Influence of Storage Conditions on Decellularized Porcine Conjunctiva
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation and Storage
2.3. Histological Analysis
2.4. Transmission Electron Microscopy
2.5. Content of Extracellular Matrix Proteins
2.6. Biomechanical Properties
2.7. Re-Epithelialization
2.8. Statistical Analysis
3. Results
3.1. Histological and Ultrastructural Analysis
3.2. Content of Extracellular Matrix Proteins
3.3. Biomechanical Properties
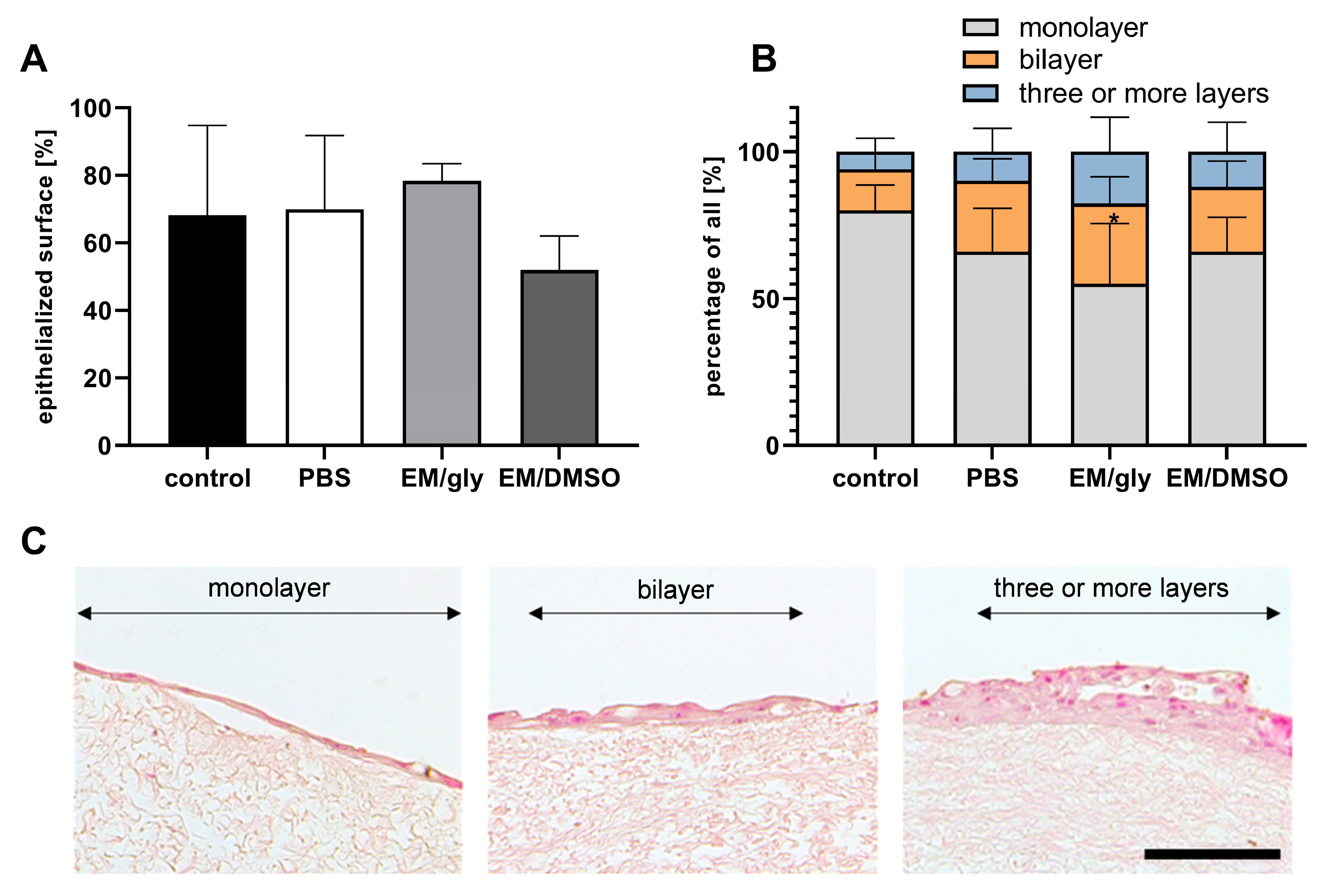
3.4. Re-Epithelialization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tseng, S.C. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology 1985, 92, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Notara, M.; Beaconsfield, M.; Tuft, S.J.; Daniels, J.T.; Geerling, G. Tissue engineering for conjunctival reconstruction: Established methods and future outlooks. Curr. Eye Res. 2009, 34, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.; Geerling, G.; Spaniol, K.; Witt, J. Eye Socket Regeneration and Reconstruction. Curr. Eye Res. 2020, 45, 253–264. [Google Scholar] [CrossRef]
- Spaniol, K.; Holtmann, C.; Geerling, G.; Schrader, S. New approaches to ocular surface reconstruction beyond the cornea. Ophthalmologe 2017, 114, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Maharajan, V.S.; Hopkinson, A. Controversies and limitations of amniotic membrane in ophthalmic surgery. In Cornea and External Eye Disease; Reinhard, T., Larkin, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 21–33. [Google Scholar]
- Witt, J.; Borrelli, M.; Mertsch, S.; Geerling, G.; Spaniol, K.; Schrader, S. Evaluation of Plastic-Compressed Collagen for Conjunctival Repair in a Rabbit Model. Tissue Eng. Part A 2019, 25, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.; Witt, J.; Roth, M.; Reichl, S.; Bradenbrink, P.; Schoppe, M.; Schrader, S.; Geerling, G. Keratin films for ocular surface reconstruction: Wound healing in an in-vivo model. Exp. Eye Res. 2022, 227, 109356. [Google Scholar] [CrossRef]
- Makuloluwa, A.K.; Hamill, K.J.; Rauz, S.; Bosworth, L.; Haneef, A.; Romano, V.; Williams, R.L.; Dartt, D.A.; Kaye, S.B. Biological tissues and components, and synthetic substrates for conjunctival cell transplantation. Ocul. Surf. 2021, 22, 15–26. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, T.; Kishida, A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater. Sci. Eng. 2017, 3, 1236–1244. [Google Scholar] [CrossRef]
- Schrader, S.; Witt, J.; Geerling, G. Plastic compressed collagen transplantation—A new option for corneal surface reconstruction? Acta Ophthalmol. 2018, 96, e757–e758. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, Y.; Zhao, C.; Li, H.; Wang, F.; Dong, M.; Liu, T.; Zhang, S.; Zhou, Q.; Shi, W. Ocular surface repair using decellularized porcine conjunctiva. Acta Biomater. 2020, 101, 344–356. [Google Scholar] [CrossRef]
- Liao, S.L.; Wei, Y.H. Correction of lower lid retraction using tarSys bioengineered grafts for graves ophthalmopathy. Am. J. Ophthalmol. 2013, 156, 387–392.e381. [Google Scholar] [CrossRef]
- Makuloluwa, A.K.; Hamill, K.J.; Rauz, S.; Bosworth, L.; Haneef, A.; Romano, V.; Williams, R.L.; Dartt, D.A.; Kaye, S.B. The conjunctival extracellular matrix, related disorders and development of substrates for conjunctival restoration. Ocul. Surf. 2021, in press. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Doherty, K.G.; Hsuan, J.D.; Cray, S.P.; D’Sa, R.A.; Pineda Molina, C.; Badylak, S.F.; Williams, R.L. Material Characterisation and Stratification of Conjunctival Epithelial Cells on Electrospun Poly(ε-Caprolactone) Fibres Loaded with Decellularised Tissue Matrices. Pharmaceutics 2021, 13, 318. [Google Scholar] [CrossRef]
- Kasbekar, S.; Kaye, S.B.; Williams, R.L.; Stewart, R.M.K.; Leow-Dyke, S.; Rooney, P. Development of decellularized conjunctiva as a substrate for the ex vivo expansion of conjunctival epithelium. J. Tissue Eng. Regen. Med. 2018, 12, e973–e982. [Google Scholar] [CrossRef]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.C.; Badylak, S.F. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.; Mertsch, S.; Borrelli, M.; Dietrich, J.; Geerling, G.; Schrader, S.; Spaniol, K. Decellularised conjunctiva for ocular surface reconstruction. Acta Biomater. 2018, 67, 259–269. [Google Scholar] [CrossRef]
- Witt, J.; Dietrich, J.; Mertsch, S.; Schrader, S.; Spaniol, K.; Geerling, G. Decellularized porcine conjunctiva as an alternative substrate for tissue-engineered epithelialized conjunctiva. Ocul. Surf. 2020, 18, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dang, H.; Xu, Y. Recent advancement of decellularization extracellular matrix for tissue engineering and biomedical application. Artif. Organs 2022, 46, 549–567. [Google Scholar] [CrossRef]
- Bakhach, J. The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues. Organogenesis 2009, 5, 119–126. [Google Scholar] [CrossRef]
- Urbani, L.; Maghsoudlou, P.; Milan, A.; Menikou, M.; Hagen, C.K.; Totonelli, G.; Camilli, C.; Eaton, S.; Burns, A.; Olivo, A.; et al. Long-term cryopreservation of decellularised oesophagi for tissue engineering clinical application. PLoS ONE 2017, 12, e0179341. [Google Scholar] [CrossRef]
- Gharenaz, N.M.; Movahedin, M.; Mazaheri, Z. Comparison of two methods for prolong storage of decellularized mouse whole testis for tissue engineering application: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Zouhair, S.; Aguiari, P.; Iop, L.; Vasquez-Rivera, A.; Filippi, A.; Romanato, F.; Korossis, S.; Wolkers, W.F.; Gerosa, G. Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability. Acta Biomater. 2019, 84, 208–221. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tseng, S.C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am. J. Ophthalmol. 1997, 123, 303–312. [Google Scholar] [CrossRef]
- Kim, J.C.; Tseng, S.C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995, 14, 473–484. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Jaus, M.O.; Polizzi, L.; Gonfiotti, A.; Comin, C.E.; Bianco, A.; Ribatti, D.; Taylor, D.A.; Macchiarini, P. Long-term changes to in vitro preserved bioengineered human trachea and their implications for decellularized tissues. Biomaterials 2012, 33, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, N.R.; Sokocevic, D.; Wagner, D.E.; Borg, Z.D.; Lathrop, M.J.; Lam, Y.W.; Deng, B.; Desarno, M.J.; Ashikaga, T.; Loi, R.; et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 2013, 34, 3231–3245. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jungebluth, P.; Go, T.; Asnaghi, A.; Bellini, S.; Martorell, J.; Calore, C.; Urbani, L.; Ostertag, H.; Mantero, S.; Conconi, M.T.; et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J. Thorac. Cardiovasc. Surg. 2009, 138, 586–593; discussion 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Y.; Yu, J.; Jiao, Z.; Ao, Y.; Yu, C.; Wang, J.; Cui, G. Effect of repeated freezing-thawing on the Achilles tendon of rabbits. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Buda, R.; Di Caprio, F.; Agati, P.; Bigi, A.; De Pasquale, V.; Ruggeri, A. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int. Orthop. 2008, 32, 145–151. [Google Scholar] [CrossRef]
- Chani, B.; Puri, V.; Sobti, R.C.; Jha, V.; Puri, S. Decellularized scaffold of cryopreserved rat kidney retains its recellularization potential. PLoS ONE 2017, 12, e0173040. [Google Scholar] [CrossRef]
- Wollmann, L.C.; Suss, P.H.; Kraft, L.; Ribeiro, V.S.; Noronha, L.; da Costa, F.D.A.; Tuon, F.F. Histological and Biomechanical Characteristics of Human Decellularized Allograft Heart Valves After Eighteen Months of Storage in Saline Solution. Biopreserv. Biobank. 2020, 18, 90–101. [Google Scholar] [CrossRef]
- Poornejad, N.; Frost, T.S.; Scott, D.R.; Elton, B.B.; Reynolds, P.R.; Roeder, B.L.; Cook, A.D. Freezing/Thawing without Cryoprotectant Damages Native but not Decellularized Porcine Renal Tissue. Organogenesis 2015, 11, 30–45. [Google Scholar] [CrossRef]
- Wagner, M.; Walter, P.; Salla, S.; Johnen, S.; Plange, N.; Rütten, S.; Goecke, T.W.; Fuest, M. Cryopreservation of amniotic membrane with and without glycerol additive. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1117–1126. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sun, T.T.; Lavker, R.M. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Investig. Ophthalmol. Vis. Sci. 1996, 37, 523–533. [Google Scholar]
- Lavker, R.M.; Sun, T.T. Epithelial stem cells: The eye provides a vision. Eye 2003, 17, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.P.; Tan, D.T.; Cajucom-Uy, H.; Beuerman, R.W. Autologous cultivated conjunctival transplantation for pterygium surgery. Am. J. Ophthalmol. 2005, 139, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.C.; Kunze, A.; Kureshi, A.; Grobe, G.; Reichl, S.; Geerling, G.; Daniels, J.T.; Schrader, S. Development of a conjunctival tissue substitute on the basis of plastic compressed collagen. J. Tissue Eng. Regen. Med. 2017, 11, 896–904. [Google Scholar] [CrossRef]
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ni Dhubhghaill, S.; Koppen, C.; Tassignon, M.J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef]
- Mallis, P.; Katsimpoulas, M.; Kostakis, A.; Dipresa, D.; Korossis, S.; Papapanagiotou, A.; Kassi, E.; Stavropoulos-Giokas, C.; Michalopoulos, E. Vitrified Human Umbilical Arteries as Potential Grafts for Vascular Tissue Engineering. Tissue Eng. Regen Med. 2020, 17, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Jose, M.V.; Thomas, V.; Dean, D.R.; Janowski, G.M. Freeze-dried acellular dermal matrix graft: Effects of rehydration on physical, chemical, and mechanical properties. Dent. Mater. 2009, 25, 1109–1115. [Google Scholar] [CrossRef]
- Díaz-Moreno, E.; Durand-Herrera, D.; Carriel, V.; Martín-Piedra, M.Á.; Sánchez-Quevedo, M.d.C.; Garzon, I.; Campos, A.; Fernández-Valadés, R.; Alaminos, M. Evaluation of freeze-drying and cryopreservation protocols for long-term storage of biomaterials based on decellularized intestine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 488–500. [Google Scholar] [CrossRef]
- Borgognoni, C.F.; Tattini Junior, V.; Ayrosa, A.M.I.B.; Polakiewicz, B.; Leirner, A.A.; Maizato, M.J.S.; Higa, O.Z.; Beppu, M.M.; Pitombo, R.N.d.M. The influence of freezing rates on bovine pericardium tissue Freeze-drying. Braz. Arch. Biol. Technol. 2009, 52, 1493–1504. [Google Scholar] [CrossRef]
- Polak, R.; Pitombo, R.N. Care during freeze-drying of bovine pericardium tissue to be used as a biomaterial: A comparative study. Cryobiology 2011, 63, 61–66. [Google Scholar] [CrossRef]
- Wang, S.; Oldenhof, H.; Goecke, T.; Ramm, R.; Harder, M.; Haverich, A.; Hilfiker, A.; Wolkers, W.F. Sucrose Diffusion in Decellularized Heart Valves for Freeze-Drying. Tissue Eng. Part C Methods 2015, 21, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Lamon, M.; Bertolin, M.; Trojan, D.; Spagnol, L.; Donisi, P.M.; Camposampiero, D.; Ponzin, D.; Ferrari, S. Cryopreservation of human amniotic membrane for ocular surface reconstruction: A comparison between protocols. Cell Tissue Bank. 2022, 23, 851–861. [Google Scholar] [CrossRef] [PubMed]




| Product | Specification | Supplier | Supplier’s Location |
|---|---|---|---|
| Phosphate-buffered saline solution | 14190250 | Thermo Fisher Scientific | Waltham, MA, USA |
| Penicillin/Streptomycin | P4333 | Sigma-Aldrich | St. Louis, MO, USA |
| Glycerol | Central Pharmacy University Hospital Duesseldorf | Duesseldorf, Germany | |
| Dulbecco’s Modified Eagle’s Medium/ F-12 Ham | D8062 | Sigma-Aldrich | St. Louis, MO, USA |
| Fetal bovine serum | S 0615 | Bio-Sell | Feucht, Germany |
| Hydrocortisone | H0888 | Sigma-Aldrich | St. Louis, MO, USA |
| Cholera toxin | C8052 | Sigma-Aldrich | St. Louis, MO, USA |
| Sodium bicarbonate | 25080060 | Thermo Fisher Scientific | Waltham, MA, USA |
| Triiodo-L-thyronine | T6397 | Sigma-Aldrich | St. Louis, MO, USA |
| Transferrin | T8158 | Sigma-Aldrich | St. Louis, MO, USA |
| Adenine | A2786 | Sigma-Aldrich | St. Louis, MO, USA |
| Insulin | I9278 | Sigma-Aldrich | St. Louis, MO, USA |
| EGF | PHG0313 | Thermo Fisher Scientific | Waltham, MA, USA |
| Freezing container | C1562 | Sigma-Aldrich | St. Louis, MO, USA |
| Paraformaldehyde Roti®Histofix | P087.1 | Carl Roth | Karlsruhe, Germany |
| Rotary microtome | RM2255 | Leica Biosystems | Nussloch, Germany |
| Sircol™ Insoluble Collagen assay kit | S2000 | Biocolor | Northern Ireland, UK |
| Fastin™ Elastin assay kit | F2000 | Biocolor | Northern Ireland, UK |
| Material testing machine | zwickiLine Z0.5 TN | ZwickRoell | Ulm, Germany |
| Cell crowns | cellcrown™24 | Scaffdex | Tampere, Finland |
| Paraffin | Surgipath Paraplast Plus | Leica Biosystems | Nussloch, Germany |
| Glutaraldehyde 25% in water | 23114 | SERVA Electrophoresis GmbH | Heidelberg, Germany |
| Cacodylic acid.Na-salt.3H2O | 15540 | SERVA Electrophoresis GmbH | Heidelberg, Germany |
| Phosphotungstic acid hydrate | P4006 | Sigma-Aldrich | St. Louis, MO, USA |
| Spurr Embedding Medium ERL-4221D | 21041 | SERVA Electrophoresis GmbH | Heidelberg, Germany |
| Ultramicrotome | Leica UC7 | Leica Microsystems | Wetzlar, Germany |
| Transmission electron microscope | Hitachi H-7100 | Hitachi High-Tech Corporation | Tokyo, Japan |
| Group | Storage Condition [Temperature] |
|---|---|
| PBS | PBS [4 °C] |
| EM/gly | Conjunctival epithelial cell medium + glycerol 85% (1:1 v/v) [−80 °C] |
| EM/DMSO | Conjunctival epithelial cell medium + 10% FBS + 10% DMSO [−196 °C, liquid nitrogen] |
| Control | Fresh PDC (stored in PBS for a maximum of 96 h) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skornia, A.; Geerling, G.; Spaniol, K.; Witt, J. Influence of Storage Conditions on Decellularized Porcine Conjunctiva. Bioengineering 2023, 10, 350. https://doi.org/10.3390/bioengineering10030350
Skornia A, Geerling G, Spaniol K, Witt J. Influence of Storage Conditions on Decellularized Porcine Conjunctiva. Bioengineering. 2023; 10(3):350. https://doi.org/10.3390/bioengineering10030350
Chicago/Turabian StyleSkornia, Adam, Gerd Geerling, Kristina Spaniol, and Joana Witt. 2023. "Influence of Storage Conditions on Decellularized Porcine Conjunctiva" Bioengineering 10, no. 3: 350. https://doi.org/10.3390/bioengineering10030350
APA StyleSkornia, A., Geerling, G., Spaniol, K., & Witt, J. (2023). Influence of Storage Conditions on Decellularized Porcine Conjunctiva. Bioengineering, 10(3), 350. https://doi.org/10.3390/bioengineering10030350








