Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration
Abstract
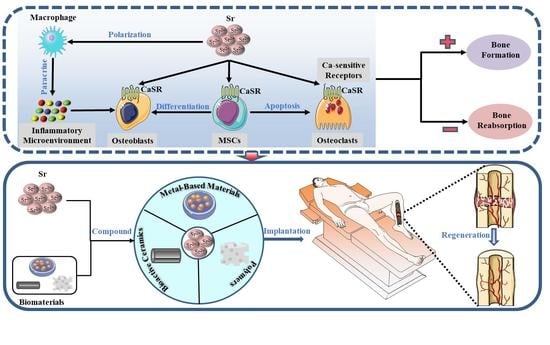
:1. Introduction
2. Mechanisms of Sr on Bone Regeneration
2.1. Inflammatory Microenvironment
2.2. Mesenchymal Stem Cells
2.3. Osteoblasts
2.4. Osteoclasts
2.5. Ca-sensitive Receptors
3. Biomaterials Compound with Sr
3.1. Bioactive Ceramics
3.1.1. Hydroxyapatite Scaffolds
3.1.2. Bioactive Glass
3.1.3. Ca Phosphate Ceramics
3.1.4. Other Bioactive Ceramics
3.2. Polymers
3.2.1. Natural Polymers
3.2.2. Synthetic Polymers
3.3. Metal-Based Materials
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baseri, N.; Meysamie, A.; Campanile, F.; Hamidieh, A.A.; Jafarian, A. Bacterial contamination of bone allografts in the tissue banks: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 123, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Yang, Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng. Part B Rev. 2021, 27, 539–547. [Google Scholar] [CrossRef]
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ullah, I.; Yu, K.; Zhang, W.; Zhou, J.; Sun, T.; Shi, L.; Yao, S.; Chen, K.; Zhang, X.; et al. Bioactive Sr2+/Fe3+co-substituted hydroxyapatite in cryogenically 3D printed porous scaffolds for bone tissue engineering. Biofabrication 2021, 13, 035007. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Wong, K.H.M.; Shen, J.; Wang, W.; Wu, J.; Li, J.; Lin, Z.; Chen, Z.; Matinlinna, J.P.; Zheng, Y.; et al. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat. Commun. 2021, 12, 2885. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M.; Amling, M.; Ignatius, A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cells Mater. 2018, 35, 365–385. [Google Scholar] [CrossRef]
- Dommeti, V.K.; Roy, S.; Pramanik, S.; Merdji, A.; Ouldyerou, A.; Özcan, M. Design and Development of Tantalum and Strontium Ion Doped Hydroxyapatite Composite Coating on Titanium Substrate: Structural and Human Osteoblast-like Cell Viability Studies. Materials 2023, 16, 1499. [Google Scholar] [CrossRef]
- Dai, L.; Chen, X.; Xiong, Y.; Chen, J.; Li, J.; Li, D.; Zhou, G.; Zou, Y.; Liu, T. Strontium gluconate potently promotes osteoblast development and restores bone formation in glucocorticoid-induced osteoporosis rats. Biochem. Biophys. Res. Commun. 2021, 554, 33–40. [Google Scholar] [CrossRef]
- Lee, N.H.; Kang, M.S.; Kim, T.H.; Yoon, D.S.; Mandakhbayar, N.; Jo, S.B.; Kim, H.S.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials 2021, 276, 121025. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Huo, M.; Lu, D.; Gao, Y.; Chen, Y.; Xu, H. Engineering Single-Atomic Iron-Catalyst-Integrated 3D-Printed Bioscaffolds for Osteosarcoma Destruction with Antibacterial and Bone Defect Regeneration Bioactivity. Adv. Mater. 2021, 33, e2100150. [Google Scholar] [CrossRef] [PubMed]
- Piette, M.; Desmet, B.; Dams, R. Determination of strontium in human whole blood by ICP-AES. Sci. Total Environ. 1994, 141, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Cheshmedzhieva, D.; Ilieva, S.; Permyakov, E.A.; Permyakov, S.E.; Dudev, T. Ca2+/Sr2+ Selectivity in Calcium-Sensing Receptor (CaSR): Implications for Strontium’s Anti-Osteoporosis Effect. Biomolecules 2021, 11, 1576. [Google Scholar] [CrossRef]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, J.; Rao, J.; Zhao, X.; Tian, X.; He, F.; Shi, H. Fabrication of strontium carbonate-based composite bioceramics as potential bone regenerative biomaterials. Colloids Surf. B Biointerfaces 2022, 218, 112755. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Deyneko, D.V.; Forysenkova, A.A.; Morozov, V.A.; Akhmedova, S.A.; Kirsanova, V.A.; Sviridova, I.K.; Sergeeva, N.S.; Rodionov, S.A.; Udyanskaya, I.L.; et al. Strontium Substituted β-Tricalcium Phosphate Ceramics: Physiochemical Properties and Cytocompatibility. Molecules 2022, 27, 6085. [Google Scholar] [CrossRef]
- Ma, F.; Xia, X.; Tang, B. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar]
- Bellucci, D.; Veronesi, E.; Strusi, V.; Petrachi, T.; Murgia, A.; Mastrolia, I.; Dominici, M.; Cannillo, V. Human Mesenchymal Stem Cell Combined with a New Strontium-Enriched Bioactive Glass: An ex-vivo Model for Bone Regeneration. Materials 2019, 12, 3633. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Chu, C.; Zhao, X.; Rung, S.; Xiao, W.; Liu, L.; Qu, Y.; Man, Y. Application of biomaterials in periodontal tissue repair and reconstruction in the presence of inflammation under periodontitis through the foreign body response: Recent progress and perspectives. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 7–17. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [Green Version]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.; Wang, Y.; Wang, Y.; Yang, R.; Liu, L.; Rung, S.; Xiang, L.; Wu, Y.; Du, S.; Man, Y.; et al. Evaluation of epigallocatechin-3-gallate (EGCG) modified collagen in guided bone regeneration (GBR) surgery and modulation of macrophage phenotype. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 73–82. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Li, S.; Pinna, A.; Barrak, F.; Chen, S.; Redpath, A.N.; Rankin, S.M.; Porter, A.E.; Jones, J.R. Interaction of monodispersed strontium containing bioactive glass nanoparticles with macrophages. Biomater. Adv. 2022, 133, 112610. [Google Scholar] [CrossRef]
- Xu, A.T.; Xie, Y.W.; Xu, J.G.; Li, J.; Wang, H.; He, F.M. Effects of strontium-incorporated micro/nano rough titanium surfaces on osseointegration via modulating polarization of macrophages. Colloids Surf. B Biointerfaces 2021, 207, 111992. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, M.W.; Wei, Y.J.; Geng, W.B.; Hu, Y.; Luo, Z.; Cai, K.Y. Construction of Wogonin Nanoparticle-Containing Strontium-Doped Nanoporous Structure on Titanium Surface to Promote Osteoporosis Fracture Repair. Adv. Healthc. Mater. 2022, 11, e2201405. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, Z.; Wu, K.; Bai, Y.; Lin, X.; Yang, H.; Yang, Q.; Wang, Z.; Ni, X.; Liu, H.; et al. Strontium-calcium phosphate hybrid cement with enhanced osteogenic and angiogenic properties for vascularised bone regeneration. J. Mater. Chem. B 2021, 9, 5982–5997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, Y.; Wang, J.; Huang, C.; Wang, Y.; Yang, H.; Liu, W.; Wang, T.; Wang, D.; Wang, G.; et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact. Mater. 2020, 5, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Y.Q.; Zhu, Y.H.; Lin, G.F.; Zhang, L.F.; Liu, X.C.; He, F.M. Antiadipogenesis and Osseointegration of Strontium-Doped Implant Surfaces. J. Dent. Res. 2019, 98, 795–802. [Google Scholar] [CrossRef]
- Lourenço, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1289–1303. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; Wang, W.; Zhang, X.; Hu, T.; He, Y.; Lin, K.; Liu, X. Strontium released bi-lineage scaffolds with immunomodulatory properties induce a pro-regenerative environment for osteochondral regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109833. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, A.T.; Wang, D.D.; Lin, G.F.; Liu, T.; He, F.M. The effects of Sr-incorporated micro/nano rough titanium surface on rBMSC migration and osteogenic differentiation for rapid osteointegration. Biomater. Sci. 2018, 6, 1946–1961. [Google Scholar] [CrossRef]
- Li, Y.; Yue, J.; Liu, Y.; Wu, J.; Guan, M.; Chen, D.; Pan, H.; Zhao, X.; Lu, W.W. Strontium regulates stem cell fate during osteogenic differentiation through asymmetric cell division. Acta Biomater. 2021, 119, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liang, Q.; Li, Y.; Fan, J.; Wang, G.; Pan, H.; Ruan, C. Strontium incorporation improves the bone-forming ability of scaffolds derived from porcine bone. Colloids Surf. B Biointerfaces 2018, 162, 279–287. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, G.J.; Yoo, H.S.; Song, D.H.; Chung, K.H.; Lee, K.J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, S.; Kim, K. Bone Tissue Engineering Strategies in Co-Delivery of Bone Morphogenetic Protein-2 and Biochemical Signaling Factors. Adv. Exp. Med. Biol. 2018, 1078, 233–244. [Google Scholar]
- Tan, S.; Zhang, B.; Zhu, X.; Ao, P.; Guo, H.; Yi, W.; Zhou, G.Q. Deregulation of bone forming cells in bone diseases and anabolic effects of strontium-containing agents and biomaterials. Biomed. Res. Int. 2014, 2014, 814057. [Google Scholar] [CrossRef] [Green Version]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium-Substituted Hydroxyapatite-Gelatin Biomimetic Scaffolds Modulate Bone Cell Response. Macromol. Biosci. 2018, 18, e1800096. [Google Scholar] [CrossRef]
- Xie, H.; Gu, Z.; He, Y.; Xu, J.; Xu, C.; Li, L.; Ye, Q. Microenvironment construction of strontium-calcium-based biomaterials for bone tissue regeneration: The equilibrium effect of calcium to strontium. J. Mater. Chem. B 2018, 6, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Hsu, Y.W.; Pan, W.L.; Hsu, F.Y. The Effect of Strontium-Substituted Hydroxyapatite Nanofibrous Matrix on Osteoblast Proliferation and Differentiation. Membranes 2021, 11, 624. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Jang, Y.S.; Kim, Y.K.; Kim, S.Y.; Lee, M.H.; Bae, T.S. Osteogenesis-Related Gene Expression and Guided Bone Regeneration of a Strontium-Doped Calcium-Phosphate-Coated Titanium Mesh. ACS Biomater. Sci. Eng. 2019, 5, 6715–6724. [Google Scholar] [CrossRef]
- Yodthong, T.; Kedjarune-Leggat, U.; Smythe, C.; Wititsuwannakul, R.; Pitakpornpreecha, T. l-Quebrachitol Promotes the Proliferation, Differentiation, and Mineralization of MC3T3-E1 Cells: Involvement of the BMP-2/Runx2/MAPK/Wnt/β-Catenin Signaling Pathway. Molecules 2018, 23, 3086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Zhao, F.; Gao, W.; Chen, X.; Guo, Z.; Zhang, W. Strontium-substituted sub-micron bioactive glasses inhibit ostoclastogenesis through suppression of RANKL-induced signaling pathway. Regen. Biomater. 2020, 7, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Boanini, E.; Torricelli, P.; Sima, F.; Axente, E.; Fini, M.; Mihailescu, I.N.; Bigi, A. Gradient coatings of strontium hydroxyapatite/zinc β-tricalcium phosphate as a tool to modulate osteoblast/osteoclast response. J. Inorg. Biochem. 2018, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moseke, C.; Wimmer, K.; Meininger, M.; Zerweck, J.; Wolf-Brandstetter, C.; Gbureck, U.; Ewald, A. Osteoclast and osteoblast response to strontium-doped struvite coatings on titanium for improved bone integration. Biomed. Tech. 2020, 65, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef] [PubMed]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Chen, S.; Shi, Y.; Ma, J. 3D printing of strontium-doped hydroxyapatite based composite scaffolds for repairing critical-sized rabbit calvarial defects. Biomed. Mater. 2018, 13, 065004. [Google Scholar] [CrossRef]
- Oryan, A.; Baghaban Eslaminejad, M.; Kamali, A.; Hosseini, S.; Sayahpour, F.A.; Baharvand, H. Synergistic effect of strontium, bioactive glass and nano-hydroxyapatite promotes bone regeneration of critical-sized radial bone defects. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Geng, Z.; Ji, L.; Li, Z.; Wang, J.; He, H.; Cui, Z.; Yang, X.; Liu, C. Nano-needle strontium-substituted apatite coating enhances osteoporotic osseointegration through promoting osteogenesis and inhibiting osteoclastogenesis. Bioact. Mater. 2021, 6, 905–915. [Google Scholar] [CrossRef]
- Chang, H.; Xiang, H.; Yao, Z.; Yang, S.; Tu, M.; Zhang, X.; Yu, B. Strontium-substituted calcium sulfate hemihydrate/hydroxyapatite scaffold enhances bone regeneration by recruiting bone mesenchymal stromal cells. J. Biomater. Appl. 2020, 35, 97–107. [Google Scholar] [CrossRef]
- Ramadas, M.; Ferreira, J.M.F.; Ballamurugan, A.M. Fabrication of three dimensional bioactive Sr2+ substituted apatite scaffolds by gel-casting technique for hard tissue regeneration. J. Tissue Eng. Regen. Med. 2021, 15, 577–585. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572–1589. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022, 10, 195–206. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, X.; Ma, Y.; Zhou, Y.; Liu, L.; Yu, F.; Fang, B.; Lin, K.; Xia, L.; Cai, M. Synergistic Effect of Micro-Nano-Hybrid Surfaces and Sr Doping on the Osteogenic and Angiogenic Capacity of Hydroxyapatite Bioceramics Scaffolds. Int. J. Nanomed. 2022, 17, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Ge, K.; Gao, F.; Yan, W.; Liu, H.; Xue, L.; Jin, Y.; Ma, H.; Zhang, J. Biomimetic mineralized strontium-doped hydroxyapatite on porous poly(l-lactic acid) scaffolds for bone defect repair. Int. J. Nanomed. 2018, 13, 1707–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Meng, Z.D.; Zhang, Y.Q.; Ye, L.Y.; Wang, C.J.; Guo, W.C. Sr-HA scaffolds fabricated by SPS technology promote the repair of segmental bone defects. Tissue Cell 2020, 66, 101386. [Google Scholar] [CrossRef]
- Denry, I.; Goudouri, O.M.; Fredericks, D.C.; Akkouch, A.; Acevedo, M.R.; Holloway, J.A. Strontium-releasing fluorapatite glass-ceramic scaffolds: Structural characterization and in vivo performance. Acta Biomater. 2018, 75, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Shenoy, S.J.; Babu, S.S.; Nair, R.P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf. B Biointerfaces 2018, 163, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Salinas, A.J. Mesoporous bioactive glasses for regenerative medicine. Mater. Today Bio 2021, 11, 100121. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109895. [Google Scholar] [CrossRef]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Manoochehri, H.; Ghorbani, M.; Moosazadeh Moghaddam, M.; Nourani, M.R.; Makvandi, P.; Sharifi, E. Strontium doped bioglass incorporated hydrogel-based scaffold for amplified bone tissue regeneration. Sci. Rep. 2022, 12, 10160. [Google Scholar] [CrossRef]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorilli, S.; Pagani, M.; Boggio, E.; Gigliotti, C.L.; Dianzani, C.; Gauthier, R.; Pontremoli, C.; Montalbano, G.; Dianzani, U.; Vitale-Brovarone, C. Sr-Containing Mesoporous Bioactive Glasses Bio-Functionalized with Recombinant ICOS-Fc: An In Vitro Study. Nanomaterials 2021, 11, 321. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, L.; Yan, R.; Shi, J.; Gu, H.; Deng, Y.; Jiang, R.; Wen, J.; Jiang, X. Strontium-incorporated bioceramic scaffolds for enhanced osteoporosis bone regeneration. Bone Res. 2022, 10, 55. [Google Scholar] [CrossRef]
- Autefage, H.; Allen, F.; Tang, H.M.; Kallepitis, C.; Gentleman, E.; Reznikov, N.; Nitiputri, K.; Nommeots-Nomm, A.; O’donnell, M.D.; Lange, C.; et al. Multiscale analyses reveal native-like lamellar bone repair and near perfect bone-contact with porous strontium-loaded bioactive glass. Biomaterials 2019, 209, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Shaltooki, M.; Dini, G.; Mehdikhani, M. Fabrication of chitosan-coated porous polycaprolactone/strontium-substituted bioactive glass nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110138. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.; Macri-Pellizzeri, L.; Zakir Hossain, K.M.; Scammell, B.E.; Grant, D.M.; Scotchford, C.A.; Hannon, A.C.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; et al. In vitro cellular testing of strontium/calcium substituted phosphate glass discs and microspheres shows potential for bone regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Midha, S.; Kumar, S.; Sharma, A.; Kaur, K.; Shi, X.; Naruphontjirakul, P.; Jones, J.R.; Ghosh, S. Silk fibroin-bioactive glass based advanced biomaterials: Towards patient-specific bone grafts. Biomed. Mater. 2018, 13, 055012. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Crawford, A.; Pires, R.A.; Hatton, P.V.; Reis, R.L. (*) Substituted Borosilicate Glasses with Improved Osteogenic Capacity for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 1331–1342. [Google Scholar] [CrossRef] [Green Version]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison Between Bioactive Sol-Gel and Melt-Derived Glasses/Glass-Ceramics Based on the Multicomponent SiO2-P2O5-CaO-MgO-Na2O-K2O System. Materials 2020, 13, 540. [Google Scholar] [CrossRef] [Green Version]
- Brauer, D.S.; Karpukhina, N.; Kedia, G.; Bhat, A.; Law, R.V.; Radecka, I.; Hill, R.G. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. J. R. Soc. Interface 2013, 10, 20120647. [Google Scholar] [CrossRef]
- Leite, Á.J.; Gonçalves, A.I.; Rodrigues, M.T.; Gomes, M.E.; Mano, J.F. Strontium-Doped Bioactive Glass Nanoparticles in Osteogenic Commitment. ACS Appl. Mater. Interfaces 2018, 10, 23311–23320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskus, A.; Kolmas, J. Ionic Substitutions in Non-Apatitic Calcium Phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.; Jiang, T.; Zou, X.; Lei, L.; Yan, W.; Yang, J.; Li, B. Strontium-substituted biphasic calcium phosphate microspheres promoted degradation performance and enhanced bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 895–905. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, J.; Sun, Z.; Deng, F.; Ning, C.; Xie, Y. Osteoblastic and anti-osteoclastic activities of strontium-substituted silicocarnotite ceramics: In vitro and in vivo studies. Bioact. Mater. 2020, 5, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Kubo, Y.; Lichte, P.; Heigl, T.; Roch, D.; Barahmand Pour, N.; Bergmann, C.; Sönmez, T.T.; Hock, J.V.P.; Fragoulis, A.; et al. Effects of Strontium-Doped β-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model. Int. J. Mol. Sci. 2020, 21, 3208. [Google Scholar] [CrossRef]
- Tao, Z.S.; Zhou, W.S.; Xu, H.G.; Yang, M. Aspirin modified strontium-doped β-tricalcium phosphate can accelerate the healing of femoral metaphyseal defects in ovariectomized rats. Biomed. Pharmacother. 2020, 132, 110911. [Google Scholar] [CrossRef]
- Liu, L.; Yu, F.; Li, L.; Zhou, L.; Zhou, T.; Xu, Y.; Lin, K.; Fang, B.; Xia, L. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: Release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater. 2021, 119, 444–457. [Google Scholar] [CrossRef]
- Li, J.J.; Dunstan, C.R.; Entezari, A.; Li, Q.; Steck, R.; Saifzadeh, S.; Sadeghpour, A.; Field, J.R.; Akey, A.; Vielreicher, M.; et al. A Novel Bone Substitute with High Bioactivity, Strength, and Porosity for Repairing Large and Load-Bearing Bone Defects. Adv. Healthc. Mater. 2019, 8, e1801298. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium(II) and mechanical loading additively augment bone formation in calcium phosphate scaffolds. J. Orthop. Res. 2018, 36, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xu, G.; Han, L.; Hu, X.; Zhao, Y.; Li, Z. Bone induction and defect repair by true bone ceramics incorporated with rhBMP-2 and Sr. J. Mater. Sci. Mater. Med. 2021, 32, 107. [Google Scholar] [CrossRef]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, Y.; Hu, L.; Peng, Y.; Deng, Y.; He, W.; Ge, Y.; Tang, B. Strontium Laminarin polysaccharide modulates osteogenesis-angiogenesis for bone regeneration. Int. J. Biol. Macromol. 2021, 181, 452–461. [Google Scholar] [CrossRef]
- Wu, T.; Liu, W.; Huang, S.; Chen, J.; He, F.; Wang, H.; Zheng, X.; Li, Z.; Zhang, H.; Zha, Z.; et al. Bioactive strontium ions/ginsenoside Rg1-incorporated biodegradable silk fibroin-gelatin scaffold promoted challenging osteoporotic bone regeneration. Mater. Today Bio 2021, 12, 100141. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Yu, L.; Lin, Q.; Wang, C.; Yang, D.; Tang, S. Strontium Modified Calcium Sulfate Hemihydrate Scaffold Incorporating Ginsenoside Rg1/Gelatin Microspheres for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 888. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ma, F.; Huang, J.; Frankie Leung, K.L.; Qin, C.; Lu, W.W.; Guo, X.E.; Tang, B. Metformin Hydrochloride Encapsulation by Alginate Strontium Hydrogel for Cartilage Regeneration by Reliving Cellular Senescence. Biomacromolecules 2021, 22, 671–680. [Google Scholar] [CrossRef]
- Xu, L.; Ma, F.; Leung, F.K.L.; Qin, C.; Lu, W.W.; Tang, B. Chitosan-strontium chondroitin sulfate scaffolds for reconstruction of bone defects in aged rats. Carbohydr. Polym. 2021, 273, 118532. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Avci, Ç.B.; Kerdar, S.N.; Amini, H.; Amini, M.; Ahmadi, M.; Sakai, S.; Bagca, B.G.; Ozates, N.P.; Rahbarghazi, R.; et al. Interaction of alginate with nano-hydroxyapatite-collagen using strontium provides suitable osteogenic platform. J. Nanobiotechnology 2022, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Huang, Z.; Yan, S.; Zhang, K.; Xu, S.; Li, G.; Cui, L.; Yin, J. Sr-HA-graft-Poly(γ-benzyl-l-glutamate) Nanocomposite Microcarriers: Controllable Sr2+ Release for Accelerating Osteogenenisis and Bony Nonunion Repair. Biomacromolecules 2017, 18, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.B.; Mccarthy, A.D.; Fernández, J.M. Evaluation of Strontium-Containing PCL-PDIPF Scaffolds for Bone Tissue Engineering: In Vitro and In Vivo Studies. Ann. Biomed. Eng. 2019, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, X.; Qiu, K.; Feng, W.; Mo, H.; Wang, M.; Wang, J.; He, C. Strontium-incorporated mineralized PLLA nanofibrous membranes for promoting bone defect repair. Colloids Surf. B Biointerfaces 2019, 179, 363–373. [Google Scholar] [CrossRef]
- Lin, S.J.; Huang, C.C. Strontium Peroxide-Loaded Composite Scaffolds Capable of Generating Oxygen and Modulating Behaviors of Osteoblasts and Osteoclasts. Int. J. Mol. Sci. 2022, 23, 6322. [Google Scholar] [CrossRef]
- Ray, S.; Thormann, U.; Eichelroth, M.; Budak, M.; Biehl, C.; Rupp, M.; Sommer, U.; El Khassawna, T.; Alagboso, F.I.; Kampschulte, M.; et al. Strontium and bisphosphonate coated iron foam scaffolds for osteoporotic fracture defect healing. Biomaterials 2018, 157, 1–16. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Mi, B.; Xiong, W.; Xu, N.; Guan, H.; Fang, Z.; Liao, H.; Zhang, Y.; Gao, B.; Xiao, X.; Fu, J.; et al. Strontium-loaded titania nanotube arrays repress osteoclast differentiation through multiple signalling pathways: In vitro and in vivo studies. Sci. Rep. 2017, 7, 2328. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Chen, W.; Zheng, M.; Lu, G.; Tang, W.; Huang, H.; Qu, D. Facile Fabrication of 3D-Printed Porous Ti6Al4V Scaffolds with a Sr-CaP Coating for Bone Regeneration. ACS Omega 2022, 7, 8391–8402. [Google Scholar] [CrossRef]
- Choi, S.M.; Park, J.W. Multifunctional effects of a modification of SLA titanium implant surface with strontium-containing nanostructures on immunoinflammatory and osteogenic cell function. J. Biomed. Mater. Res. A 2018, 106, 3009–3020. [Google Scholar] [CrossRef]
- Ding, Y.; Yuan, Z.; Liu, P.; Cai, K.; Liu, R. Fabrication of strontium-incorporated protein supramolecular nanofilm on titanium substrates for promoting osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110851. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Xu, J.; Li, J.; Wang, H.; He, F. Strontium-incorporated titanium implant surfaces treated by hydrothermal treatment enhance rapid osseointegration in diabetes: A preclinical vivo experimental study. Clin. Oral Implants Res. 2021, 32, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Zhao, L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci. Rep. 2019, 9, 14203. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, Y.; Shrestha, A.; Wang, S.; Wu, Q.; Li, L.; Guan, C.; Wang, C.; Fu, T.; Liu, W.; et al. Effects of Programmed Local Delivery from a Micro/Nano-Hierarchical Surface on Titanium Implant on Infection Clearance and Osteogenic Induction in an Infected Bone Defect. Adv. Healthc. Mater. 2019, 8, e1900002. [Google Scholar] [CrossRef]
- Li, J.; Fan, Z.; Huang, M.; Xie, Y.; Guan, Z.; Ruan, J. Enhanced healing process of tooth sockets using strontium-doped TiO2. RSC Adv. 2022, 12, 17817–17820. [Google Scholar] [CrossRef]
- Lin, G.; Zhou, C.; Lin, M.; Xu, A.; He, F. Strontium-incorporated titanium implant surface treated by hydrothermal reactions promotes early bone osseointegration in osteoporotic rabbits. Clin. Oral Implants Res. 2019, 30, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, Y.; Gu, L.; Qin, Y.; Li, D. Sol-gel-assisted micro-arc oxidation synthesis and characterization of a hierarchically rough structured Ta-Sr coating for biomaterials. RSC Adv. 2020, 10, 20020–20027. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Zhang, Z.; Qu, X.; Jia, X.; Wu, Q.; Han, Y.; Zheng, Y.; Dai, K. Biodegradable Zn-Sr alloy for bone regeneration in rat femoral condyle defect model: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 1588–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, W.; Zhang, X.; Nune, K.C.; Zhao, Y.; Liu, N.; Misra, R.D.K.; Yang, K.; Tan, L.; Yan, J. The effect of different coatings on bone response and degradation behavior of porous magnesium-strontium devices in segmental defect regeneration. Bioact. Mater. 2021, 6, 1765–1776. [Google Scholar] [CrossRef]
- Okuzu, Y.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Shimizu, T.; Sono, T.; Goto, K.; Otsuki, B.; Matsushita, T.; Kokubo, T.; et al. Strontium and magnesium ions released from bioactive titanium metal promote early bone bonding in a rabbit implant model. Acta Biomater. 2017, 63, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hengel, I.a.J.; Gelderman, F.S.A.; Athanasiadis, S.; Minneboo, M.; Weinans, H.; Fluit, A.C.; Van Der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Functionality-packed additively manufactured porous titanium implants. Mater. Today Bio 2020, 7, 100060. [Google Scholar] [CrossRef] [PubMed]


| Year | Team | Materials | Results |
|---|---|---|---|
| 2018 | Luo et al. [54] | Sr-substituted HA scaffold | Increased adhesion, proliferation, and ALP activity of MC3T3-E1 |
| 2018 | Ge et al. [62] | Sr-composited HA porous poly scaffold | Increased adhesion, proliferation, and ALP activity of MC3T3-E1 |
| 2019 | Oryan et al. [55] | Incorporation of Sr and bioglass into G/nHAp scaffold | Increased expression of OPN, OCN, and angiogenic markers of BMSCs |
| 2020 | Geng et al. [56] | Nano-needle Sr-substituted apatite coating | Increased adhesion, spreading, proliferation, and osteogenic differentiation of BMSCs, inhibited differentiation of osteoclasts |
| 2020 | Chang et al. [57] | Sr-substituted calcium sulfate hemihydrate/HA scaffold | Increased proliferation, migration, mineralized nodule area, and differentiation into osteoblast-like cells of BMSCs |
| 2020 | Zhao et al. [59] | Sr-substituted HA scaffold | Increased expression of the osteogenic marker in BMSCs |
| 2021 | Ramadas et al. [58] | Sr-substituted HA scaffold | Increased proliferation of MG-63 |
| 2022 | Zhong et al. [60] | Zn/Sr dual ion-collagen co-assembly HA | Increased osteogenic differentiation of BMSCs |
| 2022 | Jiang et al. [61] | Bioactivity of HA doped with different levels of Sr ceramics | Increased the proliferation, ALP activity, and gene expression of osteogenic and angiogenic factors in BMSCs |
| Year | Team | Materials | Results |
|---|---|---|---|
| 2017 | Fernandes et al. [79] | Mg and Sr-substituted BGs | Increased osteogenic differentiation and expression of ALP, OPN, and OCN in BMSCs |
| 2018 | Naruphontjirakul et al. [69] | Sr-containing BG nanoparticles | Increased ALP activity and expression of OCN in MC3T3-E1 |
| 2018 | Fiorilli et al. [72] | Sr-BGs | Increased osteogenic differentiation of SAOS-2 |
| 2018 | Midha et al. [78] | Sr-BGs | Increased osteogenic differentiation of BMSCs |
| 2019 | Autefage et al. [75] | PSrBG | Increased proliferation of BMSCs and MC3T3-E1 |
| 2019 | Shaltooki et al. [76] | BGs composed of PCL and different levels of Sr | Increased osteogenic activity of MG-63 |
| 2020 | Huang et al. [49] | Sr-substituted BGs | Inhibited RANKL-mediated osteoclastogenesis |
| 2021 | Baheiraei et al. [70] | Gel-BG/Sr scaffolds | Increased bone formation |
| 2021 | Fiorilli et al. [73] | Sr-containing esoporous BGs | Inhibited osteoclast differentiation and function |
| 2022 | Wu et al. [74] | Sr-BG | Increased osteogenesis and angiogenesis of BMSCs |
| Year | Team | Materials | Results |
|---|---|---|---|
| 2018 | Reitmaier et al. [92] | Sr(II)-doted CPC scaffolds | Increased bone formation |
| 2019 | Li et al. [91] | Sr-hardystonite-gahnite bioactive ceramic scaffold | Induced substantial bone formation and defect bridging |
| 2020 | Chen et al. [86] | Sr-substituted biphasic calcium phosphate microspheres | Increased proliferation and osteogenic inductivity of BMSCs |
| 2020 | Zeng et al. [87] | Sr-substituted calcium phosphate silicate bioactive ceramic | Increased proliferation and ALP activity of BMSCs, inhibited osteoclast differentiation |
| 2020 | Tohidnezhad et al. [88] | Sr-composited β-tricalcium phosphate scaffold | Increased bone fracture gap bridging |
| 2020 | Tao et al. [89] | Aspirin-modified Sr-composited β-tricalcium phosphate | Increased osteogenic viability of MC3T3-E1 |
| 2020 | Wu et al. [31] | Sr-reinforced calcium phosphate hybrid cement | Increased ALP activity and osteogenic gene expression of BMSCs, and promoted bone regeneration |
| 2021 | Liu et al. [90] | Sr-substituted calcium silicate ceramics | Increased angiogenesis of BMSCs and accelerated bone regeneration |
| Year | Team | Materials | Results |
|---|---|---|---|
| 2018 | Cheng et al. [39] | SrCl-coated surface porous CPB scaffold containing PCL | Increased osteogenic differentiation of BMSCs |
| 2019 | Ye et al. [96] | Sr-composited calcium phosphate/polycaprolactone/chitosan nanohybrid films | Increased adhesion, proliferation, and vascular differentiation of BMSCs |
| 2020 | Luo et al. [99] | Sr-calcium sulfate hemihydrate scaffold containing ginsenoside Rg1-encapsulated gelatin microspheres | Increased osteogenic differentiation and ALP activity of MC3T3-E1 |
| 2021 | Ma et al. [97] | Sr Laminarin polysaccharide | Increased expression of OCN in MC3T3-E1 |
| 2021 | Wu et al. [98] | Biodegradable silk protein-gelatin scaffolds doped with SrP and ginsenoside Rg1 | Increased osteogenic differentiation of BMSCs |
| 2021 | Xu et al. [100] | Metformin hydrochloride encapsulated Sralginate hydrogel | Increased chondrocyte repair, inhibited expression of senescence apoptosis, oxidative, and inflammatory genes |
| 2021 | Xu et al. [101] | Chitosan-Sr sulfate chondroitin scaffold | Increased BMP-2 expression of MC3T3-E1 |
| 2022 | Hassani et al. [102] | Alginate-nano-hydroxyapatite-collagen microspheres mixed with Ca2+, Ba2+, and Sr2+ | Increased the viability and osteogenic capacity of osteoblasts |
| Year | Team | Materials | Results |
|---|---|---|---|
| 2017 | Gao et al. [103] | Sr-HA-graft-Poly (γ-benzyl-l-glutamate) nanocomposite microcarriers | Increased adhesion, proliferation, and osteogenic gene expression of ADSCs |
| 2019 | Lourenço et al. [35] | Sr-crosslinked RGD-alginate hydrogel reinforced with Sr-doped hydroxyapatite microspheres | Induced osteogenic differentiation of BMSCs and reduced osteoclast function |
| 2019 | Lino et al. [104] | A compatibilized blend of poly-ε-caprolactone and polydiisopropyl fumarate enriched with 1% or 5% Sr2+ | Increased expression of ALP in BMSCs |
| 2019 | Han et al. [105] | Mineralized electrostatic spun poly (lactic acid) nanofiber membranes with different amounts of Sr | Increased proliferation and osteogenic differentiation of BMSCs |
| 2022 | Lin et al. [106] | Sr peroxide-loaded poly (lactic-co-glycolic acid)-gelatin scaffold system | Increased proliferation of osteoblast and inhibited formation of osteoclast |
| Year | Team | Materials | Results |
|---|---|---|---|
| 2017 | Mi et al. [109] | Sr-loaded Ti dioxide nanotube | Inhibited osteoclast differentiation |
| 2018 | Choi et al. [111] | Sandblasted/acid-etched titanium implants with Sr-containing nanostructures | Increased osteogenic differentiation of BMSCs and expression of osteogenic genes in osteoblasts |
| 2019 | Zhou et al. [114] | Sr-composited titanium dioxide coating | Increased proliferation and osteogenic differentiation of BMSCs |
| 2019 | Li et al. [115] | Dual delivery system coated on Ti surface | Manipulated macrophage polarization to activate pre-osteoblast differentiation |
| 2019 | Lin et al. [117] | Sr-incorporated titanium implant | Increased effect of early bone healing |
| 2020 | Ding et al. [112] | Protein supramolecular nanomembranes doped with Sr on Ti base | Increased early adhesion, proliferation, osteogenic differentiation, and expression of osteogenic genes in BMSCs |
| 2020 | Jia et al. [118] | Zn-Sr alloy | Increased cytocompatibility and osteogenesis of MC3T3-E1 |
| 2020 | Zhang et al. [119] | Mg-Sr alloy | Increased proliferation, mineralization, and ALP activity of BMSCs |
| 2021 | Xu et al. [113] | Sr-Ti implants | Increased OPG expression and lowered inflammatory factors expression |
| 2022 | Su et al. [110] | Sr calcium phosphate coating on Ti6Al4V scaffolds | Increased adhesion, spreading, and osteogenesis of BMSCs |
| 2022 | Li et al. [116] | Sr-doped titanium dioxide mesoporous nanospheres | Increased the formation of new bone tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Huang, H.; Zhang, J.; Sun, T.; Zhang, W.; Li, Z. Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration. Bioengineering 2023, 10, 414. https://doi.org/10.3390/bioengineering10040414
Liu X, Huang H, Zhang J, Sun T, Zhang W, Li Z. Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration. Bioengineering. 2023; 10(4):414. https://doi.org/10.3390/bioengineering10040414
Chicago/Turabian StyleLiu, Xin, Huagui Huang, Jing Zhang, Tianze Sun, Wentao Zhang, and Zhonghai Li. 2023. "Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration" Bioengineering 10, no. 4: 414. https://doi.org/10.3390/bioengineering10040414
APA StyleLiu, X., Huang, H., Zhang, J., Sun, T., Zhang, W., & Li, Z. (2023). Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration. Bioengineering, 10(4), 414. https://doi.org/10.3390/bioengineering10040414