [CrossRef]
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The Role of Neuropeptide Y in Cardiovascular Health and Disease. Front. Physiol. 2018, 9, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsell, A.; Michalkiewicz, M.; Dumont, Y.; Quirion, R.; Caberlotto, L.; Rimondini, R.; Mathé, A.A.; Heilig, M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc. Natl. Acad. Sci. USA 2000, 97, 12852–12857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, H.; Onishi, H.; Koide, S.; Toshihiro, K.; Yamagami, S. Plasma neuropeptide Y in patients with major depressive disorder. Neurosci. Lett. 1996, 216, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; Zachrisson, O.; Thorsell, A.; Ehnvall, A.; Mottagui-Tabar, S.; Sjogren, M.; Åsberg, M.; Ekman, R.; Wahlestedt, C.; Ågren, H. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: Preliminary evidence for association with preproNPY gene polymorphism. J. Psychiatr. Res. 2003, 38, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ren, X.; Zhang, H.; Pandey, G.N. Effect of depression and suicidal behavior on neuropeptide Y (NPY) and its receptors in the adult human brain: A postmortem study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 112, 110428. [Google Scholar] [CrossRef] [PubMed]
- Frisch, C.; Hanke, J.; Kleinerüschkamp, S.; Röske, S.; Kaaden, S.; Elger, C.E.; Schramm, J.; Yilmazer-Hanke, D.M.; Helmstaedter, C. Positive Correlation Between the Density of Neuropeptide Y Positive Neurons in the Amygdala and Parameters of Self-Reported Anxiety and Depression in Mesiotemporal Lobe Epilepsy Patients. Biol. Psychiatry 2009, 66, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, S.; Eker, O.O.; Abdulrezzak, U. The Effects of Antidepressants on Neuropeptide Y in Patients with Depression and Anxiety. Pharmacopsychiatry 2016, 49, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Serova, L.; Laukova, M.; Alaluf, L.; Pucillo, L.; Sabban, E. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014, 24, 142–147. [Google Scholar] [CrossRef]
- Sabban, E.L.; Serova, L.I.; Alaluf, L.G.; Laukova, M.; Peddu, C. Comparative effects of intranasal neuropeptide Y and HS014 in preventing anxiety and depressive-like behavior elicited by single prolonged stress. Behav. Brain Res. 2015, 295, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Nwokafor, C.; Serova, L.I.; Nahvi, R.J.; McCloskey, J.; Sabban, E.L. Activation of NPY receptor subtype 1 by [D-His26]NPY is sufficient to prevent development of anxiety and depressive like effects in the single prolonged stress rodent model of PTSD. Neuropeptides 2019, 80, 102001. [Google Scholar] [CrossRef]
- Bjørnebekk, A.; Mathé, A.A.; Brené, S. The Antidepressant Effects of Running and Escitalopram Are Associated with Levels of Hippocampal NPY and Y1 Receptor but Not Cell Proliferation in a Rat Model of Depression. Hippocampus 2009, 20, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Hori, A.; Yabe, T.; Nagai, T.; Oikawa, T.; Yamada, H.; Hanawa, T. Involvement of neuropeptide y signaling in the antidepressant-like effect and hippocampal cell proliferation induced by kososan, a kampo medicine, in the stress-induced depression-like model mice. Biol. Pharm. Bull. 2012, 35, 1775–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domschke, K.; Dannlowski, U.; Hohoff, C.; Ohrmann, P.; Bauer, J.; Kugel, H.; Zwanzger, P.; Heindel, W.; Deckert, J.; Arolt, V.; et al. Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur. Neuropsychopharmacol. 2010, 20, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, K.; Kawabe, M.; Karasuyama, K.; Kurachi, T.; Hayashi, A.; Ataka, K.; Iwai, H.; Takeno, H.; Hayasaka, O.; Kotani, T.; et al. Neuropeptide Y deficiency induces anxiety-like behaviours in zebrafish (Danio rerio). Sci. Rep. 2020, 10, 5913. [Google Scholar] [CrossRef] [Green Version]
- Kawabe, M.; Hayashi, A.; Komatsu, M.; Inui, A.; Shiozaki, K. Ninjinyoeito improves anxiety behavior in neuropeptide Y deficient zebrafish. Neuropeptides 2021, 87, 102136. [Google Scholar] [CrossRef]
- Deo, G.S.; Dandekar, M.P.; Upadhya, M.A.; Kokare, D.M.; Subhedar, N.K. Neuropeptide Y Y1 receptors in the central nucleus of amygdala mediate the anxiolytic-like effect of allopregnanolone in mice: Behavioral and immunocytochemical evidences. Brain Res. 2010, 1318, 77–86. [Google Scholar] [CrossRef]
- Taksande, B.; Kotagale, N.R.; Gawande, D.; Bharne, A.P.; Chopde, C.T.; Kokare, D.M. Neuropeptide Y in the central nucleus of amygdala regulates the anxiolytic effect of agmatine in rats. Eur. Neuropsychopharmacol. 2014, 24, 955–963. [Google Scholar] [CrossRef]
- Yamada, S.; Islam, M.S.; van Kooten, N.; Bovee, S.; Oh, Y.-M.; Tsujimura, A.; Watanabe, Y.; Tanaka, M. Neuropeptide Y neurons in the nucleus accumbens modulate anxiety-like behavior. Exp. Neurol. 2020, 327, 113216. [Google Scholar] [CrossRef]
- Chang, M.M.; Leeman, S.E.; Niall, H.D. Amino-acid Sequence of Substance P. Nat. New Biol. 1971, 232, 86–87. [Google Scholar] [CrossRef]
- Hökfelt, T.; Pernow, B.; Wahren, J. Substance P: A pioneer amongst neuropeptides. J. Intern. Med. 2001, 249, 27–40. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Zhao, J.; Ji, X.; Wei, H.; Luo, Y. Plasma and cerebrospinal fluid substance P in post-stroke patients with depression. Psychiatry Clin. Neurosci. 2009, 63, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-M.; Yu, L.; Jin, H.-J.; Zhao, H. Substance P receptor antagonist in lateral habenula improves rat depression-like behavior. Brain Res. Bull. 2014, 100, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Kerr, D.; Hunt, S.; Kelly, J. Neurokinin-1 receptor deletion modulates behavioural and neurochemical alterations in an animal model of depression. Behav. Brain Res. 2012, 228, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebner, K.; Muigg, P.; Singewald, G.; Singewald, N. Substance P in Stress and Anxiety. Ann. N. Y. Acad. Sci. 2008, 1144, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Bassi, G.S.; de Carvalho, M.C.; Brandão, M.L. Effects of substance P and Sar-Met-SP, a NK1 agonist, in distinct amygdaloid nuclei on anxiety-like behavior in rats. Neurosci. Lett. 2014, 569, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.; Masson, S.; Brandão, M.; Silva, M.D.S. Anxiolytic-like effects of substance P administration into the dorsal, but not ventral, hippocampus and its influence on serotonin. Peptides 2008, 29, 1191–1200. [Google Scholar] [CrossRef]
- Carey, L.M.; Rice, R.J.; Prus, A.J. The Neurotensin NTS1 Receptor Agonist PD149163 Produces Antidepressant-Like Effects in the Forced Swim Test: Further Support for Neurotensin as a Novel Pharmacologic Strategy for Antidepressant Drugs. Drug Dev. Res. 2017, 78, 196–202. [Google Scholar] [CrossRef]
- Wölk, E.; Stengel, A.; Schaper, S.; Rose, M.; Hofmann, T. Neurotensin and Xenin Show Positive Correlations With Perceived Stress, Anxiety, Depressiveness and Eating Disorder Symptoms in Female Obese Patients. Front. Behav. Neurosci. 2021, 15, 629729. [Google Scholar] [CrossRef]
- Ellenbroek, B.A.; Angelucci, F.; Husum, H.; Mathé, A.A. Gene-environment interactions in a rat model of depression. Maternal separation affects neurotensin in selected brain regions. Neuropeptides 2016, 59, 83–88. [Google Scholar] [CrossRef]
- László, K.; Tóth, K.; Kertes, E.; Péczely, L.; Lénárd, L. The role of neurotensin in positive reinforcement in the rat central nucleus of amygdala. Behav. Brain Res. 2010, 208, 430–435. [Google Scholar] [CrossRef]
- Melander, T.; Hökfelt, T.; Rökaeus, A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J. Comp. Neurol. 1986, 248, 475–517. [Google Scholar] [CrossRef] [PubMed]
- Branchek, A.T.; Smith, E.K.; Gerald, C.; Walker, M.W. Galanin receptor subtypes. Trends Pharmacol. Sci. 2000, 21, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kuteeva, E.; Hokfelt, T.; Wardi, T.; Ögren, S.O. Galanin—25 years with a multitalented neuropeptide. Cell. Mol. Life Sci. 2008, 65, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, Signaling, and Pharmacology of Galanin Peptides and Receptors: Three Decades of Emerging Diversity. Pharmacol. Rev. 2014, 67, 118–175. [Google Scholar] [CrossRef] [Green Version]
- Machado, F.D.C.; De Souza, L.V.; Rangel, M.; Jara, Z.P.; Franco, M.D.C. Implication of galanin gene rs948854 polymorphism in depressive symptoms in adolescents. Horm. Behav. 2018, 97, 14–17. [Google Scholar] [CrossRef]
- Locker, F.; Bieler, L.; Nowack, L.; Leitner, J.; Brunner, S.; Zaunmair, P.; Kofler, B.; Couillard-Despres, S. Involvement of Neuropeptide Galanin Receptors 2 and 3 in Learning, Memory and Anxiety in Aging Mice. Molecules 2021, 26, 1978. [Google Scholar] [CrossRef]
- Keszler, G.; Molnár, Z.; Rónai, Z.; Sasvári-Székely, M.; Székely, A.; Kótyuk, E. Association between anxiety and non-coding genetic variants of the galanin neuropeptide. PLoS ONE 2019, 14, e0226228. [Google Scholar] [CrossRef]
- Shahid, Z.; Asuka, E.; Singh, G. Physiology, Hypothalamus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535380/ (accessed on 14 July 2022).
- Ventura-Silva, A.P.; Borges, S.; Sousa, N.; Rodrigues, A.J.; Pêgo, J.M. Amygdalar corticotropin-releasing factor mediates stress-induced anxiety. Brain Res. 2019, 1729, 146622. [Google Scholar] [CrossRef]
- Backström, T.; Pettersson, A.; Johansson, V.; Winberg, S. CRF and urotensin I effects on aggression and anxiety-like behavior in rainbow trout. J. Exp. Biol. 2011, 214, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.M.; Vecchiarelli, H.A.; Morena, M.; Lee, T.T.Y.; Hermanson, D.J.; Kim, A.B.; McLaughlin, R.J.; Hassan, K.I.; Kuhne, C.; Wotjak, C.T.; et al. Corticotropin-Releasing Hormone Drives Anandamide Hydrolysis in the Amygdala to Promote Anxiety. J. Neurosci. 2015, 35, 3879–3892. [Google Scholar] [CrossRef] [Green Version]
- Palotai, M.; Telegdy, G.; Jászberényi, M. Orexin A-induced anxiety-like behavior is mediated through GABA-ergic, α- and β-adrenergic neurotransmissions in mice. Peptides 2014, 57, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Arendt, D.H.; Ronan, P.J.; Oliver, K.D.; Callahan, L.B.; Summers, T.R.; Summers, C.H. Depressive behavior and activation of the orexin/hypocretin system. Behav. Neurosci. 2013, 127, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nollet, M.; Gaillard, P.; Minier, F.; Tanti, A.; Belzung, C.; Leman, S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 2011, 61, 336–346. [Google Scholar] [CrossRef]
- Nocjar, C.; Zhang, J.; Feng, P.; Panksepp, J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 2012, 218, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, Y.; Liu, C.; Yan, Z.; Ma, Q.; Chen, J.; Zhang, M.; Yan, Q.; Li, X.; Chen, J. Xiaoyaosan regulates depression-related behaviors with physical symptoms by modulating Orexin A/OxR1 in the hypothalamus. Anat. Rec. 2020, 303, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Fendt, M. Increased anxiety but normal fear and safety learning in orexin-deficient mice. Behav. Brain Res. 2017, 320, 210–218. [Google Scholar] [CrossRef]
- Akça, F.; Uzun, N.; Kılınç, I. Orexin A in adolescents with anxiety disorders. Int. J. Psychiatry Clin. Pract. 2020, 24, 127–134. [Google Scholar] [CrossRef]
- Tsunematsu, T.; Ueno, T.; Tabuchi, S.; Inutsuka, A.; Tanaka, K.F.; Hasuwa, H.; Kilduff, T.S.; Terao, A.; Yamanaka, A. Optogenetic Manipulation of Activity and Temporally Controlled Cell-Specific Ablation Reveal a Role for MCH Neurons in Sleep/Wake Regulation. J. Neurosci. 2014, 34, 6896–6909. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.; Jurek, B. The interplay between oxytocin and the CRF system: Regulation of the stress response. Cell Tissue Res. 2018, 375, 85–91. [Google Scholar] [CrossRef]
- Ozsoy, S.; Esel, E.; Kula, M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009, 169, 249–252. [Google Scholar] [CrossRef]
- Lara-Cinisomo, S.; D’Anna-Hernandez, K.; Fujimoto, E.M.; Pedersen, C.A. Exploring associations between perinatal depression, anxiety, and urinary oxytocin levels in Latinas. Arch. Women’s Ment. Health 2018, 22, 447–455. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Robins, S.; Chen, G.; Yerko, V.; Zhou, Y.; Nagy, C.; Feeley, N.; Gold, I.; Hayton, B.; Turecki, G.; et al. Perinatal depression and DNA methylation of oxytocin-related genes: A study of mothers and their children. Horm. Behav. 2017, 96, 84–94. [Google Scholar] [CrossRef] [PubMed]
- La Fratta, I.; Franceschelli, S.; Speranza, L.; Patruno, A.; Michetti, C.; D’Ercole, P.; Ballerini, P.; Grilli, A.; Pesce, M. Salivary oxytocin, cognitive anxiety and self-confidence in pre-competition athletes. Sci. Rep. 2021, 11, 16877. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Watson, S.J.; Young, E.; Lewis, M.E.; Khachaturian, H.; Walker, J.M. Endogenous Opioids: Biology and Function. Annu. Rev. Neurosci. 1984, 7, 223–255. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.S.; Gendron, L.; Tremblay, M.-E.; Drolet, G. Enkephalins: Endogenous Analgesics with an Emerging Role in Stress Resilience. Neural Plast. 2017, 2017, e1546125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossier, J.; Guillemin, R.; Bloom, F. Foot shock induced stress decreases LEU5-enkephalin immunoreactivity in rat hypothalamus. Eur. J. Pharmacol. 1978, 48, 465–466. [Google Scholar] [CrossRef]
- Nabeshima, T.; Katoh, A.; Wada, M.; Kameyama, T. Stress-induced changes in brain Met-enkephalin, Leu-enkephalin and dynorphin concentrations. Life Sci. 1992, 51, 211–217. [Google Scholar] [CrossRef]
- Felipe, M.D.C.D.; Jiménez, I.; Castro, A.; Fuentes, J. Antidepressant action of imipramine and iprindole in mice is enhanced by inhibitors of enkephalin-degrading peptidases. Eur. J. Pharmacol. 1989, 159, 175–180. [Google Scholar] [CrossRef]
- Nam, H.; Chandra, R.; Francis, T.C.; Dias, C.; Cheer, J.F.; Lobo, M.K. Reduced nucleus accumbens enkephalins underlie vulnerability to social defeat stress. Neuropsychopharmacology 2019, 44, 1876–1885. [Google Scholar] [CrossRef]
- Bérubé, P.; Laforest, S.; Bhatnagar, S.; Drolet, G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol. Behav. 2013, 122, 237–245. [Google Scholar] [CrossRef]
- Bergström, A.; Jayatissa, M.; Mørk, A.; Wiborg, O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008, 1196, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, P.; Poulin, J.-F.; Laforest, S.; Drolet, G. Enkephalin Knockdown in the Basolateral Amygdala Reproduces Vulnerable Anxiety-Like Responses to Chronic Unpredictable Stress. Neuropsychopharmacology 2013, 39, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Giri, A.K.; Hruby, V.J. Investigational peptide and peptidomimetic μ and δ opioid receptor agonists in the relief of pain. Expert Opin. Investig. Drugs 2013, 23, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.H.; Kitchen, I.; Bailey, A. The endogenous opioid system in cocaine addiction: What lessons have opioid peptide and receptor knockout mice taught us? Br. J. Pharmacol. 2012, 166, 1993–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinas, P.C.; Koutedakis, Y.; Flouris, A.D. Effects of exercise and physical activity on depression. Ir. J. Med. Sci. 2010, 180, 319–325. [Google Scholar] [CrossRef]
- Navinés, R.; Martín-Santos, R.; Gómez-Gil, E.; de Osaba, M.J.M.; Gastó, C. Interaction between serotonin 5-HT1A receptors and β-endorphins modulates antidepressant response. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1804–1809. [Google Scholar] [CrossRef]
- Grisel, J.E.; Bartels, J.L.; Allen, S.A.; Turgeon, V.L. Influence of β-Endorphin on anxious behavior in mice: Interaction with EtOH. Psychopharmacology 2008, 200, 105–115. [Google Scholar] [CrossRef] [Green Version]
- McWilliams, L.A.; Asmundson, G.J. Is there a negative association between anxiety sensitivity and arousal-increasing substances and activities? J. Anxiety Disord. 2001, 15, 161–170. [Google Scholar] [CrossRef]
- Thyer, B.; Matthews, J. The effect of phobic anxiety on plasma β-endorphin: A single-subject experiment. Behav. Res. Ther. 1986, 24, 237–241. [Google Scholar] [CrossRef]
- Sadiq, N.M.; Tadi, P. Physiology, Pituitary Hormones. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Henriquez-Hernandez, L.A.; Flores-Morales, A.; Santana-Farré, R.; Axelson, M.; Nilsson, P.; Norstedt, G.; Fernandez-Perez, L. Role of Pituitary Hormones on 17α-Ethinylestradiol-Induced Cholestasis in Rat. Experiment 2006, 320, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Boone, M.; Deen, P.M.T. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflügers Arch. -Eur. J. Physiol. 2008, 456, 1005–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.B.; Landgraf, R.; Keck, E.M. Vasopressin, major depression, and hypothalamic–pituitary–adrenocortical desensitization. Biol. Psychiatry 2000, 48, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A.; Hage, B.; Carter, C.S.; Nazarloo, H.P.; Singh, J. Does Arginine Vasopressin Reflect HPA-axis Activation in Major Depressive Disorder? Clin. Exp. Investig. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Van Londen, L.; Goekoop, J.G.; Van Kempen, G.M.; Frankhuijzen-Sierevogel, A.C.; Wiegant, V.M.; Van Der Velde, E.A.; De Wied, D. Plasma Levels of Arginine Vasopressin Elevated in Patients with Major Depression. Neuropsychopharmacology 1997, 17, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Landgraf, R. The Involvement of the Vasopressin System in Stress-Related Disorders. CNS Neurol. Disord.-Drug Targets 2006, 5, 167–179. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Hu, S.-B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology 2003, 29, 483–493. [Google Scholar] [CrossRef]
- Miller, W.L. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm. Res. Paediatr. 2018, 89, 212–223. [Google Scholar] [CrossRef]
- Choi, K.W.; Na, E.J.; Fava, M.; Mischoulon, D.; Cho, H.; Jeon, H.J. Increased adrenocorticotropic hormone (ACTH) levels predict severity of depression after six months of follow-up in outpatients with major depressive disorder. Psychiatry Res. 2018, 270, 246–252. [Google Scholar] [CrossRef]
- Inder, W.J.; Prickett, T.C.; Joyce, P.R.; Mulder, R.T.; Donald, R.A. Reduction in basal afternoon plasma ACTH during early treatment of depression with fluoxetine. Psychopharmacology 2001, 156, 73–78. [Google Scholar] [CrossRef]
- Daniels, W.M.U.; Pietersen, C.Y.; Carstens, M.E.; Stein, D. Maternal Separation in Rats Leads to Anxiety-Like Behavior and a Blunted ACTH Response and Altered Neurotransmitter Levels in Response to a Subsequent Stressor. Metab. Brain Dis. 2004, 19, 3–14. [Google Scholar] [CrossRef]
- File, E.S.; Vellucci, S.V. Studies on the role of ACTH and of 5-HT in anxiety, using an animal model. J. Pharm. Pharmacol. 1978, 30, 105–110. [Google Scholar] [CrossRef] [PubMed]
| Target Neuropeptide | Administered Substance | Model | Species | Administration | Effect | Reference |
|---|---|---|---|---|---|---|
| CRF | CP1544526 (CRF1 receptor blocker) | CUMS | Rat | ↓ | [13] | |
| CRF | CRF | TST, FST | Mouse | i.c.v | ↓ | [14] |
| CRF | CRF | FST | Rat | i.c.v | ↑ | [15] |
| CRF | CRF | mTBI | Mouse | i.c.v | + | [16] |
| CRF | CF1R antagonist, antalarmin | mTBI | Mouse | i.c.v | − | [16] |
| CRF | CRF, stressin 1 (CF1R agonist) | EPM | Rat | Bilateral injection | + | [17] |
| CRF | NBI 27914 (CF1R antagonist) | EPM | Rat | Bilateral injection | − | [17] |
| ENK | Enkephalinase inhibitors | Social Interaction Test | Mouse | Intra-NC | ↓ | [18] |
| GAL | Galanin, GALR1 agonist (M617), GALR2 antagonist (M817) | FST | Rat | i.c.v | ↑ | [19] |
| GAL | GALR2 agonist AR-M1896 | FST | Rat | i.c.v | ↓ | [19] |
| GAL | Galanin, AR-M1896 | FST | Rat | Intra-DRN | ↓ | [20] |
| GAL | M617 | FST | Rat | Intra-DRN | 0 | [20] |
| GAL | AR-M1896, M617 | OFT | Rat | Intra-DRN | 0 | [20] |
| GAL | GAL (1-15) | OB | Rat | i.c.v | ↓ | [21] |
| GAL | M871 | OB | Rat | i.c.v | ↑ | [21] |
| GAL | GalR2 selective agonist M1160 | TST | Mouse | i.c.v | ↓ | [22] |
| GAL | Galanin + NPYY1R agonist | FST | Rat | i.c.v | ↓ | [23] |
| GAL | M617 | ETM, OF | Rat | Intra-DRN | + | [24] |
| GAL | AR-M1896 | ETM, OF | Rat | Intra-DRN | − | [24] |
| GAL | Galanin | EPM | Rat | Intra-dorsal hippocampus | + | [25] |
| GAL | M871 | EPM | Rat | Intra-dorsal hippocampus | − | [25] |
| MCH | MCH | FST | Rat | Intra-DRN | ↑ | [26] |
| MCH | MCH-1 receptor antagonist ATC0175 | FST | Rat | Intra-DRN | ↓ | [27] |
| MCH | MCH | FST | Rat | Intra-LC | ↑ | [28] |
| MCH | SNAP-94847, a MCH receptor 1 antagonist | FST | Rat | Intra-LC | ↓ | [28] |
| MCH | MCH | Stress model | Rat, Mouse | Intranasal | ↓ | [29] |
| MCH | MCH | EPM | Mouse | Intra-BLA | + | [30] |
| MCH | SNAP-94847, a MCH receptor 1 antagonist | EPM | Mouse | Intra-BLA | − | [30] |
| MCH | MCH | Chronic acute combining stress | Mouse | Intra-BLA | − | [30] |
| MCH | TPI 1361-17, a MCH1R antagonist | EPM, LBD | Mouse | Central administration | − | [31] |
| MCH | MCH | EPM | Rat | Intra-DRN | 0 | [27] |
| NT | NT1R antagonist (SR 48,692) + NT after 15 min | OPF | Rat | Bilateral micro- injection in the VP | + | [32] |
| NT | NTS1 agonist, NT | EPM, LBD, OFT | Rat | Injection into the PrL | + | [33] |
| NT | NTS1 antagonist | EPM, LBD, OFT | Rat | Injection into the PrL | 0 | [33] |
| OT | OT | Common peroneal nerve (CPN) ligation | Mouse | Intra-ACC | − | [34] |
| OX | OX | FRL | Rat | Microinjection in VP | ↓ | [35] |
| OX | TCS1102, ORX1 and ORX2 receptor antagonist | FRL | Rat | Microinjection in VP | ↑ | [36] |
| OX | OxA, OxB | EPM | Rat | Microinjection into the PVT | + | [37] |
| OX | TCSOX229 (Ox2R antagonist) | EPM | Rat | Microinjection into the PVT | − | [37] |
| OX | OxA | Black and white test | Goldfish | i.c.v | − | [38] |
| OX | SB334867 (OX1R antagonist) | Black and white test | Goldfish | i.c.v | + | [38] |
| OX | OX | EPM, LBD | Hamster | i.c.v | − | [39] |
| OX | Flunitrazepam (GABA receptor 2 subunit agonist) | EPM, LBD | Hamster | i.c.v | + | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okdeh, N.; Mahfouz, G.; Harb, J.; Sabatier, J.-M.; Roufayel, R.; Gazo Hanna, E.; Kovacic, H.; Fajloun, Z. Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview. Bioengineering 2023, 10, 258. https://doi.org/10.3390/bioengineering10020258
Okdeh N, Mahfouz G, Harb J, Sabatier J-M, Roufayel R, Gazo Hanna E, Kovacic H, Fajloun Z. Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview. Bioengineering. 2023; 10(2):258. https://doi.org/10.3390/bioengineering10020258
Chicago/Turabian StyleOkdeh, Nathalie, Georges Mahfouz, Julien Harb, Jean-Marc Sabatier, Rabih Roufayel, Eddie Gazo Hanna, Hervé Kovacic, and Ziad Fajloun. 2023. "Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview" Bioengineering 10, no. 2: 258. https://doi.org/10.3390/bioengineering10020258
APA StyleOkdeh, N., Mahfouz, G., Harb, J., Sabatier, J.-M., Roufayel, R., Gazo Hanna, E., Kovacic, H., & Fajloun, Z. (2023). Protective Role and Functional Engineering of Neuropeptides in Depression and Anxiety: An Overview. Bioengineering, 10(2), 258. https://doi.org/10.3390/bioengineering10020258









