Rotational Distortion and Compensation in Optical Coherence Tomography with Anisotropic Pixel Resolution
Abstract
:1. Introduction
2. Theoretical Simulation of Rotational Distortion and Compensation
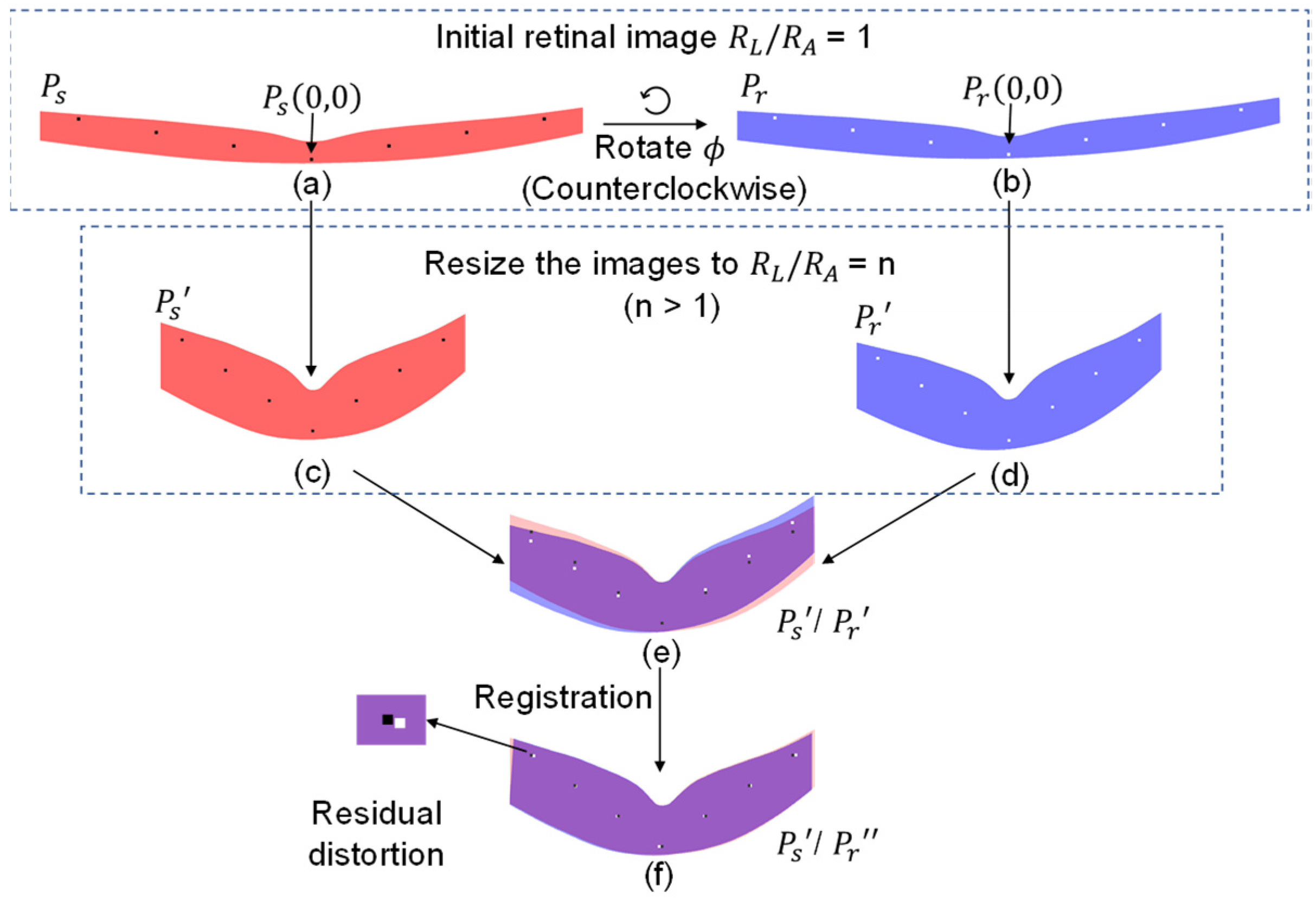
2.1. Conceptual Illustration of Rotational Distortion
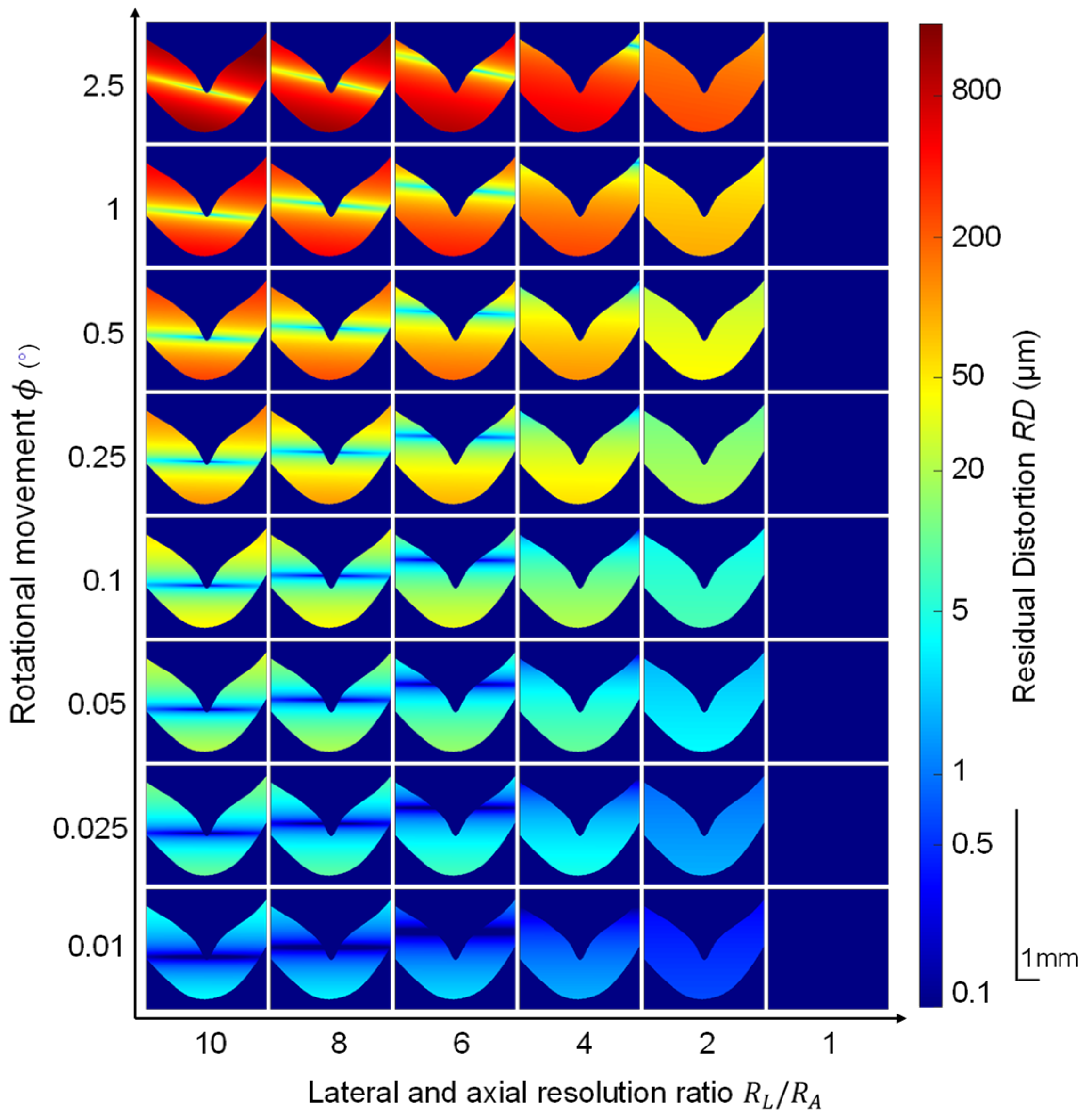
2.2. Quantitative Simulation of Rotational Movement
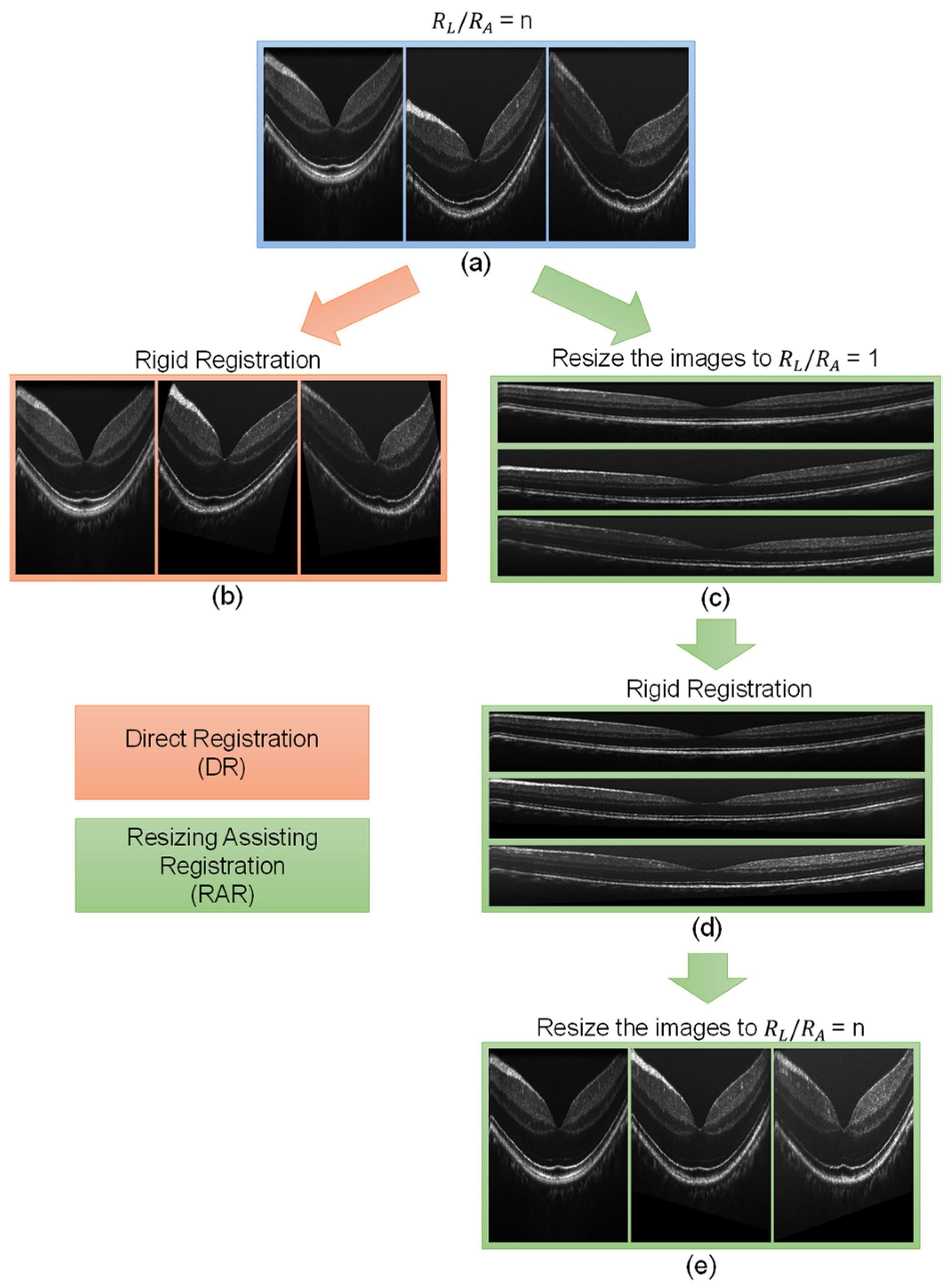
2.3. Image Registration
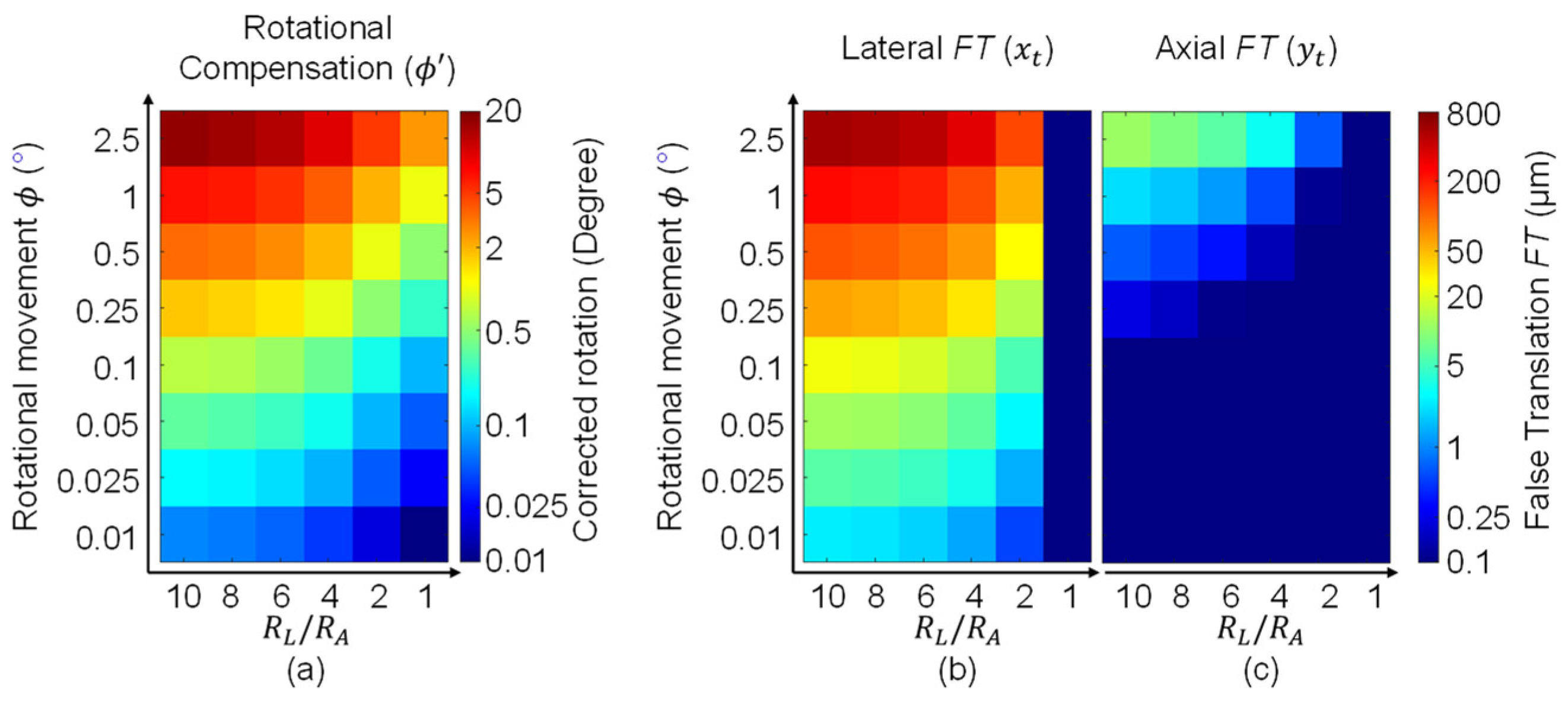
2.4. Displacement Characterization
2.5. Characterization of the Transformation Occurred during Registration
2.6. Summary
3. Materials and Methods for Experimental Validation
3.1. Human Subjects
3.2. Imaging System and Data Acquisition
3.3. Data Processing
4. Experimental Results
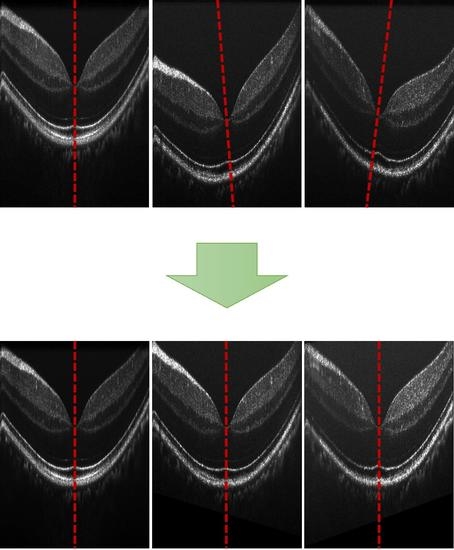
4.1. Registration of Repeated B-Scans
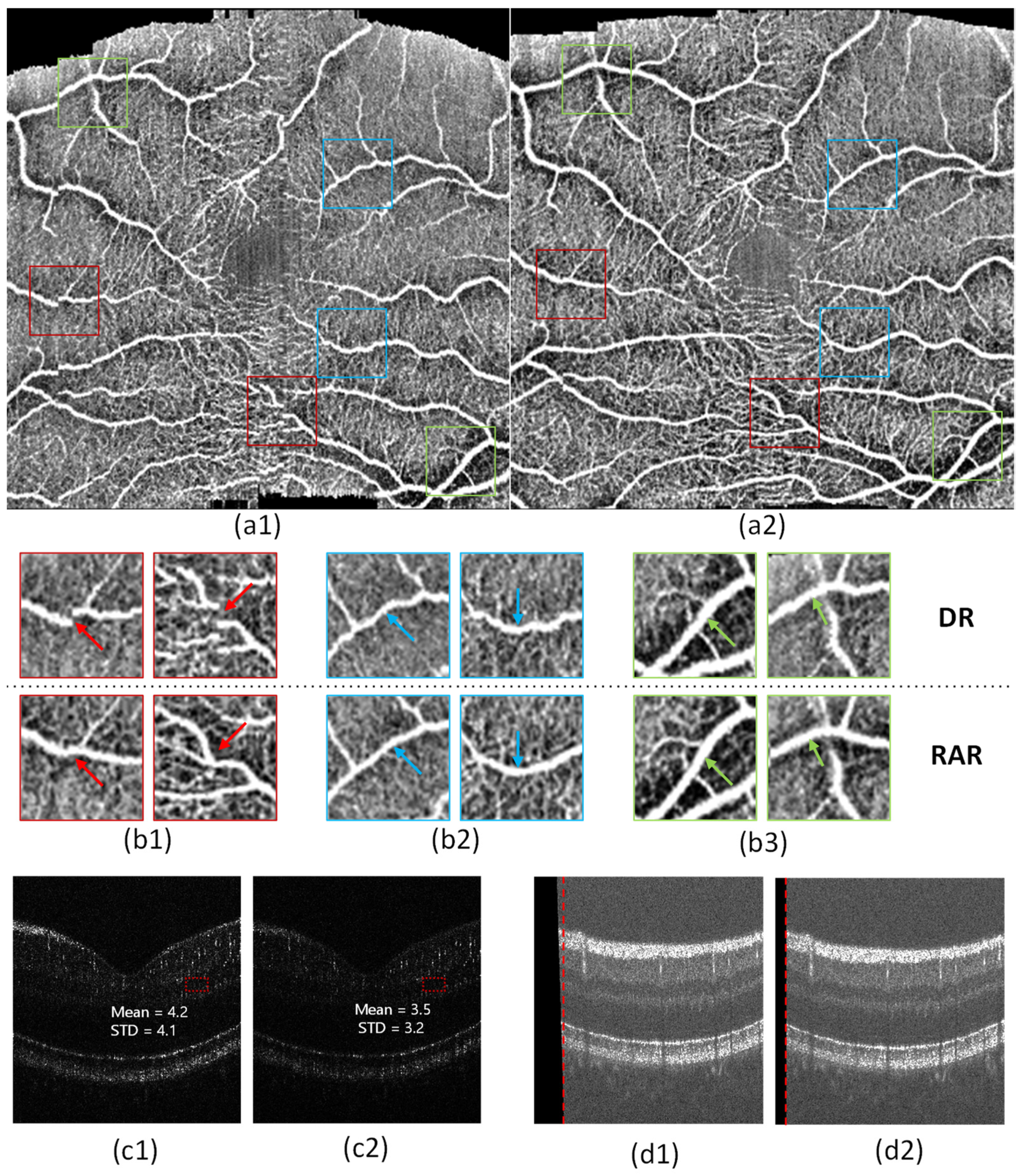
4.2. Registration of 3D OCT Image
5. Discussion
- RAR can effectively compensate for the image displacement caused by eye movement.
- RAR can effectively correct the image distortion caused by retinal direction differences.
- RAR can improve the fidelity of OCTA.
- RAR can preserve a large useful area of the image.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2015; Volume 2. [Google Scholar]
- Swanson, E.A.; Izatt, J.A.; Hee, M.R.; Huang, D.; Lin, C.; Schuman, J.; Puliafito, C.; Fujimoto, J.G. In vivo retinal imaging by optical coherence tomography. J. Opt. Lett. 1993, 18, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, D.F.; Mieler, W.F.; Hariprasad, S.M. Spectral-domain optical coherence tomography: A comparison of modern high-resolution retinal imaging systems. J. Am. J. Ophthalmol. 2010, 149, 18–31.e12. [Google Scholar] [PubMed]
- Engbert, R.; Kliegl, R. Microsaccades uncover the orientation of covert attention. Vis. Res. 2003, 43, 1035–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, M.F.; Hornegger, J. OCT Motion Correction. In Optical Coherence Tomography: Technology and Applications; Drexler, W., Fujimoto, J.G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 459–476. [Google Scholar]
- Pan, L.; Chen, X. Retinal OCT Image Registration: Methods and Applications. J. IEEE Rev. Biomed. Eng. 2021, 16, 307–318. [Google Scholar]
- Cheng, J.; Tao, D.; Quan, Y.; Wong, D.W.K.; Cheung, G.C.M.; Akiba, M.; Liu, J. Speckle reduction in 3D optical coherence tomography of retina by A-scan reconstruction. J. IEEE Trans. Med. Imaging 2016, 35, 2270–2279. [Google Scholar]
- Pan, L.; Shi, F.; Xiang, D.; Yu, K.; Duan, L.; Zheng, J.; Chen, X. OCTRexpert: A feature-based 3D registration method for retinal OCT images. J. IEEE Trans. Image Process. 2020, 29, 3885–3897. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Hangai, M.; Yoshimura, N. Spectral-domain optical coherence tomography with multiple B-scan averaging for enhanced imaging of retinal diseases. J. Ophthalmol. 2008, 115, 1071–1078. [Google Scholar]
- Pappuru, R.R.; Briceno, C.; Ouyang, Y.; Walsh, A.C.; Sadda, S.R. Clinical significance of B-scan averaging with SD-OCT. J Ophthalm. Surg. Lasers Imaging Retin. 2012, 43, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Kurokawa, K.; Zhang, F.; Lee, J.J.; Miller, D.T. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proc. Natl. Acad. Sci. USA 2017, 114, 12803–12808. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Miller, E.B.; Manna, S.K.; Meleppat, R.K.; Pugh, E.N.; Zawadzki, R. Temporal speckle-averaging of optical coherence tomography volumes for in-vivo cellular resolution neuronal and vascular retinal imaging. J. Neurophoton. 2019, 6, 041105. [Google Scholar] [CrossRef]
- Ma, G.; Ding, J.; Kim, T.-H.; Yao, X. Quantitative Optical Coherence Tomography for Longitudinal Monitoring of Postnatal Retinal Development in Developing Mouse Eyes. Vis. Res. 2022, 12, 1860. [Google Scholar] [CrossRef]
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative optical coherence tomography angiography: A review. J. Exp. Biol. Med. 2020, 245, 301–312. [Google Scholar] [CrossRef]
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. J. Ophthalmol. 2014, 121, 1435–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, J.K.; Stromski, S. Flow measurement without phase information in optical coherence tomography images. J. Opt. Express 2005, 13, 5234–5239. [Google Scholar] [CrossRef] [PubMed]
- Fingler, J.; Zawadzki, R.J.; Werner, J.S.; Schwartz, D.; Fraser, S.E. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. J. Opt. Express 2009, 17, 22190–22200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.K. Optical microangiography: A label-free 3-D imaging technology to visualize and quantify blood circulations within tissue beds in vivo. IEEE J. Sel. Top. Quantum Electron. 2009, 16, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. J. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [Green Version]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. J. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Fingler, J.; Schwartz, D.; Yang, C.; Fraser, S.E. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. J. Opt. Express 2007, 15, 12636–12653. [Google Scholar] [CrossRef] [Green Version]
- Kraus, M.F.; Potsaid, B.; Mayer, M.A.; Bock, R.; Baumann, B.; Liu, J.J.; Hornegger, J.; Fujimoto, J.G. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. J. Biomed. Opt. Express 2012, 3, 1182–1199. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-H.; Ma, G.; Son, T.; Yao, X. Functional Optical Coherence Tomography for Intrinsic Signal Optoretinography: Recent Developments and Deployment Challenges. Front. Med. 2022, 9, 864824. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Son, T.; Kim, T.-H.; Yao, X. Functional optoretinography: Concurrent OCT monitoring of intrinsic signal amplitude and phase dynamics in human photoreceptors. J. Biomed. Opt. Express 2021, 12, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Son, T.; Kim, T.-H.; Ma, G.; Kim, H.; Yao, X. Functional intrinsic optical signal imaging for objective optoretinography of human photoreceptors. J. Exp. Biol. Med. 2021, 246, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, V.P.; Maloney-Bertelli, A.; Kuchenbecker, J.A.; Boyle, K.C.; Ling, T.; Chen, Z.C.; Park, B.H.; Roorda, A.; Palanker, D.; Sabesan, R. The optoretinogram reveals the primary steps of phototransduction in the living human eye. J. Sci. Adv. 2020, 6, eabc1124. [Google Scholar] [CrossRef]
- Cooper, R.F.; Brainard, D.H.; Morgan, J.I. Optoretinography of individual human cone photoreceptors. J. Opt. Express 2020, 28, 39326–39339. [Google Scholar] [CrossRef]
- Hillmann, D.; Spahr, H.; Pfäffle, C.; Sudkamp, H.; Franke, G.; Hüttmann, G. In vivo optical imaging of physiological responses to photostimulation in human photoreceptors. Proc. Natl. Acad. Sci. USA 2016, 113, 13138–13143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Kurokawa, K.; Lassoued, A.; Crowell, J.A.; Miller, D.T. Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 7951–7956. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Son, T.; Kim, T.H.; Yao, X. In vivo optoretinography of phototransduction activation and energy metabolism in retinal photoreceptors. J. Biophoton. 2021, 14, e202000462. [Google Scholar] [CrossRef]
- Lassoued, A.; Zhang, F.; Kurokawa, K.; Liu, Y.; Bernucci, M.T.; Crowell, J.A.; Miller, D.T. Cone photoreceptor dysfunction in retinitis pigmentosa revealed by optoretinography. Proc. Natl. Acad. Sci. USA 2021, 118, e2107444118. [Google Scholar] [CrossRef]
- Zawadzki, R.J.; Fuller, A.R.; Choi, S.S.; Wiley, D.F.; Hamann, B.; Werner, J.S. Correction of motion artifacts and scanning beam distortions in 3D ophthalmic optical coherence tomography imaging. In Proceedings of the Ophthalmic Technologies XVII, San Jose, CA, USA, 20–23 January 2007; pp. 35–45. [Google Scholar]
- Potsaid, B.; Gorczynska, I.; Srinivasan, V.J.; Chen, Y.; Jiang, J.; Cable, A.; Fujimoto, J.G. Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second. J. Opt. Express 2008, 16, 15149–15169. [Google Scholar] [CrossRef] [Green Version]
- Camino, A.; Zhang, M.; Dongye, C.; Pechauer, A.D.; Hwang, T.S.; Bailey, S.T.; Lujan, B.; Wilson, D.J.; Huang, D.; Jia, Y. Automated registration and enhanced processing of clinical optical coherence tomography angiography. J. Quant. Imaging Med. Surg. 2016, 6, 391. [Google Scholar] [CrossRef] [Green Version]
- Thevenaz, P.; Ruttimann, U.E.; Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998, 7, 27–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, S.; Hormel, T.T.; Wei, X.; Cepurna, W.; Morrison, J.C.; Jia, Y. Imaging retinal structures at cellular-level resolution by visible-light optical coherence tomography. J. Opt. Lett. 2020, 45, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Birdal, T. Sharpness Estimation from Image Gradients. MATLAB Central File Exchange. Retrieved 28 February 2023. Available online: https://www.mathworks.com/matlabcentral/fileexchange/32397-sharpness-estimation-from-image-gradients (accessed on 26 February 2023).
- Le, D.; Alam, M.; Miao, B.A.; Lim, J.I.; Yao, X. Fully automated geometric feature analysis in optical coherence tomography angiography for objective classification of diabetic retinopathy. Biomed. Opt. Express 2019, 10, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Reif, R.; Qin, J.; An, L.; Zhi, Z.; Dziennis, S.; Wang, R. Quantifying optical microangiography images obtained from a spectral domain optical coherence tomography system. Int. J. Biomed. Imaging 2012, 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, E2395–E2402. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Thapa, D.; Lim, J.I.; Cao, D.; Yao, X. Computer-aided classification of sickle cell retinopathy using quantitative features in optical coherence tomography angiography. J. Biomed. Opt. Express 2017, 8, 4206–4216. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Thapa, D.; Lim, J.I.; Cao, D.; Yao, X. Quantitative characteristics of sickle cell retinopathy in optical coherence tomography angiography. J. Biomed. Opt. Express 2017, 8, 1741–1753. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Son, T.; Adejumo, T.; Yao, X. Rotational Distortion and Compensation in Optical Coherence Tomography with Anisotropic Pixel Resolution. Bioengineering 2023, 10, 313. https://doi.org/10.3390/bioengineering10030313
Ma G, Son T, Adejumo T, Yao X. Rotational Distortion and Compensation in Optical Coherence Tomography with Anisotropic Pixel Resolution. Bioengineering. 2023; 10(3):313. https://doi.org/10.3390/bioengineering10030313
Chicago/Turabian StyleMa, Guangying, Taeyoon Son, Tobiloba Adejumo, and Xincheng Yao. 2023. "Rotational Distortion and Compensation in Optical Coherence Tomography with Anisotropic Pixel Resolution" Bioengineering 10, no. 3: 313. https://doi.org/10.3390/bioengineering10030313
APA StyleMa, G., Son, T., Adejumo, T., & Yao, X. (2023). Rotational Distortion and Compensation in Optical Coherence Tomography with Anisotropic Pixel Resolution. Bioengineering, 10(3), 313. https://doi.org/10.3390/bioengineering10030313