Bioceramics in Endodontics: Updates and Future Perspectives
Abstract
1. Introduction
2. Search Methodology
3. Characteristics of Bioceramics
3.1. Chemical Properties
3.2. Biocompatibility and Bioactivity
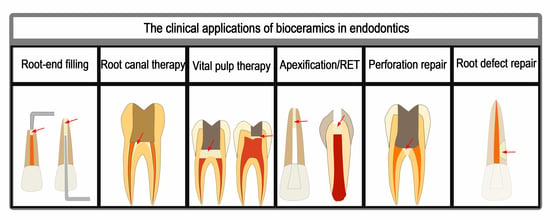
4. Clinical Applications in Endodontics
4.1. Root-End Filling
4.1.1. Orthograde Filling
4.1.2. Retrograde Filling
Endodontic Microsurgery
Intentional Replantation
4.2. Root Canal Therapy
4.3. Vital Pulp Therapy
4.3.1. Pulp Capping
4.3.2. Pulpotomy
4.4. Apexification and Regenerative Endodontic Treatment
4.4.1. Apexification
4.4.2. Regenerative Endodontic Treatment
4.5. Perforation Repair
4.6. Root Defect Repair
4.6.1. Palatal-Radicular Groove
4.6.2. Root Resorption
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; Ur Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent J. 2021, 33, 229–238. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics—From Concept To Clinic. Am. Ceram. Soc. Bullet. 1993, 72, 93–98. [Google Scholar] [CrossRef]
- Raghavendra, S.S.; Jadhav, G.R.; Gathani, K.M.; Kotadia, P. Bioceramics in endodontics—A review. J. Istanb. Univ. Fac. Dent. 2017, 51, S128–S137. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The use of bioceramics in endodontics—Literature review. Clujul. Med. 2016, 89, 470–473. [Google Scholar] [CrossRef]
- Song, W.; Li, S.; Tang, Q.; Chen, L.; Yuan, Z. In vitro biocompatibility and bioactivity of calcium silicate-based bioceramics in endodontics (Review). Int. J. Mol. Med. 2021, 48, 128. [Google Scholar] [CrossRef]
- Khan, A.S.; Ur Rehman, S.; Ahmad, S.; AlMaimouni, Y.K.; Alzamil, M.A.S.; Dummer, P.M.H. Five decades of the International Endodontic Journal: Bibliometric overview 1967-2020. Int. Endod. J. 2021, 54, 1819–1839. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Koulaouzidou, E.A.; Economides, N.; Beltes, P.; Geromichalos, G.; Papazisis, K. In vitro evaluation of the cytotoxicity of ProRoot MTA and MTA Angelus. J. Oral Sci. 2008, 50, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Lolayekar, N.; Bhat, S.S.; Hegde, S. Sealing ability of ProRoot MTA and MTA-Angelus simulating a one-step apical barrier technique—An in vitro study. J. Clin. Pediatr. Dent. 2009, 33, 305–310. [Google Scholar] [CrossRef]
- de Oliveira, N.G.; de Souza Araújo, P.R.; da Silveira, M.T.; Sobral, A.P.V.; Carvalho, M.V. Comparison of the biocompatibility of calcium silicate-based materials to mineral trioxide aggregate: Systematic review. Eur. J. Dent. 2018, 12, 317–326. [Google Scholar] [CrossRef]
- Jerez-Olate, C.; Araya, N.; Alcántara, R.; Luengo, L.; Bello-Toledo, H.; González-Rocha, G.; Sánchez-Sanhueza, G. In vitro antibacterial activity of endodontic bioceramic materials against dual and multispecies aerobic-anaerobic biofilm models. Aust. Endod. J. 2021, 48, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Zafar, K.; Jamal, S.; Ghafoor, R. Bio-active cements-Mineral Trioxide Aggregate based calcium silicate materials: A narrative review. J. Pak. Med. Assoc. 2020, 70, 497–504. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus Biodentine: Review of Literature with a Comparative Analysis. J. Clin. Diagn. Res. 2017, 11, Zg01–Zg05. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.; Anthonappa, R.P. Biodentine™ material characteristics and clinical applications: A 3 year literature review and update. Eur. Arch. Paediatr. Dent. 2018, 19, 1–22. [Google Scholar] [CrossRef]
- Eren, S.K.; Örs, S.A.; Aksel, H.; Canay, Ş.; Karasan, D. Effect of irrigants on the color stability, solubility, and surface characteristics of calcium-silicate based cements. Restor. Dent. Endod. 2022, 47, e10. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Nazari, A.; Garcia-Godoy, F.; Asatourian, A.; Malekzadeh, M.; Elyasi, M. Mechanical response of dental cements as determined by nanoindentation and scanning electron microscopy. Microsc. Microanal. 2013, 19, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.; Saeed, M. In vitro comparative study of sealing ability of Diadent BioAggregate and other root-end filling materials. J. Conserv. Dent. 2012, 15, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Shahabi, S.; Jafarzadeh, T.; Amini, S.; Kheirieh, S. The properties of a new endodontic material. J. Endod. 2008, 34, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.Z.; Rabi, T. TheraCal LC: From Biochemical and Bioactive Properties to Clinical Applications. Int. J. Dent. 2018, 2018, 3484653. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.B.; Inada, R.N.H.; Jampani, J.L.A.; Guerreiro-Tanomaru, J.M.; Sasso-Cerri, E.; Tanomaru-Filho, M.; Cerri, P.S. Biocompatibility and bioactive potential of an experimental tricalcium silicate-based cement in comparison with Bio-C repair and MTA Repair HP materials. Int. Endod. J. 2023, 56, 259–277. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef]
- Eskandari, F.; Razavian, A.; Hamidi, R.; Yousefi, K.; Borzou, S. An Updated Review on Properties and Indications of Calcium Silicate-Based Cements in Endodontic Therapy. Int. J. Dent. 2022, 2022, 6858088. [Google Scholar] [CrossRef]
- Jafari, F.; Jafari, S. Composition and physicochemical properties of calcium silicate based sealers: A review article. J. Clin. Exp. Dent. 2017, 9, e1249–e1255. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reichert, T.E. Dental stem cells in tooth regeneration and repair in the future. Expert Opin. Biol. Ther. 2018, 18, 187–196. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef]
- Orti, V.; Collart-Dutilleul, P.Y.; Piglionico, S.; Pall, O.; Cuisinier, F.; Panayotov, I. Pulp Regeneration Concepts for Nonvital Teeth: From Tissue Engineering to Clinical Approaches. Tissue Eng. Part B Rev. 2018, 24, 419–442. [Google Scholar] [CrossRef]
- Sismanoglu, S.; Ercal, P. Effects of calcium silicate-based cements on odonto/osteogenic differentiation potential in mesenchymal stem cells. Aust. Endod. J. 2022. [Google Scholar] [CrossRef]
- Millan, C.; Vivanco, J.F.; Benjumeda-Wijnhoven, I.M.; Bjelica, S.; Santibanez, J.F. Mesenchymal Stem Cells and Calcium Phosphate Bioceramics: Implications in Periodontal Bone Regeneration. Adv. Exp. Med. Biol. 2018, 1107, 91–112. [Google Scholar]
- Kim, Y.; Lee, D.; Kim, H.M.; Kye, M.; Kim, S.Y. Biological Characteristics and Odontogenic Differentiation Effects of Calcium Silicate-Based Pulp Capping Materials. Materials 2021, 14, 4661. [Google Scholar] [CrossRef]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W.X. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef]
- Abou ElReash, A.; Hamama, H.; Grawish, M.; Saeed, M.; Zaen El-Din, A.M.; Shahin, M.A.; Zhenhuan, W.; Xiaoli, X. A laboratory study to test the responses of human dental pulp stem cells to extracts from three dental pulp capping biomaterials. Int. Endod. J. 2021, 54, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fangteng, J.Z.; Liu, H. Effect of iRoot BP Plus on biological behavior of deciduous tooth pulp stem cells and human pulp stem cells. Shanghai J. Stomatol. 2019, 28, 251–258. [Google Scholar]
- Sanz, J.L.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Melo, M.; Rengo, S.; Spagnuolo, G.; Rodríguez-Lozano, F.J. Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies. J. Clin. Med. 2020, 9, 3872. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Forner, L.; Almudéver, A.; Guerrero-Gironés, J.; Llena, C. Viability and Stimulation of Human Stem Cells from the Apical Papilla (hSCAPs) Induced by Silicate-Based Materials for Their Potential Use in Regenerative Endodontics: A Systematic Review. Materials 2020, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Takimoto, K.; Wealleans, J.; Diogenes, A. Effect of 3 Bioceramic Materials on Stem Cells of the Apical Papilla Proliferation and Differentiation Using a Dentin Disk Model. J. Endod. 2018, 44, 599–603. [Google Scholar] [CrossRef]
- Garrido, M.; Morales, D.; Saldías, M.P.; Fernández, C.; Villalobos, V.; Cerda, O.; Cáceres, M. Cellular response of human apical papilla cells to calcium hydroxide and tricalcium silicate-based cements. BMC Oral Health 2021, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xue, K.; Hu, G.; Du, H.; Gan, K.; Zhu, J.; Du, T. Effects of iRoot SP on osteogenic differentiation of human stem cells from apical papilla. BMC Oral Health 2021, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Songhee Song, K.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar] [PubMed]
- Zhou, Y.; Wu, C.; Xiao, Y. Silicate-based bioceramics for periodontal regeneration. J. Mater. Chem. B 2014, 2, 3907–3910. [Google Scholar] [CrossRef] [PubMed]
- Rezende, T.M.B.; Ribeiro Sobrinho, A.P.; Vieira, L.Q.; Sousa, M.; Kawai, T. Mineral trioxide aggregate (MTA) inhibits osteoclastogenesis and osteoclast activation through calcium and aluminum activities. Clin. Oral Investig. 2021, 25, 1805–1814. [Google Scholar] [CrossRef]
- Tian, J.; Qi, W.; Zhang, Y.; Glogauer, M.; Wang, Y.; Lai, Z.; Jiang, H. Bioaggregate Inhibits Osteoclast Differentiation, Fusion, and Bone Resorption In Vitro. J. Endod. 2015, 41, 1500–1506. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Peng, B. Effect of BioAggregate on osteoclast differentiation and inflammatory bone resorption in vivo. Int. Endod. J. 2015, 48, 1077–1085. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Yan, P.; Peng, B. Effect of BioAggregate on Receptor Activator of Nuclear Factor-Kappa B Ligand-induced Osteoclastogenesis from Murine Macrophage Cell Line In Vitro. J. Endod. 2015, 41, 1265–1271. [Google Scholar] [CrossRef]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Rosenberg, P.A. Repair and regeneration in endodontics. Int. Endod. J. 2011, 44, 889–906. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Fan, M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int. Endod. J. 2013, 46, 923–929. [Google Scholar] [CrossRef]
- Öncel Torun, Z.; Torun, D.; Demirkaya, K.; Yavuz, S.T.; Elçi, M.P.; Sarper, M.; Avcu, F. Effects of iRoot BP and white mineral trioxide aggregate on cell viability and the expression of genes associated with mineralization. Int. Endod. J. 2015, 48, 986–993. [Google Scholar] [CrossRef]
- Jung, J.Y.; Woo, S.M.; Lee, B.N.; Koh, J.T.; Nör, J.E.; Hwang, Y.C. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int. Endod. J. 2015, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Martens, L.; De Coster, P. Gene Expression Profiling and Molecular Signaling of Dental Pulp Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J. Endod. 2015, 41, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Huan, Z.; Pei, G.; Li, J.; Cao, Y.; Jiang, L.; Zhu, Y. Silicate bioceramics elicit proliferation and odonto-genic differentiation of human dental pulp cells. Dent. Mater. J. 2022, 41, 27–36. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, J.; Zhang, J.; Peng, B. A comparative study of BioAggregate and ProRoot MTA on adhesion, migration, and attachment of human dental pulp cells. J. Endod. 2014, 40, 1118–1123. [Google Scholar] [CrossRef]
- Adıgüzel, M.; Ahmetoğlu, F.; Eldeniz, A.; Tekin, M.G.; Göğebakan, B. Comparison of cytotoxic effects of calcium silicate-based materials on human pulp fibroblasts Mehmet. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 241–246. [Google Scholar] [CrossRef]
- Zakerzadeh, A.; Esnaashari, E.; Dadfar, S. In Vitro Comparison of Cytotoxicity and Genotoxicity of Three Vital Pulp Capping Materials. Iran. Endod. J. 2017, 12, 419–425. [Google Scholar]
- Emara, R.; Elhennawy, K.; Schwendicke, F. Effects of calcium silicate cements on dental pulp cells: A systematic review. J. Dent. 2018, 77, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, S.Y.; Kang, S.K.; Kum, K.Y.; Kim, E.C. In vitro biocompatibility, inflammatory response, and osteogenic potential of 4 root canal sealers: Sealapex, Sankin apatite root sealer, MTA Fillapex, and iRoot SP root canal sealer. J. Endod. 2014, 40, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Kou, P.M.; Babensee, J.E. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J. Biomed. Mater. Res. A 2011, 96, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A.; Guillot, P.; Hakiy, N.; Politz, O.; Orfanos, C.E.; Schledzewski, K.; Goerdt, S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand. J. Immunol. 2001, 53, 386–392. [Google Scholar] [CrossRef]
- Brackett, M.G.; Lewis, J.B.; Messer, R.L.; Lei, L.; Lockwood, P.E.; Wataha, J.C. Dysregulation of monocytic cytokine secretion by endodontic sealers. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, H.; Nagata, K.; Maeda, K.; Iijima, T. Involvement of substance P, mast cells, TNF-alpha and ICAM-1 in the infiltration of inflammatory cells in human periapical granulomas. J. Oral. Pathol. Med. 2002, 31, 175–180. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Li, Y.; Yan, P.; Jiang, H. Influence of iRoot SP and mineral trioxide aggregate on the activation and polarization of macrophages induced by lipopolysaccharide. BMC Oral Health 2018, 18, 56. [Google Scholar] [CrossRef]
- Tu, M.G.; Sun, K.T.; Wang, T.H.; He, Y.Z.; Hsia, S.M.; Tsai, B.H.; Shih, Y.H.; Shieh, T.M. Effects of mineral trioxide aggregate and bioceramics on macrophage differentiation and polarization in vitro. J. Formos. Med. Assoc. 2019, 118, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yuan, Z.; Yan, P.; Li, Y.; Jiang, H.; Huang, S. Effect of iRoot SP and mineral trioxide aggregate (MTA) on the viability and polarization of macrophages. Arch. Oral. Biol. 2017, 80, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Q.; Yin, C.; Jia, X.; Zhao, Z.; Zhang, X.; Yuan, G.; Hu, H.; Zhao, Q. Sustained calcium ion release from bioceramics promotes CaSR-mediated M2 macrophage polarization for osteoinduction. J. Leukoc. Biol. 2021, 110, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Bodrumlu, E. Biocompatibility of retrograde root filling materials: A review. Aust. Endod. J. 2008, 34, 30–35. [Google Scholar] [CrossRef]
- Murata, K.; Washio, A.; Morotomi, T.; Rojasawasthien, T.; Kokabu, S.; Kitamura, C. Physicochemical Properties, Cytocompatibility, and Biocompatibility of a Bioactive Glass Based Retrograde Filling Material. Nanomaterials 2021, 11, 1828. [Google Scholar] [CrossRef]
- Chao, Y.C.; Chen, P.H.; Su, W.S.; Yeh, H.W.; Su, C.C.; Wu, Y.C.; Chiang, H.S.; Jhou, H.J.; Shieh, Y.S. Effectiveness of different root-end filling materials in modern surgical endodontic treatment: A systematic review and network meta-analysis. J. Dent. Sci. 2022, 17, 1731–1743. [Google Scholar] [CrossRef]
- Tabiyar, K.; Logani, A. The Apical Extent of Mineral Trioxide Aggregate Apical Barrier Does not Influence the Treatment Outcome in a Nonvital Immature Permanent Anterior Tooth: A Split-Mouth Clinical Study. Eur. Endod. J. 2021, 6, 44–49. [Google Scholar]
- Pace, R.; Giuliani, V.; Nieri, M.; Di Nasso, L.; Pagavino, G. Mineral trioxide aggregate as apical plug in teeth with necrotic pulp and immature apices: A 10-year case series. J. Endod. 2014, 40, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, S.; Mungara, J. Efficacy of mineral trioxide aggregate as an apical plug in non-vital young permanent teeth: Preliminary results. J. Clin. Pediatr. Dent. 2010, 35, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Holden, D.T.; Schwartz, S.A.; Kirkpatrick, T.C.; Schindler, W.G. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J. Endod. 2008, 34, 812–817. [Google Scholar] [CrossRef]
- Sarris, S.; Tahmassebi, J.F.; Duggal, M.S.; Cross, I.A. A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children-a pilot study. Dent. Traumatol. 2008, 24, 79–85. [Google Scholar] [CrossRef]
- Van Pham, K.; Tran, T.A. Effectiveness of MTA apical plug in dens evaginatus with open apices. BMC Oral Health 2021, 21, 566. [Google Scholar] [CrossRef]
- Ree, M.H.; Schwartz, R.S. Long-term Success of Nonvital, Immature Permanent Incisors Treated with a Mineral Trioxide Aggregate Plug and Adhesive Restorations: A Case Series from a Private Endodontic Practice. J. Endod. 2017, 43, 1370–1377. [Google Scholar] [CrossRef]
- Abbas, A.; Kethineni, B.; Puppala, R.; Birapu, U.C.; Raghavendra, K.J.; Reddy, P. Efficacy of Mineral Trioxide Aggregate and Biodentine as Apical Barriers in Immature Permanent Teeth: A Microbiological Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 656–662. [Google Scholar]
- Refaei, P.; Jahromi, M.Z.; Moughari, A.A.K. Comparison of the microleakage of mineral trioxide aggregate, calcium-enriched mixture cement, and Biodentine orthograde apical plug. Dent. Res. J. 2020, 17, 66–72. [Google Scholar]
- Ok, E.; Altunsoy, M.; Tanriver, M.; Capar, I.D.; Kalkan, A.; Gok, T. Fracture resistance of simulated immature teeth after apexification with calcium silicate-based materials. Eur. J. Dent. 2016, 10, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, A.; Asgary, S.; Eghbal, M.J.; Ghoddusi, J.; Bayat-Movahed, S. Calcium-enriched mixture cement as artificial apical barrier: A case series. J. Conserv. Dent. 2011, 14, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Tabrizizade, M.; Asadi, Y.; Sooratgar, A.; Moradi, S.; Sooratgar, H.; Ayatollahi, F. Sealing ability of mineral trioxide aggregate and calcium-enriched mixture cement as apical barriers with different obturation techniques. Iran. Endod. J. 2014, 9, 261–265. [Google Scholar]
- Ayatollahi, F.; Tabrizizadeh, M.; Hazeri Baqdad Abad, M.; Ayatollahi, R.; Zarebidoki, F. Comparison of Microleakage of MTA and CEM Cement Apical Plugs in Three Different Media. Iran. Endod. J. 2016, 11, 198–201. [Google Scholar]
- Ayatollahi, F.; Hazeri Baqdad Abad, M.; Razavi, S.H.; Tabrizizadeh, M.; Ayatollahi, R.; Zarebidoki, F. Evaluating the Accuracy of Two Microleakage Assessment Methods for Mineral Trioxide Aggregate and Calcium-enriched Mixture Cement. Iran. Endod. J. 2017, 12, 497–501. [Google Scholar]
- Memiş Özgül, B.; Bezgin, T.; Şahin, C.; Sarı, Ş. Resistance to leakage of various thicknesses of apical plugs of Bioaggregate using liquid filtration model. Dent. Traumatol. 2015, 31, 250–254. [Google Scholar] [CrossRef]
- Tuloglu, N.; Bayrak, S. Comparative evaluation of mineral trioxide aggregate and bioaggregate as apical barrier material in traumatized nonvital, immature teeth: A clinical pilot study. Niger. J. Clin. Pract. 2016, 19, 52–57. [Google Scholar] [CrossRef]
- Paños-Crespo, A.; Sánchez-Torres, A.; Gay-Escoda, C. Retrograde filling material in periapical surgery: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e422–e429. [Google Scholar] [CrossRef] [PubMed]
- Floratos, S.; Kim, S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent. Clin. North. Am. 2017, 61, 81–91. [Google Scholar] [CrossRef]
- Jadun, S.; Monaghan, L.; Darcey, J. Endodontic microsurgery. Part two: Armamentarium and technique. Br. Dent. J. 2019, 227, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Connert, T.; Weiger, R.; Krastl, G. Present status and future directions—Guided endodontics. Int. Endod. J. 2022, 10, 995–1002. [Google Scholar] [CrossRef]
- Abusrewil, S.M.; McLean, W.; Scott, J.A. The use of Bioceramics as root-end filling materials in periradicular surgery: A literature review. Saudi Dent. J. 2018, 30, 273–282. [Google Scholar] [CrossRef]
- Kohli, M.R.; Berenji, H.; Setzer, F.C.; Lee, S.M.; Karabucak, B. Outcome of Endodontic Surgery: A Meta-analysis of the Literature-Part 3: Comparison of Endodontic Microsurgical Techniques with 2 Different Root-end Filling Materials. J. Endod. 2018, 44, 923–931. [Google Scholar] [CrossRef]
- Schutte, H.; van Hooft, E. The unresolved contest between MTA and IRM as apical barrier material in apicoectomies. A retrospective cohort study. Ned. Tijdschr. Tandheelkd. 2022, 129, 33–40. [Google Scholar] [CrossRef]
- Tang, J.J.; Shen, Z.S.; Qin, W.; Lin, Z. A comparison of the sealing abilities between Biodentine and MTA as root-end filling materials and their effects on bone healing in dogs after periradicular surgery. J. Appl. Oral. Sci. 2019, 27, e20180693. [Google Scholar] [CrossRef]
- Bani, M.; Sungurtekin-Ekçi, E.; Odabaş, M.E. Efficacy of Biodentine as an Apical Plug in Nonvital Permanent Teeth with Open Apices: An In Vitro Study. Biomed. Res. Int. 2015, 2015, 359275. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Chung, M.; Kim, S.; Song, M.; Kim, E. Effects of fast- and slow-setting calcium silicate-based root-end filling materials on the outcome of endodontic microsurgery: A retrospective study up to 6 years. Clin. Oral. Investig. 2020, 24, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Karabucak, B.; Wang, C.; Wang, H.G.; Koyama, E.; Kohli, M.R.; Nah, H.D.; Kim, S. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J. Endod. 2015, 41, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rencher, B.; Chang, A.M.; Fong, H.; Johnson, J.D.; Paranjpe, A. Comparison of the sealing ability of various bioceramic materials for endodontic surgery. Restor. Dent. Endod. 2021, 46, e35. [Google Scholar] [CrossRef]
- Leal, F.; De-Deus, G.; Brandão, C.; Luna, A.; Souza, E.; Fidel, S. Similar sealability between bioceramic putty ready-to-use repair cement and white MTA. Braz. Dent. J. 2013, 24, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Toia, C.C.; Teixeira, F.B.; Cucco, C.; Valera, M.C.; Cavalcanti, B.N. Filling ability of three bioceramic root-end filling materials: A micro-computed tomography analysis. Aust. Endod. J. 2020, 46, 424–431. [Google Scholar] [CrossRef]
- Chan, S.; Glickman, G.N.; Woodmansey, K.F.; He, J. Retrospective Analysis of Root-end Microsurgery Outcomes in a Postgraduate Program in Endodontics Using Calcium Silicate-based Cements as Root-end Filling Materials. J. Endod. 2020, 46, 345–351. [Google Scholar] [CrossRef]
- Shinbori, N.; Grama, A.M.; Patel, Y.; Woodmansey, K.; He, J. Clinical outcome of endodontic microsurgery that uses EndoSequence BC root repair material as the root-end filling material. J. Endod. 2015, 41, 607–612. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Janner, S.F.M.; Haenni, S.; Bornstein, M.M. Bioceramic root repair material (BCRRM) for root-end obturation in apical surgery. An analysis of 174 teeth after 1 year. Swiss. Dent. J. 2020, 130, 390–396. [Google Scholar] [PubMed]
- Zhou, W.; Zheng, Q.; Tan, X.; Song, D.; Zhang, L.; Huang, D. Comparison of Mineral Trioxide Aggregate and iRoot BP Plus Root Repair Material as Root-end Filling Materials in Endodontic Microsurgery: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, C.; Jia, L.; Wang, Y.; Liu, W.; Zhou, X.; Johnson, T.M.; Huang, D. Materials for retrograde filling in root canal therapy. Cochrane Database Syst. Rev. 2016, 12, Cd005517. [Google Scholar] [CrossRef]
- Ayup, H.; Duane, B. Limited evidence on best material for retrograde root fillings. Evid. Based. Dent. 2018, 19, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Abella Sans, F.; Duggal, M.S.; Grande, N.M.; Krastl, G.; Nagendrababu, V.; Gambarini, G. European Society of Endodontology position statement: Surgical extrusion, intentional replantation and tooth autotransplantation: European Society of Endodontology developed by. Int. Endod. J. 2021, 54, 655–659. [Google Scholar] [CrossRef]
- Becker, B.D. Intentional Replantation Techniques: A Critical Review. J. Endod. 2018, 44, 14–21. [Google Scholar] [CrossRef]
- Torabinejad, M.; Dinsbach, N.A.; Turman, M.; Handysides, R.; Bahjri, K.; White, S.N. Survival of Intentionally Replanted Teeth and Implant-supported Single Crowns: A Systematic Review. J. Endod. 2015, 41, 992–998. [Google Scholar] [CrossRef]
- Cunliffe, J.; Ayub, K.; Darcey, J.; Foster-Thomas, E. Intentional replantation—A clinical review of cases undertaken at a major UK dental school. Br. Dent. J. 2020, 229, 230–238. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, N.; Miao, D.; Ying, X.; Chen, Y. Intentional replantation of periodontally involved hopeless teeth: A case series study. Clin. Oral Investig. 2020, 24, 1769–1777. [Google Scholar] [CrossRef]
- Chogle, S.; Chatha, N.; Bukhari, S. Intentional Replantation of Teeth is a Viable and Cost-effective Alternative Treatment to Single-Tooth Implants. J. Evid. Based Dent. Pract. 2019, 19, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Mainkar, A. A Systematic Review of the Survival of Teeth Intentionally Replanted with a Modern Technique and Cost-effectiveness Compared with Single-tooth Implants. J. Endod. 2017, 43, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Devji, T. Uncertainty about survival rates and cost-effectiveness of intentional replantation for persistent apical periodontitis. J. Am. Dent. Assoc. 2018, 149, e122. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Abella Sans, F.; Bastos, J.V.; Nagendrababu, V. Effectiveness of intentional replantation in managing teeth with apical periodontitis: A systematic review. Int. Endod. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, H.; Bai, Y.; Luo, Q.; Wu, H.; Liu, H. Clinical outcomes after intentional replantation of permanent teeth: A systematic review. Bosn. J. Basic Med. Sci. 2020, 20, 13–20. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, S.J.; Yoon, T.C.; Roh, B.D.; Kim, E. Survival Rate of Teeth with a C-shaped Canal after Intentional Replantation: A Study of 41 Cases for up to 11 Years. J. Endod. 2016, 42, 1320–1325. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, Y.; Shin, S.J.; Kim, E.; Jung, I.Y.; Friedman, S.; Lee, S.J. Retention and Healing Outcomes after Intentional Replantation. J. Endod. 2016, 42, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Grzanich, D.; Rizzo, G.; Silva, R.M. Saving Natural Teeth: Intentional Replantation-Protocol and Case Series. J. Endod. 2017, 43, 2119–2124. [Google Scholar] [CrossRef]
- Asgary, S.; Talebzadeh, B. Intentional replantation of a molar with several endodontic complications. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 489–492. [Google Scholar] [CrossRef]
- Asgary, S.; Nosrat, A. Concurrent intentional replantation of maxillary molars using a novel root-end filling. Gen. Dent. 2014, 62, 30–33. [Google Scholar]
- Kheirieh, S.; Fazlyab, M.; Torabzadeh, H.; Eghbal, M.J. Extraoral Retrograde Root Canal Filling of an Orthodontic-induced External Root Resorption Using CEM Cement. Iran. Endod. J. 2014, 9, 149–152. [Google Scholar] [PubMed]
- Asgary, S.; Alim Marvasti, L.; Kolahdouzan, A. Indications and case series of intentional replantation of teeth. Iran. Endod. J. 2014, 9, 71–78. [Google Scholar] [PubMed]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef]
- Yang, X.Q.; Yang, R.Q.; Tian, J.; Wei, X. Application status and prospect of single-cone obturation technique with bioceramic sealers. Chin. J. Stomatol. 2022, 57, 424–429. [Google Scholar]
- Coaguila-Llerena, H.; Ordinola-Zapata, R.; Staley, C.; Dietz, M.; Chen, R.; Faria, G. Multispecies biofilm removal by a multisonic irrigation system in mandibular molars. Int. Endod. J. 2022, 55, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Coaguila-Llerena, H.; Gaeta, E.; Faria, G. Outcomes of the GentleWave system on root canal treatment: A narrative review. Restor. Dent. Endod. 2022, 47, e11. [Google Scholar] [CrossRef] [PubMed]
- Zanza, A.; Seracchiani, M.; Reda, R.; Miccoli, G.; Testarelli, L.; Di Nardo, D. Metallurgical Tests in Endodontics: A Narrative Review. Bioengineering 2022, 9, 30. [Google Scholar] [CrossRef]
- Heran, J.; Khalid, S.; Albaaj, F.; Tomson, P.L.; Camilleri, J. The single cone obturation technique with a modified warm filler. J. Dent. 2019, 89, 103181. [Google Scholar] [CrossRef] [PubMed]
- Santos-Junior, A.O.; Tanomaru-Filho, M.; Pinto, J.C.; Tavares, K.; Torres, F.F.E.; Guerreiro-Tanomaru, J.M. Effect of obturation technique using a new bioceramic sealer on the presence of voids in flattened root canals. Braz. Oral Res. 2021, 35, e028. [Google Scholar] [CrossRef]
- Girelli, C.F.; Lacerda, M.F.; Lemos, C.A.; Amaral, M.R.; Lima, C.O.; Silveira, F.F.; Nunes, E. The thermoplastic techniques or single-cone technique on the quality of root canal filling with tricalcium silicate-based sealer: An integrative review. J. Clin. Exp. Dent. 2022, 14, e566–e572. [Google Scholar] [CrossRef] [PubMed]
- Mekhdieva, E.; Del Fabbro, M.; Alovisi, M.; Comba, A.; Scotti, N.; Tumedei, M.; Carossa, M.; Berutti, E.; Pasqualini, D. Postoperative Pain following Root Canal Filling with Bioceramic vs. Traditional Filling Techniques: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4509. [Google Scholar] [CrossRef]
- Aslan, T.; Dönmez Özkan, H. The effect of two calcium silicate-based and one epoxy resin-based root canal sealer on postoperative pain: A randomized controlled trial. Int. Endod. J. 2021, 54, 190–197. [Google Scholar] [CrossRef]
- Chybowski, E.A.; Glickman, G.N.; Patel, Y.; Fleury, A.; Solomon, E.; He, J. Clinical Outcome of Non-Surgical Root Canal Treatment Using a Single-cone Technique with Endosequence Bioceramic Sealer: A Retrospective Analysis. J. Endod. 2018, 44, 941–945. [Google Scholar] [CrossRef] [PubMed]
- AlBakhakh, B.; Al-Saedi, A.; Al-Taee, R.; Nahidh, M. Rapid Apical Healing with Simple Obturation Technique in Response to a Calcium Silicate-Based Filling Material. Int. J. Dent. 2022, 2022, 6958135. [Google Scholar] [CrossRef]
- Zavattini, A.; Knight, A.; Foschi, F.; Mannocci, F. Outcome of Root Canal Treatments Using a New Calcium Silicate Root Canal Sealer: A Non-Randomized Clinical Trial. J. Clin. Med. 2020, 9, 782. [Google Scholar] [CrossRef]
- Bardini, G.; Casula, L.; Ambu, E.; Musu, D.; Mercadè, M.; Cotti, E. A 12-month follow-up of primary and secondary root canal treatment in teeth obturated with a hydraulic sealer. Clin. Oral Investig. 2021, 25, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, M.G.; Kwak, S.W.; Kim, R.H.; Ha, J.H.; Kim, H.C. Pilot Evaluation of Sealer-Based Root Canal Obturation Using Epoxy-Resin-Based and Calcium-Silicate-Based Sealers: A Randomized Clinical Trial. Materials 2022, 15, 5146. [Google Scholar] [CrossRef]
- Cao, Y.; Bogen, G.; Lim, J.; Shon, W.J.; Kang, M.K. Bioceramic Materials and the Changing Concepts in Vital Pulp Therapy. J. Calif. Dent. Assoc. 2016, 44, 278–290. [Google Scholar] [CrossRef]
- Duncan, H.F. Present status and future directions-Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55 (Suppl. S3), 497–511. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Li, Y.; Tay, F.R. Vital pulp therapy: Histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.J.; Seremidi, K.; Stratigaki, E.; Kloukos, D.; Duggal, M.; Gizani, S. Deep dentine caries management of immature permanent posterior teeth with vital pulp: A systematic review and meta-analysis. J. Dent. 2022, 124, 104214. [Google Scholar] [CrossRef] [PubMed]
- AAE Position Statement on Vital Pulp Therapy. J. Endod. 2021, 47, 1340–1344. [CrossRef] [PubMed]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, P.; Cabanillas-Balsera, D.; Saúco-Márquez, J.J.; Segura-Egea, J.J. Outcome of Direct Pulp Capping in Teeth Diagnosed as Irreversible Pulpitis: Systematic Review and Meta-Analysis. J. Clin. Exp. Dent. 2022, 14, e594–e603. [Google Scholar] [CrossRef]
- Matsuura, T.; Kawata-Matsuura, V.K.; Yamada, S. Long-term clinical and radiographic evaluation of the effectiveness of direct pulp-capping materials. J. Oral Sci. 2019, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, M.K.; Güneri, P. Prognostic factors in direct pulp capping with mineral trioxide aggregate or calcium hydroxide: 2- to 6-year follow-up. Clin. Oral Investig. 2017, 21, 357–367. [Google Scholar] [CrossRef]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef]
- Kundzina, R.; Stangvaltaite, L.; Eriksen, H.M.; Kerosuo, E. Capping carious exposures in adults: A randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int. Endod. J. 2017, 50, 924–932. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Khademi, A. Outcomes of vital pulp therapy in permanent teeth with different medicaments based on review of the literature. Dent. Res. J. 2015, 12, 406–417. [Google Scholar]
- Liu, S.; Wang, S.; Dong, Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J. Endod. 2015, 41, 652–657. [Google Scholar] [CrossRef]
- Mahgoub, N.; Alqadasi, B.; Aldhorae, K.; Assiry, A.; Altawili, Z.M.; Tao, H. Comparison between iRoot BP Plus (EndoSequence Root Repair Material) and Mineral Trioxide Aggregate as Pulp-capping Agents: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 542–552. [Google Scholar]
- Motwani, N.; Ikhar, A.; Nikhade, P.; Chandak, M.; Rathi, S.; Dugar, M.; Rajnekar, R. Premixed bioceramics: A novel pulp capping agent. J. Conserv. Dent. 2021, 24, 124–129. [Google Scholar] [CrossRef]
- Reis, M.S.; Scarparo, R.K.; Signor, B.; Bolzan, J.T.; Steier, L.; Figueiredo, J.A.P. Pulp capping with mineral trioxide aggregate or Biodentine: A comparison of mineralized barrier formation and inflammatory and degenerative events. Braz. Oral Res. 2021, 35, e118. [Google Scholar] [CrossRef]
- Hoseinifar, R.; Eskandarizadeh, A.; Parirokh, M.; Torabi, M.; Safarian, F.; Rahmanian, E. Histological Evaluation of Human Pulp Response to Direct Pulp Capping with MTA, CEM Cement, and Biodentine. J. Dent. 2020, 21, 177–183. [Google Scholar]
- Abdul, M.S.M.; Murali, N.; Rai, P.; Mirza, M.B.; Salim, S.; Aparna, M.; Singh, S. Clinico-Histological Evaluation of Dentino-Pulpal Complex of Direct Pulp Capping Agents: A Clinical Study. J. Pharm. Bioallied. Sci. 2021, 13, S194–S198. [Google Scholar] [CrossRef]
- Jalan, A.L.; Warhadpande, M.M.; Dakshindas, D.M. A comparison of human dental pulp response to calcium hydroxide and Biodentine as direct pulp-capping agents. J. Conserv. Dent. 2017, 20, 129–133. [Google Scholar] [CrossRef]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 556–571. [Google Scholar] [CrossRef]
- Linu, S.; Lekshmi, M.S.; Varunkumar, V.S.; Sam Joseph, V.G. Treatment Outcome Following Direct Pulp Capping Using Bioceramic Materials in Mature Permanent Teeth with Carious Exposure: A Pilot Retrospective Study. J. Endod. 2017, 43, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Parinyaprom, N.; Nirunsittirat, A.; Chuveera, P.; Na Lampang, S.; Srisuwan, T.; Sastraruji, T.; Bua-On, P.; Simprasert, S.; Khoipanich, I.; Sutharaphan, T.; et al. Outcomes of Direct Pulp Capping by Using Either ProRoot Mineral Trioxide Aggregate or Biodentine in Permanent Teeth with Carious Pulp Exposure in 6- to 18-Year-Old Patients: A Randomized Controlled Trial. J. Endod. 2018, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Katge, F.A.; Patil, D.P. Comparative Analysis of 2 Calcium Silicate-based Cements (Biodentine and Mineral Trioxide Aggregate) as Direct Pulp-capping Agent in Young Permanent Molars: A Split Mouth Study. J. Endod. 2017, 43, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.H.; El-Negoly, S.A.; Zaen El-Din, A.M.; El-Zekrid, M.H.; Grawish, L.M.; Grawish, H.M.; Grawish, M.E. Biodentine versus mineral trioxide aggregate as a direct pulp capping material for human mature permanent teeth—A systematic review. J. Conserv. Dent. 2018, 21, 466–473. [Google Scholar] [PubMed]
- Zafar, K.; Nazeer, M.R.; Ghafoor, R.; Khan, F.R. Success of pulpotomy in mature permanent teeth with irreversible pulpitis: A systematic review. J. Conserv. Dent. 2020, 23, 121–125. [Google Scholar] [CrossRef]
- Sadaf, D. Success of Coronal Pulpotomy in Permanent Teeth with Irreversible Pulpitis: An Evidence-based Review. Cureus 2020, 12, e6747. [Google Scholar] [CrossRef]
- Coll, J.A.; Seale, N.S.; Vargas, K.; Marghalani, A.A.; Al Shamali, S.; Graham, L. Primary Tooth Vital Pulp Therapy: A Systematic Review and Meta-analysis. Pediatr. Dent. 2017, 39, 16–123. [Google Scholar]
- Li, Y.; Sui, B.; Dahl, C.; Bergeron, B.; Shipman, P.; Niu, L.; Chen, J.; Tay, F.R. Pulpotomy for carious pulp exposures in permanent teeth: A systematic review and meta-analysis. J. Dent. 2019, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Linsuwanont, P.; Wimonsutthikul, K.; Pothimoke, U.; Santiwong, B. Treatment Outcomes of Mineral Trioxide Aggregate Pulpotomy in Vital Permanent Teeth with Carious Pulp Exposure: The Retrospective Study. J. Endod. 2017, 43, 225–230. [Google Scholar] [CrossRef]
- Alqaderi, H.E.; Al-Mutawa, S.A.; Qudeimat, M.A. MTA pulpotomy as an alternative to root canal treatment in children’s permanent teeth in a dental public health setting. J. Dent. 2014, 42, 1390–1395. [Google Scholar] [CrossRef]
- Taha, N.A.; Ahmad, M.B.; Ghanim, A. Assessment of Mineral Trioxide Aggregate pulpotomy in mature permanent teeth with carious exposures. Int. Endod. J. 2017, 50, 117–125. [Google Scholar] [CrossRef]
- Kang, C.M.; Sun, Y.; Song, J.S.; Pang, N.S.; Roh, B.D.; Lee, C.Y.; Shin, Y. A randomized controlled trial of various MTA materials for partial pulpotomy in permanent teeth. J. Dent. 2017, 60, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Khazali, M.A. Partial Pulpotomy in Mature Permanent Teeth with Clinical Signs Indicative of Irreversible Pulpitis: A Randomized Clinical Trial. J. Endod. 2017, 43, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Brignardello-Petersen, R. Mineral trioxide aggregate likely to have a better success rate than calcium hydroxide in mature permanent teeth undergoing partial pulpotomy. J. Am. Dent. Assoc. 2017, 148, e159. [Google Scholar] [CrossRef]
- Bakhurji, E. Mineral Trioxide Aggregate Could Have a Better Success Rate Than Calcium Hydroxide for Partial Pulpotomy of Symptomatic Mature Permanent Molars. J. Evid. Based Dent. Pract. 2020, 20, 101341. [Google Scholar] [CrossRef]
- Tran, X.V.; Ngo, L.T.Q.; Boukpessi, T. Biodentine(TM) Full Pulpotomy in Mature Permanent Teeth with Irreversible Pulpitis and Apical Periodontitis. Healthcare 2021, 9, 720. [Google Scholar] [CrossRef]
- Uesrichai, N.; Nirunsittirat, A.; Chuveera, P.; Srisuwan, T.; Sastraruji, T.; Chompu-Inwai, P. Partial pulpotomy with two bioactive cements in permanent teeth of 6- to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: A noninferiority randomized controlled trial. Int. Endod. J. 2019, 52, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.N.; Mutluay, M.S.; Arıkan, V.; Sarı, Ş. The evaluation of MTA and Biodentine as a pulpotomy materials for carious exposures in primary teeth. Clin. Oral Investig. 2019, 23, 661–666. [Google Scholar] [CrossRef]
- Taha, N.A.; Abdelkhader, S.Z. Outcome of full pulpotomy using Biodentine in adult patients with symptoms indicative of irreversible pulpitis. Int. Endod. J. 2018, 51, 819–828. [Google Scholar] [CrossRef]
- Taha, N.A.; Abdulkhader, S.Z. Full Pulpotomy with Biodentine in Symptomatic Young Permanent Teeth with Carious Exposure. J. Endod. 2018, 44, 932–937. [Google Scholar] [CrossRef]
- Santos, J.M.; Pereira, J.F.; Marques, A.; Sequeira, D.B.; Friedman, S. Vital Pulp Therapy in Permanent Mature Posterior Teeth with Symptomatic Irreversible Pulpitis: A Systematic Review of Treatment Outcomes. Medicina 2021, 57, 573. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, B.; Xu, Z.; Dou, G.; Lei, Y.; Yong, W. The effect of partial pulpotomy with iRoot BP Plus in traumatized immature permanent teeth: A randomized prospective controlled trial. Dent. Traumatol. 2020, 36, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Fazlyab, M.; Sadri, D.; Saghiri, M.A.; Khosravanifard, B.; Asgary, S. Comparison of pulp response to mineral trioxide aggregate and a bioceramic paste in partial pulpotomy of sound human premolars: A randomized controlled trial. Int. Endod. J. 2014, 47, 873–881. [Google Scholar] [CrossRef]
- Rao, Q.; Kuang, J.; Mao, C.; Dai, J.; Hu, L.; Lei, Z.; Song, G.; Yuan, G. Comparison of iRoot BP Plus and Calcium Hydroxide as Pulpotomy Materials in Permanent Incisors with Complicated Crown Fractures: A Retrospective Study. J. Endod. 2020, 46, 352–357. [Google Scholar] [CrossRef]
- Guan, X.; Zhou, Y.; Yang, Q.; Zhu, T.; Chen, X.; Deng, S.; Zhang, D. Vital Pulp Therapy in Permanent Teeth with Irreversible Pulpitis Caused by Caries: A Prospective Cohort Study. J. Pers. Med. 2021, 11, 1125. [Google Scholar] [CrossRef]
- Nosrat, A.; Seifi, A.; Asgary, S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: A randomized clinical trial. Int. J. Paediatr. Dent. 2013, 23, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Eghbal, M.J.; Bagheban, A.A. Long-term outcomes of pulpotomy in permanent teeth with irreversible pulpitis: A multi-center randomized controlled trial. Am. J. Dent. 2017, 30, 151–155. [Google Scholar]
- Asgary, S.; Eghbal, M.J.; Shahravan, A.; Saberi, E.; Baghban, A.A.; Parhizkar, A. Outcomes of root canal therapy or full pulpotomy using two endodontic biomaterials in mature permanent teeth: A randomized controlled trial. Clin. Oral Investig. 2021, 26, 3287–3297. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J. The effect of pulpotomy using a calcium-enriched mixture cement versus one-visit root canal therapy on postoperative pain relief in irreversible pulpitis: A randomized clinical trial. Odontology 2010, 98, 126–133. [Google Scholar] [CrossRef]
- Eghbal, M.J.; Haeri, A.; Shahravan, A.; Kazemi, A.; Moazami, F.; Mozayeni, M.A.; Saberi, E.; Samiei, M.; Vatanpour, M.; Akbarzade Baghban, A.; et al. Postendodontic Pain after Pulpotomy or Root Canal Treatment in Mature Teeth with Carious Pulp Exposure: A Multicenter Randomized Controlled Trial. Pain Res. Manag. 2020, 2020, 5853412. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Eghbal, M.J. Treatment outcomes of pulpotomy in permanent molars with irreversible pulpitis using biomaterials: A multi-center randomized controlled trial. Acta Odontol. Scand. 2013, 71, 130–136. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Ghoddusi, J.; Yazdani, S. One-year results of vital pulp therapy in permanent molars with irreversible pulpitis: An ongoing multicenter, randomized, non-inferiority clinical trial. Clin. Oral Investig. 2013, 17, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Eghbal, M.J.; Ghoddusi, J. Two-year results of vital pulp therapy in permanent molars with irreversible pulpitis: An ongoing multicenter randomized clinical trial. Clin. Oral Investig. 2014, 18, 635–641. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Fazlyab, M.; Baghban, A.A.; Ghoddusi, J. Five-year results of vital pulp therapy in permanent molars with irreversible pulpitis: A non-inferiority multicenter randomized clinical trial. Clin. Oral Investig. 2015, 19, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Bossù, M.; Iaculli, F.; Di Giorgio, G.; Salucci, A.; Polimeni, A.; Di Carlo, S. Different Pulp Dressing Materials for the Pulpotomy of Primary Teeth: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 838. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Zhang, Y.; Zhou, F.; Deng, J.; Zou, J.; Wang, Y. Materials for pulpotomy in immature permanent teeth: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 227. [Google Scholar] [CrossRef]
- Li, W.; Mao, M.; Hu, N.; Wang, J.; Huang, J.; Gu, S. In vitro evaluation of periapical lesion-derived stem cells for dental pulp tissue engineering. FEBS Open Bio. 2021, 12, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gan, L.; Cui, D.X.; Yu, S.H.; Pan, Y.; Zheng, L.W.; Wan, M. Epigenetic regulation of dental pulp stem cells and its potential in regenerative endodontics. World. J. Stem. Cells 2021, 13, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Liang, C.; Liao, L.; Tian, W.D. Advances in Research on Stem Cell-Based Pulp Regeneration. Tissue Eng. Regen. Med. 2021, 18, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, F.; Hong, Y.; He, J.; Lin, Y. Revascularisation versus apexification for treatment of immature teeth based on periapical healing and root development: A systematic review and meta-analysis. Eur. J. Paediatr. Dent. 2021, 22, 207–214. [Google Scholar]
- Anthrayose, P.; Nawal, R.R.; Yadav, S.; Talwar, S.; Yadav, S. Effect of revascularisation and apexification procedures on biomechanical behaviour of immature maxillary central incisor teeth: A three-dimensional finite element analysis study. Clin. Oral Investig. 2021, 25, 6671–6679. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Oliveira, M.L.; Cerqueira-Neto, A.; Vargas-Neto, J.; Nagata, J.Y.; Gomes, B.; Ferraz, C.C.R.; de Almeida, J.F.A.; de-Jesus-Soares, A. Outcomes of traumatised immature teeth treated with apexification or regenerative endodontic procedure: A retrospective study. Aust. Endod. J. 2021, 47, 178–187. [Google Scholar] [CrossRef]
- Nicoloso, G.F.; Goldenfum, G.M.; Pizzol, T.; Scarparo, R.K.; Montagner, F.; de Almeida Rodrigues, J.; Casagrande, L. Pulp Revascularization or Apexification for the Treatment of Immature Necrotic Permanent Teeth: Systematic Review and Meta-Analysis. J. Clin. Pediatr. Dent. 2019, 43, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Guerrero, F.; Mendoza, A.; Ribas, D.; Aspiazu, K. Apexification: A systematic review. J. Conserv. Dent. 2018, 21, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Lu, J.X.; Zeng, Q.; Zhao, W.; Li, W.Q.; Ling, J.Q. Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. J. Formos. Med. Assoc. 2016, 115, 523–530. [Google Scholar] [CrossRef]
- Bonte, E.; Beslot, A.; Boukpessi, T.; Lasfargues, J.J. MTA versus Ca(OH)2 in apexification of non-vital immature permanent teeth: A randomized clinical trial comparison. Clin. Oral Investig. 2015, 19, 1381–1388. [Google Scholar] [CrossRef]
- Bücher, K.; Meier, F.; Diegritz, C.; Kaaden, C.; Hickel, R.; Kühnisch, J. Long-term outcome of MTA apexification in teeth with open apices. Quintessence Int. 2016, 47, 473–482. [Google Scholar] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Ürkmez, E.; Pınar Erdem, A. Bioactivity evaluation of calcium silicate-based endodontic materials used for apexification. Aust. Endod. J. 2020, 46, 60–67. [Google Scholar] [CrossRef]
- Vidal, K.; Martin, G.; Lozano, O.; Salas, M.; Trigueros, J.; Aguilar, G. Apical Closure in Apexification: A Review and Case Report of Apexification Treatment of an Immature Permanent Tooth with Biodentine. J. Endod. 2016, 42, 730–734. [Google Scholar] [CrossRef]
- Darak, P.; Likhitkar, M.; Goenka, S.; Kumar, A.; Madale, P.; Kelode, A. Comparative evaluation of fracture resistance of simulated immature teeth and its effect on single visit apexification versus complete obturation using MTA and biodentine. J. Family Med. Prim. Care 2020, 9, 2011–2015. [Google Scholar] [CrossRef]
- Bajwa, N.K.; Jingarwar, M.M.; Pathak, A. Single Visit Apexification Procedure of a Traumatically Injured Tooth with a Novel Bioinductive Material (Biodentine). Int. J. Clin. Pediatr. Dent. 2015, 8, 58–61. [Google Scholar] [CrossRef]
- Niranjan, B.; Shashikiran, N.D.; Dubey, A.; Singla, S.; Gupta, N. Biodentine-A New Novel Bio-Inductive Material For Treatment of Traumatically Injured Tooth (Single Visit Apexification). J. Clin. Diagn Res. 2016, 10, Zj03–Zj04. [Google Scholar] [CrossRef]
- Nayak, G.; Hasan, M.F. Biodentine-a novel dentinal substitute for single visit apexification. Restor. Dent. Endod. 2014, 39, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Singh, B.; Patil, S. Cone beam-computed topographic evaluation of a central incisor with an open apex and a failed root canal treatment using one-step apexification with Biodentine™: A case report. J. Conserv. Dent. 2014, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Tolibah, Y.A.; Kouchaji, C.; Lazkani, T.; Ahmad, I.A.; Baghdadi, Z.D. Comparison of MTA versus Biodentine in Apexification Procedure for Nonvital Immature First Permanent Molars: A Randomized Clinical Trial. Children 2022, 9, 410. [Google Scholar] [CrossRef]
- Juez, M.; Ballester, M.L.; Berástegui, E. In vitro comparison of apical microleakage by spectrophotometry in simulated apexification using White Mineral Trioxide Aggregate, TotalFill Bioceramic Root Repair material, and BioDentine. J. Conserv. Dent. 2019, 22, 237–240. [Google Scholar]
- Pereira, I.R.; Carvalho, C.; Paulo, S.; Martinho, J.P.; Coelho, A.S.; Paula, A.B.; Marto, C.M.; Carrilho, E.; Botelho, M.F.; Abrantes, A.M.; et al. Apical Sealing Ability of Two Calcium Silicate-Based Sealers Using a Radioactive Isotope Method: An In Vitro Apexification Model. Materials 2021, 14, 6456. [Google Scholar] [CrossRef] [PubMed]
- Sockalingam, S.; Awang Talip, M.; Zakaria, A.S.I. Maturogenesis of an Immature Dens Evaginatus Nonvital Premolar with an Apically Placed Bioceramic Material (EndoSequence Root Repair Material®): An Unexpected Finding. Case Rep. Dent. 2018, 2018, 6535480. [Google Scholar] [CrossRef]
- Shaik, I.; Dasari, B.; Kolichala, R.; Doos, M.; Qadri, F.; Arokiyasamy, J.L.; Tiwari, R.V.C. Comparison of the Success Rate of Mineral Trioxide Aggregate, Endosequence Bioceramic Root Repair Material, and Calcium Hydroxide for Apexification of Immature Permanent Teeth: Systematic Review and Meta-Analysis. J. Pharm. Bioallied. Sci. 2021, 13, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef]
- Wigler, R.; Kaufman, A.Y.; Lin, S.; Steinbock, N.; Hazan-Molina, H.; Torneck, C.D. Revascularization: A treatment for permanent teeth with necrotic pulp and incomplete root development. J. Endod. 2013, 39, 319–326. [Google Scholar] [CrossRef]
- El Ashiry, E.A.; Farsi, N.M.; Abuzeid, S.T.; El Ashiry, M.M.; Bahammam, H.A. Dental Pulp Revascularization of Necrotic Permanent Teeth with Immature Apices. J. Clin. Pediatr. Dent. 2016, 40, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Staffoli, S.; Plotino, G.; Nunez Torrijos, B.G.; Grande, N.M.; Bossù, M.; Gambarini, G.; Polimeni, A. Regenerative Endodontic Procedures Using Contemporary Endodontic Materials. Materials 2019, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, Y.; Mei, L.; Li, J. Clinical and Radiographic Outcomes in Immature Permanent Necrotic Evaginated Teeth Treated with Regenerative Endodontic Procedures. J. Endod. 2017, 43, 246–251. [Google Scholar] [CrossRef]
- Hameed, M.H.; Gul, M.; Ghafoor, R.; Badar, S.B. Management of immature necrotic permanent teeth with regenerative endodontic procedures—A review of literature. J. Pak. Med. Assoc. 2019, 69, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Wongwatanasanti, N.; Jantarat, J.; Sritanaudomchai, H.; Hargreaves, K.M. Effect of Bioceramic Materials on Proliferation and Odontoblast Differentiation of Human Stem Cells from the Apical Papilla. J. Endod. 2018, 44, 1270–1275. [Google Scholar] [CrossRef]
- Wattanapakkavong, K.; Srisuwan, T. Release of Transforming Growth Factor Beta 1 from Human Tooth Dentin after Application of Either ProRoot MTA or Biodentine as a Coronal Barrier. J. Endod. 2019, 45, 701–705. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kim, Y.H.; Chae, Y.K.; Jo, S.S.; Choi, S.C.; Nam, O.H. Void characteristics and tortuosity of calcium silicate-based cements for regenerative endodontics: A micro-computed tomography analysis. BMC Oral Health 2021, 21, 565. [Google Scholar] [CrossRef]
- Topçuoğlu, G.; Topçuoğlu, H.S. Regenerative Endodontic Therapy in a Single Visit Using Platelet-rich Plasma and Biodentine in Necrotic and Asymptomatic Immature Molar Teeth: A Report of 3 Cases. J. Endod. 2016, 42, 1344–1346. [Google Scholar] [CrossRef]
- Aldakak, M.M.; Capar, I.D.; Rekab, M.S.; Abboud, S. Single-Visit Pulp Revascularization of a Nonvital Immature Permanent Tooth Using Biodentine. Iran. Endod. J. 2016, 11, 246–249. [Google Scholar]
- Ambu, E.; Caruso, S.; Gatto, R.; Tecco, S.; Severino, M. Regenerative endodontics procedure of an immature permanent mandibular molar with a necrotic pulp using biodentine: A 16 months radiographic follow-up. J. Biol. Regul. Homeost. Agents 2020, 34, 33–37, dental supplement. [Google Scholar]
- Cymerman, J.J.; Nosrat, A. Regenerative Endodontic Treatment as a Biologically Based Approach for Non-Surgical Retreatment of Immature Teeth. J. Endod. 2020, 46, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Kohli, M.R.; Setzer, F.; Karabucak, B. Outcome of Revascularization Procedure: A Retrospective Case Series. J. Endod. 2016, 42, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Ullah, R. Top-cited Articles in Regenerative Endodontics: A Bibliometric Analysis. J. Endod. 2018, 44, 1650–1664. [Google Scholar] [CrossRef]
- Saed, S.M.; Ashley, M.P.; Darcey, J. Root perforations: Aetiology, management strategies and outcomes. The hole truth. Br. Dent. J. 2016, 220, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Decurcio, D.A.; Rossi-Fedele, G.; Silva, J.A.; Guedes, O.A.; Borges, Á.H. Root perforations: A review of diagnosis, prognosis and materials. Braz. Oral Res. 2018, 32, e73. [Google Scholar] [CrossRef]
- Pinheiro, L.S.; Kopper, P.M.P.; Quintana, R.M.; Scarparo, R.K.; Grecca, F.S. Does MTA provide a more favourable histological response than other materials in the repair of furcal perforations? A systematic review. Int. Endod. J. 2021, 54, 2195–2218. [Google Scholar] [CrossRef] [PubMed]
- Abboud, K.M.; Abu-Seida, A.M.; Hassanien, E.E.; Tawfik, H.M. Biocompatibility of NeoMTA Plus® versus MTA Angelus as delayed furcation perforation repair materials in a dog model. BMC Oral Health 2021, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Dastorani, M.; Shourvarzi, B.; Nojoumi, F.; Ajami, M. Comparison of Bacterial Microleakage of Endoseal MTA Sealer and Pro-Root MTA in Root Perforation. J. Dent. 2021, 22, 96–101. [Google Scholar]
- de Sousa Reis, M.; Scarparo, R.K.; Steier, L.; de Figueiredo, J.A.P. Periradicular inflammatory response, bone resorption, and cementum repair after sealing of furcation perforation with mineral trioxide aggregate (MTA Angelus™) or Biodentine™. Clin. Oral Investig. 2019, 23, 4019–4027. [Google Scholar] [CrossRef]
- Silva, L.A.B.; Pieroni, K.; Nelson-Filho, P.; Silva, R.A.B.; Hernandéz-Gatón, P.; Lucisano, M.P.; Paula-Silva, F.W.G.; de Queiroz, A.M. Furcation Perforation: Periradicular Tissue Response to Biodentine as a Repair Material by Histopathologic and Indirect Immunofluorescence Analyses. J. Endod. 2017, 43, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, M.; Zogheib, C.; Makhlouf, A.C.; Kaloustian, M.K.; El Hachem, C.; Habib, M. Sealing Ability of Calcium Silicate-based Materials in the Repair of Furcal Perforations: A Laboratory Comparative Study. J. Contemp. Dent. Pract. 2020, 21, 1091–1097. [Google Scholar]
- Mohan, D.; Singh, A.K.; Kuriakose, F.; Malik, R.; Joy, J.; John, D. Evaluation of Sealing Potential of Different Repair Materials in Furcation Perforations Using Dye Penetration: An In Vitro Study. J. Contemp. Dent. Pract. 2021, 22, 80–83. [Google Scholar]
- Katge, F.A.; Shivasharan, P.R.; Patil, D. Sealing ability of mineral trioxide aggregate Plus™ and Biodentine™ for repair of furcal perforation in primary molars: An in vitro study. Contemp. Clin. Dent. 2016, 7, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Sinkar, R.C.; Patil, S.S.; Jogad, N.P.; Gade, V.J. Comparison of sealing ability of ProRoot MTA, RetroMTA, and Biodentine as furcation repair materials: An ultraviolet spectrophotometric analysis. J. Conserv. Dent. 2015, 18, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Aslan, T.; Esim, E.; Üstün, Y.; Dönmez Özkan, H. Evaluation of Stress Distributions in Mandibular Molar Teeth with Different Iatrogenic Root Perforations Repaired with Biodentine or Mineral Trioxide Aggregate: A Finite Element Analysis Study. J. Endod. 2021, 47, 631–640. [Google Scholar] [CrossRef]
- Kakani, A.K.; Veeramachaneni, C. Sealing ability of three different root repair materials for furcation perforation repair: An in vitro study. J. Conserv. Dent. 2020, 23, 62–65. [Google Scholar] [CrossRef]
- Koç, C.; Aslan, B.; Ulusoy, Z.; Oruçoğlu, H. Sealing ability of three different materials to repair furcation perforations using computerized fluid filtration method. J. Dent. Res. Dent. Clin. Dent. Prospects. 2021, 15, 183–187. [Google Scholar] [CrossRef]
- Nazari Moghadam, K.; Aghili, H.; Rashed Mohassel, A.; Zahedpasha, S.; Moghadamnia, A.A. A comparative study on sealing ability of mineral trioxide aggregate, calcium enriched cement and bone cement in furcal perforations. Minerva Stomatol. 2014, 63, 203–210. [Google Scholar]
- Haghgoo, R.; Arfa, S.; Asgary, S. Microleakage of CEM Cement and ProRoot MTA as Furcal Perforation Repair Materials in Primary Teeth. Iran. Endod. J. 2013, 8, 187–190. [Google Scholar]
- Ramazani, N.; Sadeghi, P. Bacterial Leakage of Mineral Trioxide Aggregate, Calcium-Enriched Mixture and Biodentine as Furcation Perforation Repair Materials in Primary Molars. Iran. Endod. J. 2016, 11, 214–218. [Google Scholar]
- Abdelmotelb, M.A.; Gomaa, Y.F.; Khattab, N.M.A.; Elheeny, A.A.H. Premixed bioceramics versus mineral trioxide aggregate in furcal perforation repair of primary molars: In vitro and in vivo study. Clin. Oral Investig. 2021, 25, 4915–4925. [Google Scholar] [CrossRef] [PubMed]
- Gorni, F.G.; Andreano, A.; Ambrogi, F.; Brambilla, E.; Gagliani, M. Patient and Clinical Characteristics Associated with Primary Healing of Iatrogenic Perforations after Root Canal Treatment: Results of a Long-term Italian Study. J. Endod. 2016, 42, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Mente, J.; Leo, M.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate: Repair of root perforations-long-term results. J. Endod. 2014, 40, 790–796. [Google Scholar] [CrossRef]
- Rabinovich, I.M.; Snegirev, M.V.; Markheev, C.I. Dental root resorption etiology, diagnosis and treatment. Stomatologiia 2019, 98, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Erber, R.; Frese, C.; Gehrig, H.; Saure, D.; Mente, J. In vitro evaluation of different dental materials used for the treatment of extensive cervical root defects using human periodontal cells. Clin. Oral Investig. 2017, 21, 753–761. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar, A. Palatoradicular groove: The hidden predator and etiological factor—Advanced proposed classification and literature review. Indian J. Dent. Res. 2020, 31, 656–661. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, E.C.; Poon, K.Y. Palato-gingival grooves in maxillary incisors. A possible predisposing factor to localised periodontal disease. Br. Dent. J. 1968, 124, 14–18. [Google Scholar]
- Kim, H.J.; Choi, Y.; Yu, M.K.; Lee, K.W.; Min, K.S. Recognition and management of palatogingival groove for tooth survival: A literature review. Restor. Dent. Endod. 2017, 42, 77–86. [Google Scholar] [CrossRef]
- Narmatha, V.J.; Thakur, S.; Shetty, S.; Bali, P.K. The complex radicular groove: Interdisciplinary management with mineral trioxide aggregate and bone substitute. J. Contemp. Dent. Pract. 2014, 15, 792–796. [Google Scholar] [PubMed]
- Miao, H.; Chen, M.; Otgonbayar, T.; Zhang, S.S.; Hou, M.H.; Wu, Z.; Wang, Y.L.; Wu, L.G. Papillary reconstruction and guided tissue regeneration for combined periodontal-endodontic lesions caused by palatogingival groove and additional root: A case report. Clin. Case Rep. 2015, 3, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Vashisth, P.; Arora, R.; Dwivedi, S. Combined endodontic therapy and periapical surgery with MTA and bone graft in treating palatogingival groove. BMJ Case Rep. 2013, 2013, bcr2013009056. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; de Ataide Ide, N.; Fernandes, M.; Lambor, R. Treatment of combined endodontic: Periodontic lesion by sealing of palato-radicular groove using biodentine. J. Conserv. Dent. 2014, 17, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Nadig, P.P.; Agrawal, I.S.; Agrawal, V.S.; Srinivasan, S.C. Palato-Radicular Groove: A Rare Entity in Maxillary Central Incisor Leading To Endo-Perio Lesion. J. Clin. Diagn. Res. 2016, 10, Zj14–Zj15. [Google Scholar] [CrossRef]
- Sharma, S.; Deepak, P.; Vivek, S.; Ranjan Dutta, S. Palatogingival Groove: Recognizing and Managing the Hidden Tract in a Maxillary Incisor: A Case Report. J. Int. Oral Health 2015, 7, 110–114. [Google Scholar] [PubMed]
- Johns, D.A.; Shivashankar, V.Y.; Shobha, K.; Johns, M. An innovative approach in the management of palatogingival groove using Biodentine™ and platelet-rich fibrin membrane. J. Conserv. Dent. 2014, 17, 75–79. [Google Scholar] [CrossRef]
- Yan, H.; Xu, N.; Wang, H.; Yu, Q. Intentional Replantation with a 2-segment Restoration Method to Treat Severe Palatogingival Grooves in the Maxillary Lateral Incisor: A Report of 3 Cases. J. Endod. 2019, 45, 1543–1549. [Google Scholar] [CrossRef]
- Xuelian, T.; Lan, Z.; Dingming, H. Intentional replantation for the treatment of palatal radicular groove with endo-periodontal lesion in the maxillary lateral incisor: A case report. West China J. Stomatol. 2017, 35, 448–452. [Google Scholar]
- Aidos, H.; Diogo, P.; Santos, J.M. Root Resorption Classifications: A Narrative Review and a Clinical Aid Proposal for Routine Assessment. Eur. Endod. J. 2018, 3, 134–145. [Google Scholar] [CrossRef]
- Abbott, P.V.; Lin, S. Tooth resorption-Part 2: A clinical classification. Dent. Traumatol. 2022, 38, 267–285. [Google Scholar] [CrossRef]
- Patel, S.; Foschi, F.; Condon, R.; Pimentel, T.; Bhuva, B. External cervical resorption: Part 2—Management. Int. Endod. J. 2018, 51, 1224–1238. [Google Scholar] [CrossRef]
- Arnold, M. Reparative Endodontic Treatment of a Perforating Internal Inflammatory Root Resorption: A Case Report. J. Endod. 2021, 47, 146–155. [Google Scholar] [CrossRef]
- Abuabara, A.; Costa, R.G.; Morais, E.C.; Furuse, A.Y.; Gonzaga, C.C.; Filho, F.B. Prosthetic rehabilitation and management of an MTA-treated maxillary central incisor with root perforation and severe internal resorption. J. Prosthodont. 2013, 22, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Agnoletto, M.; Engelke Back, E.D.; Tomazinho, L.F.; Paganini, F.A.; Vansan, L.P. Surgical resolution of an aggressive iatrogenic root perforation in a maxillary central incisor: A case report with a 4-year follow-up. Gen. Dent. 2017, 65, e1–e4. [Google Scholar]
- Büttel, L.; Weiger, R.; Krastl, G. Repair of a root perforation with MTA: A case report. Schweiz. Monatsschr. Zahnmed. 2013, 123, 549–563. [Google Scholar] [PubMed]
- Froughreyhani, M.; Salem Milani, A.; Barakatein, B.; Shiezadeh, V. Treatment of Strip Perforation Using Root MTA: A Case Report. Iran. Endod. J. 2013, 8, 80–83. [Google Scholar]
- Subay, R.K.; Subay, M.O.; Tuzcu, S.B. Endodontic management of root perforating internal replacement resorption. Eur. J. Dent. 2018, 12, 450–453. [Google Scholar] [CrossRef]
- Bendyk-Szeffer, M.; Łagocka, R.; Trusewicz, M.; Lipski, M.; Buczkowska-Radlińska, J. Perforating internal root resorption repaired with mineral trioxide aggregate caused complete resolution of odontogenic sinus mucositis: A case report. J. Endod. 2015, 41, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, D.; Eziana Hussein, F.; Abd Ghani, H. Management of Perforating Idiopathic Internal Root Resorption. Iran. Endod. J. 2017, 12, 257–260. [Google Scholar] [PubMed]
- Khalil, W.A.; Alghamdi, F.; Aljahdali, E. Strengthening effect of bioceramic cement when used to repair simulated internal resorption cavities in endodontically treated teeth. Dent. Med. Probl. 2020, 57, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Aktemur Türker, S.; Uzunoğlu, E.; Deniz Sungur, D.; Tek, V. Fracture Resistance of Teeth with Simulated Perforating Internal Resorption Cavities Repaired with Different Calcium Silicate-based Cements and Backfilling Materials. J. Endod. 2018, 44, 860–863. [Google Scholar] [CrossRef]
- Karypidou, A.; Chatzinikolaou, I.D.; Kouros, P.; Koulaouzidou, E.; Economides, N. Management of bilateral invasive cervical resorption lesions in maxillary incisors using a novel calcium silicate-based cement: A case report. Quintessence Int. 2016, 47, 637–642. [Google Scholar] [PubMed]
- Pruthi, P.J.; Dharmani, U.; Roongta, R.; Talwar, S. Management of external perforating root resorption by intentional replantation followed by Biodentine restoration. Dent. Res. J. 2015, 12, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Esnaashari, E.; Pezeshkfar, A.; Fazlyab, M. Nonsurgical management of an extensive perforative internal root resorption with calcium-enriched mixture cement. Iran. Endod. J. 2015, 10, 75–78. [Google Scholar] [PubMed]
- Asgary, S.; Eghbal, M.J.; Mehrdad, L.; Kheirieh, S.; Nosrat, A. Surgical management of a failed internal root resorption treatment: A histological and clinical report. Restor. Dent. Endod. 2014, 39, 137–142. [Google Scholar] [CrossRef]
- Asgary, S.; Nosrat, A. Conservative Management of Class 4 Invasive Cervical Root Resorption Using Calcium-enriched Mixture Cement. J. Endod. 2016, 42, 1291–1294. [Google Scholar] [CrossRef]
- Asgary, S.; Nosrat, A.; Seifi, A. Management of inflammatory external root resorption by using calcium-enriched mixture cement: A case report. J. Endod. 2011, 37, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Fazlyab, M. Surgical repair of invasive cervical root resorption with calcium-enriched mixture cement: A case report. Gen. Dent. 2015, 63, 37–40. [Google Scholar]
- Howait, M.; Shaker, M.; Aljuhani, H.; AlMohnna, M. External Cervical Resorption: A Case Report and Brief Review of the Literature, and Treatment Algorithms. J. Contemp. Dent. Pract. 2021, 22, 298–303. [Google Scholar] [CrossRef]
- Dudeja, C.; Taneja, S.; Kumari, M.; Singh, N. An in vitro comparison of effect on fracture strength, pH and calcium ion diffusion from various biomimetic materials when used for repair of simulated root resorption defects. J. Conserv. Dent. 2015, 18, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Linares, M.; de Oliveira Fagundes, J.; Solana, C.; Baca, P.; Ferrer-Luque, C.M. Current status on antimicrobial activity of a tricalcium silicate cement. J. Oral Sci. 2022, 64, 113–117. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, W.; Zhu, M.; Wu, C.; Zhu, Y. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: A review. Bioact. Mater. 2022, 18, 383–398. [Google Scholar] [CrossRef]
- Zhao, Y.; Wee, C.Y.; Zhang, H.; Yang, Z.; Wang, W.E.J.; Thian, E.S. Silver-substituted hydroxyapatite inhibits Pseudomonas aeruginosa outer membrane protein F: A potential antibacterial mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 134, 112713. [Google Scholar] [CrossRef]
- Nayak, V.V.; Tovar, N.; Hacquebord, J.H.; Duarte, S.; Panariello, B.H.D.; Tonon, C.; Atria, P.J.; Coelho, P.G.; Witek, L. Physiochemical and bactericidal activity evaluation: Silver-augmented 3D-printed scaffolds-An in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 195–209. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Q.; Guo, Y.; Ning, C.; Dai, K. Copper containing silicocarnotite bioceramic with improved mechanical strength and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111493. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. In vitro antibacterial capacity and cytocompatibility of SiO2-CaO-P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B 2014, 2, 4836–4847. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Garg, A.; Farooq, U.; Panda, A.K.; Mirza, M.A.; Noureldeen, A.; Darwish, H.; Iqbal, Z. Preparation and quality by design assisted (Qb-d) optimization of bioceramic loaded microspheres for periodontal delivery of doxycycline hyclate. Saudi J. Biol. Sci. 2021, 28, 2677–2685. [Google Scholar] [CrossRef]
- Marashdeh, M.; Stewart, C.; Kishen, A.; Levesque, C.; Finer, Y. Drug-Silica Coassembled Particles Improve Antimicrobial Properties of Endodontic Sealers. J. Endod. 2021, 47, 793–799. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, W.F.; Zhai, W. Toward stronger robocast calcium phosphate scaffolds for bone tissue engineering: A mini-review and meta-analysis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 134, 112578. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, F.; Liu, X.; Ge, Y.; Zeng, Y.; Zhai, Z.; Ning, C.; Li, H. Favorable osteogenic activity of iron doped in silicocarnotite bioceramic: In vitro and in vivo Studies. J. Orthop. Translat. 2022, 32, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Myat-Htun, M.; Mohd Noor, A.F.; Kawashita, M.; Baba Ismail, Y.M. Tailoring mechanical and in vitro biological properties of calcium–silicate based bioceramic through iron doping in developing future material. J. Mech. Behav. Biomed. Mater. 2022, 128, 105122. [Google Scholar] [CrossRef]
- Qin, H.; Wei, Y.; Han, J.; Jiang, X.; Yang, X.; Wu, Y.; Gou, Z.; Chen, L. 3D printed bioceramic scaffolds: Adjusting pore dimension is beneficial for mandibular bone defects repair. J. Tissue Eng. Regen. Med. 2022, 16, 409–421. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Y.; Lu, T.; He, F.; Zhang, L.; He, Q.; Ye, J. Enhancing the bioactivity of hydroxyapatite bioceramic via encapsulating with silica-based bioactive glass sol. J. Mech. Behav. Biomed. Mater. 2022, 128, 105104. [Google Scholar] [CrossRef]
- Taymour, N.; Fahmy, A.E.; Gepreel, M.A.H.; Kandil, S.; El-Fattah, A.A. Improved Mechanical Properties and Bioactivity of Silicate Based Bioceramics Reinforced Poly(ether-ether-ketone) Nanocomposites for Prosthetic Dental Implantology. Polymers 2022, 14, 1632. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.N.; Nicholson, T.; Kahler, B.; Walsh, L.J. Mineral Trioxide Aggregate-A Review of Properties and Testing Methodologies. Materials 2017, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Du, T.F.; Li, H.B.; Shen, Y.; Mobuchon, C.; Hieawy, A.; Wang, Z.J.; Yang, Y.; Ma, J.; Haapasalo, M. Physical properties and hydration behavior of a fast-setting bioceramic endodontic material. BMC Oral Health 2016, 16, 23. [Google Scholar] [CrossRef]
- Baghdadi, I.; Zaazou, A.; Tarboush, B.A.; Zakhour, M.; Özcan, M.; Salameh, Z. Physiochemical properties of a bioceramic-based root canal sealer reinforced with multi-walled carbon nanotubes, titanium carbide and boron nitride biomaterials. J. Mech. Behav. Biomed. Mater. 2020, 110, 103892. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. A Microstructure Insight of MTA Repair HP of Rapid Setting Capacity and Bioactive Response. Materials 2020, 13, 1641. [Google Scholar] [CrossRef]
- Abdalla, M.M.; Lung, C.Y.K.; Neelakantan, P.; Matinlinna, J.P. A novel, doped calcium silicate bioceramic synthesized by sol-gel method: Investigation of setting time and biological properties. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sony, S.; Suresh Babu, S.; Nishad, K.V.; Varma, H.; Komath, M. Development of an injectable bioactive bone filler cement with hydrogen orthophosphate incorporated calcium sulfate. J. Mater. Sci. Mater. Med. 2015, 26, 5355. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C-S-H Gel Structure of White Portland Cement. J. Funct. Biomater. 2019, 10, 46. [Google Scholar] [CrossRef]
- Koju, N.; Sikder, P.; Gaihre, B.; Bhaduri, B.S. Smart Injectable Self-Setting Monetite Based Bioceramics for Orthopedic Applications. Materials 2018, 11, 1258. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Schemkämper, P.; Bürklein, S.; Schäfer, E. Short and Long-Term Solubility, Alkalizing Effect, and Thermal Persistence of Premixed Calcium Silicate-Based Sealers: AH Plus Bioceramic Sealer vs. Total Fill BC Sealer. Materials 2022, 15, 7320. [Google Scholar] [CrossRef] [PubMed]
- Chaves de Souza, L.; Teixeira Neves, G.S.; Kirkpatrick, T.; Letra, A.; Silva, R. Physico-chemical and biological properties of AH Plus Bioceramic. J. Endod. 2022, 49, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Cardoso, M.L.; Rodrigues, J.P.; De-Deus, G.; Fidalgo, T. Solubility of bioceramic- and epoxy resin-based root canal sealers: A systematic review and meta-analysis. Aust. Endod. J. 2021, 47, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.T.; Silva, P.B.D.; Só, B.B.; Hashizume, L.N.; Vivan, R.R.; Rosa, R.A.D.; Duarte, M.A.H.; Só, M.V.R. Evaluation of Physicochemical Properties of New Calcium Silicate-Based Sealer. Braz. Dent. J. 2018, 29, 536–540. [Google Scholar] [CrossRef]
- Placek, L.M.; Keenan, T.J.; Coughlan, A.; Wren, A.W. Synthesis, Processing and the Effect of Thermal Treatment on the Solubility, Antioxidant Potential and Cytocompatibility of Y2O3 and CeO2 doped SiO2-SrO-Na2O Glass-Ceramics. J. Biomater. Appl. 2022, 37, 102–117. [Google Scholar] [CrossRef]
- Baghdadi, I.; AbuTarboush, B.; Zaazou, A.; Skienhe, H.; Özcan, M.; Zakhour, M.; Salameh, Z. Effect of sintering temperature on the physiochemical properties, microstructure, and compressive strength of a bioceramic root canal sealer reinforced with multi-walled carbon nanotubes and titanium carbide. J. Mech. Behav. Biomed. Mater. 2021, 119, 104524. [Google Scholar] [CrossRef] [PubMed]


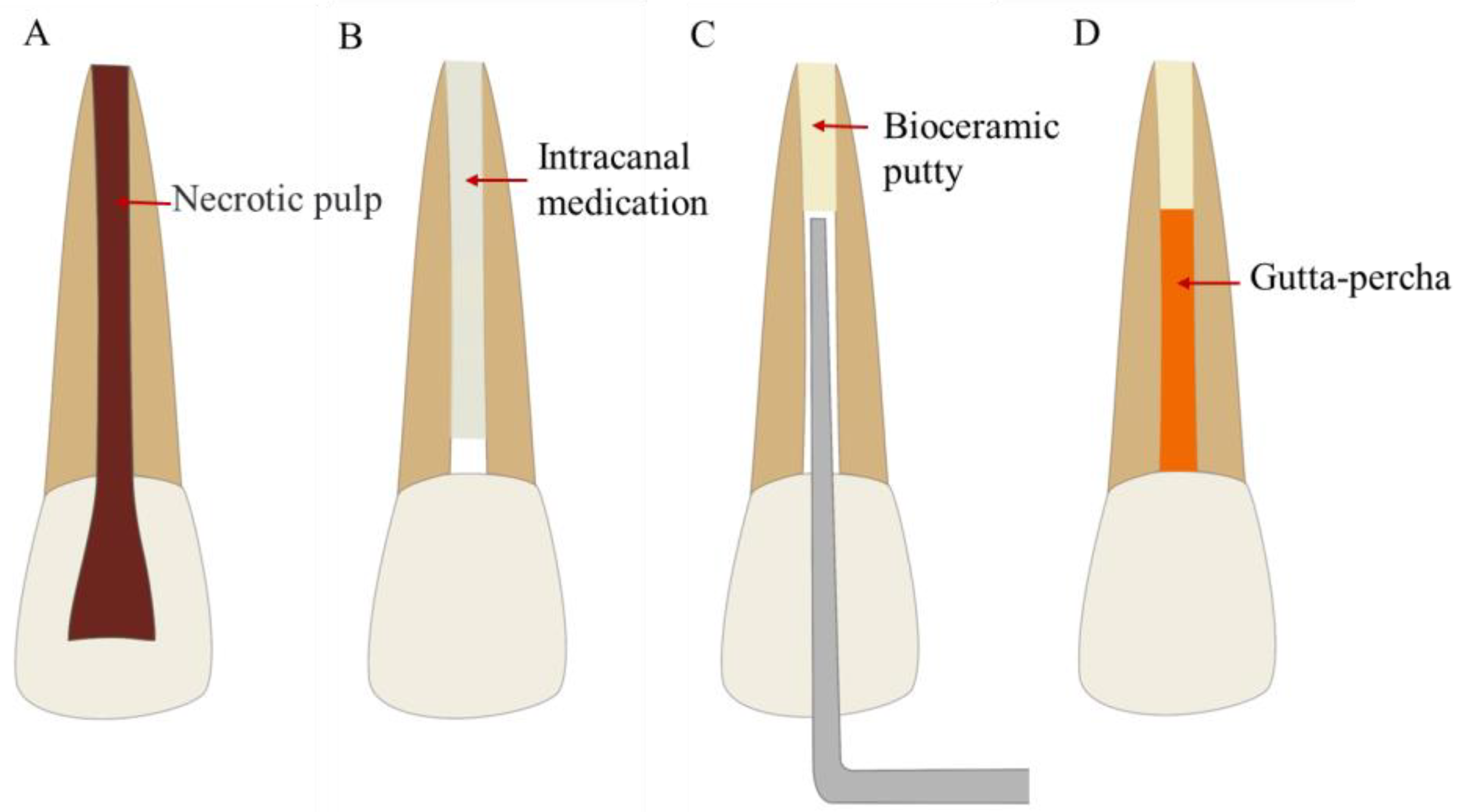








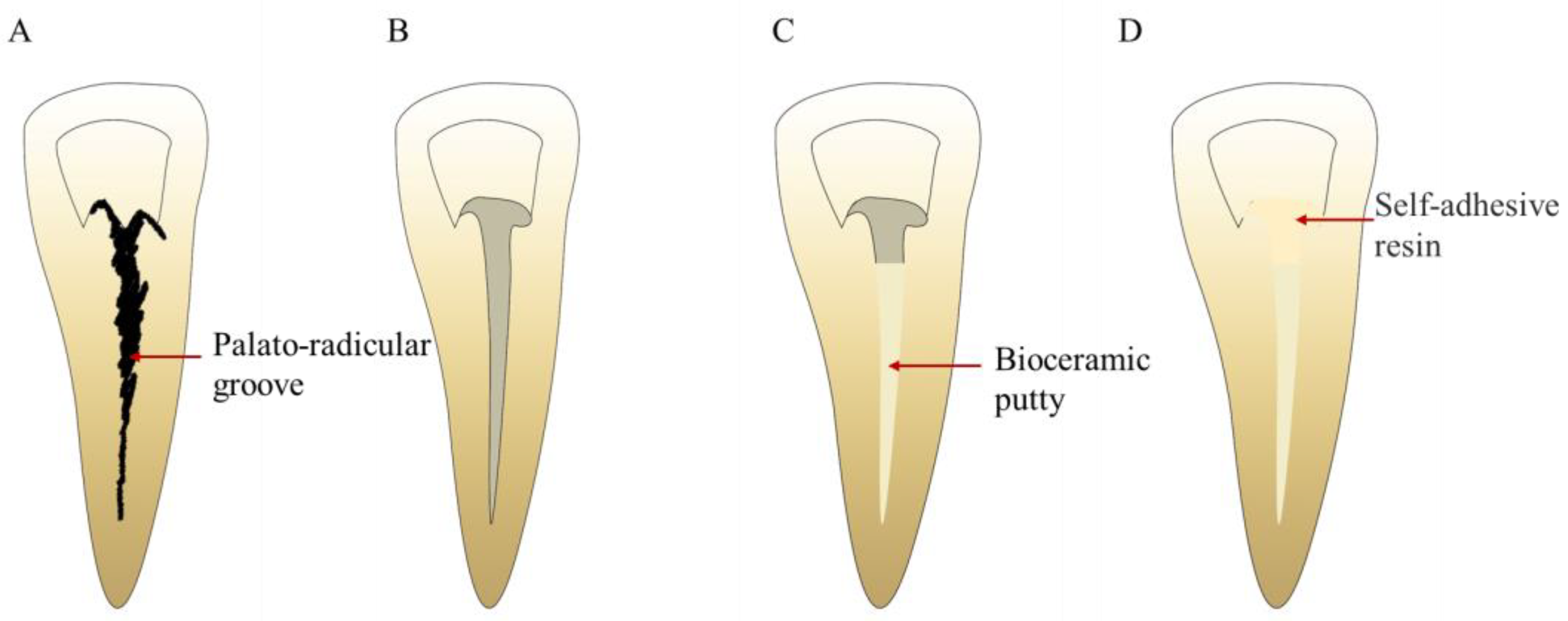

| Bioceramics | Chemical Composition | |
|---|---|---|
| ProRoot MTA (gray) | Powder | Dicalcium silicate, tricalcium silicate, tricalcium aluminate, calcium sulfate, bismuth oxide, and calcium aluminoferrite |
| Liquid | Sterile water | |
| ProRoot MTA (white) | Powder | Dicalcium silicate, tricalcium silicate, tricalcium aluminate, calcium sulfate, and bismuth oxide |
| Liquid | Sterile water | |
| Biodentine | Powder | Tricalcium silicate, dicalcium silicate, calcium carbonate, calcium oxide, zirconium oxide, and iron oxides |
| Liquid | Water, calcium chloride, and hydrosoluble polymer | |
| BC Putty | Putty | Calcium silicates, monobasic calcium phosphate, zirconium oxide, tantalum oxide, proprietary fillers, and thickening agents |
| BioAggregate | Powder | Tricalcium silicate, dicalcium silicate, calcium phosphate, calcium hydroxide, hydroxyapatite, silicon dioxide, and tantalum oxide |
| Liquid | Deionized water | |
| CEM | Powder | Calcium oxide, sulfur trioxide, phosphorous pentoxide, silicon dioxide, trace amounts of aluminum trioxide, sodium oxide, magnesium oxide, and chloride |
| Liquid | Water-based solution | |
| TheraCal LC | Paste | Type III Portland cement, Sr glass, fumed silica, barium sulfate, barium zirconate, a resin containing bisphenol A glycidyl methacrylate (Bis-GMA), and poly dimethacrylate (PEGDMA) |
| BC Sealer | Paste | Calcium silicates, calcium phosphate, zirconium oxide, tantalum oxide, and thickening agents |
| MTA Fillapex | Paste A | Salicylate resin, bismuth trioxide, and fumed silica |
| Paste B | Fumed silica, titanium dioxide, MTA (40%), and base resin | |
| BioRoot RCS | Powder | Tricalcium silicate, zirconium oxide, and povidone |
| Liquid | Water, calcium chloride, and hydrosoluble polymer | |
| Tech BioSealer | Powder | CEM, calcium sulfate, calcium chloride, bismuth oxide, and montmorillonite |
| Liquid | Dulbecco’s phosphate-buffered saline (DPBS) | |
| EndoSeal MTA | Paste | Calcium silicates, calcium aluminates, calcium aluminoferrite, calcium sulfates, radiopacifier, and thickening agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. https://doi.org/10.3390/bioengineering10030354
Dong X, Xu X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering. 2023; 10(3):354. https://doi.org/10.3390/bioengineering10030354
Chicago/Turabian StyleDong, Xu, and Xin Xu. 2023. "Bioceramics in Endodontics: Updates and Future Perspectives" Bioengineering 10, no. 3: 354. https://doi.org/10.3390/bioengineering10030354
APA StyleDong, X., & Xu, X. (2023). Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering, 10(3), 354. https://doi.org/10.3390/bioengineering10030354