Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Artificial Incipient Caries Like-Lesion Formation
2.3. 28-Day pH Cycling Protocol
2.4. SEM-EDX Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlGhannam, M.I.; AlAbbas, M.S.; AlJishi, J.A.; AlRuwaili, M.A.; AlHumaid, J.; Ibrahim, M.S. Remineralizing Effects of Resin-Based Dental Sealants: A Systematic Review of In Vitro Studies. Polymers 2022, 14, 779. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The Use of Hydroxyapatite Toothpaste to Prevent Dental Caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Ismail, A.I.; Tellez, M.; Pitts, N.B.; Ekstrand, K.R.; Ricketts, D.; Longbottom, C.; Eggertsson, H.; Deery, C.; Fisher, J.; Young, D.A.; et al. Caries Management Pathways Preserve Dental Tissues and Promote Oral Health. Community Dent. Oral Epidemiol. 2013, 41, e12–e40. [Google Scholar] [CrossRef]
- Gjorgievska, E.S.; Nicholson, J.W.; Slipper, I.J.; Stevanovic, M.M. Remineralization of Demineralized Enamel by Toothpastes: A Scanning Electron Microscopy, Energy Dispersive X-Ray Analysis, and Three-Dimensional Stereo-Micrographic Study. Microsc. Microanal. 2013, 19, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Damle, S.G.; Vidya, I.; Yadav, R.; Bhattal, H.; Loomba, A. Quantitative Determination of Inorganic Constituents in Saliva and Their Relationship with Dental Caries Experience in Children. Dentistry 2012, 2, 1000131. [Google Scholar] [CrossRef]
- Puleio, F.; Fiorillo, L.; Gorassini, F.; Iandolo, A.; Meto, A.; D’Amico, C.; Cervino, G.; Pinizzotto, M.; Bruno, G.; Portelli, M.; et al. Systematic Review on White Spot Lesions Treatments. Eur. J. Dent. 2022, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, H.; Luo, J.; Yao, H.; Wang, P.; Li, Z.; Wei, J. Advanced Materials for Enamel Remineralization. Front. Bioeng. Biotechnol. 2022, 10, 985881. [Google Scholar] [CrossRef] [PubMed]
- Coceska, E.; Gjorgievska, E.; Coleman, N.J.; Gabric, D.; Slipper, I.J.; Stevanovic, M.; Nicholson, J.W. Enamel Alteration Following Tooth Bleaching and Remineralization. J. Microsc. 2016, 262, 232–244. [Google Scholar] [CrossRef]
- Guntermann, L.; Rohrbach, A.; Schäfer, E.; Dammaschke, T. Remineralization and Protection from Demineralization: Effects of a Hydroxyapatite-Containing, a Fluoride-Containing and a Fluoride- and Hydroxyapatite-Free Toothpaste on Human Enamel in Vitro. Head Face Med. 2022, 18, 26. [Google Scholar] [CrossRef]
- ten Cate, J.M. Current Concepts on the Theories of the Mechanism of Action of Fluoride. Acta Odontol. Scand. 1999, 57, 325–329. [Google Scholar] [CrossRef]
- Goldberg, M. Fluorides in Dental Tissues: Caries Prevention and Fluorosis. JSM Dent. 2020, 8, 1123. [Google Scholar] [CrossRef]
- Shellis, R.P.; Wilson, R.M. Apparent Solubility Distributions of Hydroxyapatite and Enamel Apatite. J. Colloid Interface Sci. 2004, 278, 325–332. [Google Scholar] [CrossRef]
- Walsh, T.; Worthington, H.V.; Glenny, A.-M.; Marinho, V.C.; Jeroncic, A. Fluoride Toothpastes of Different Concentrations for Preventing Dental Caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralization and Repair of Enamel Surface by Biomimetic Zn-Carbonate Hydroxyapatite Containing Toothpaste: A Comparative in Vivo Study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Aimutis, W.R. Bioactive Properties of Milk Proteins with Particular Focus on Anticariogenesis. J. Nutr. 2004, 134, 989S–995S. [Google Scholar] [CrossRef]
- Zero, D.T.; Hara, A.T.; Kelly, S.A.; González-Cabezas, C.; Eckert, G.J.; Barlow, A.P.; Mason, S.C. Evaluation of a Desensitizing Test Dentifrice Using an in Situ Erosion Remineralization Model. J. Clin. Dent. 2006, 17, 112–116. [Google Scholar]
- Fowler, C.; Willson, R.; Rees, G.D. In Vitro Microhardness Studies on a New Anti-Erosion Desensitizing Toothpaste. J. Clin. Dent. 2006, 17, 100–105. [Google Scholar]
- Nardi, G.M.; Guerra, F.; Ndokaj, A.; Corridore, D.; Straker, M.A.; Sportelli, P.; Di Giorgio, R.; Grassi, F.R.; Grassi, R.; Ottolenghi, L. Phototherapy and Tailored Brushing Method. Personalized Oral Care in Patients with Facial and Dental Trauma. A Report of a Case. Healthcare 2021, 9, 561. [Google Scholar] [CrossRef]
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-Doped Amorphous Calcium Phosphate Nanoparticles as a Promising Biomimetic Material for Dental Remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef]
- Leventouri, T. Synthetic and Biological Hydroxyapatites: Crystal Structure Questions. Biomaterials 2006, 27, 3339–3342. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Tao, J.; Xu, X.; Mao, C.; Gu, X.; Tang, R. Repair of Enamel by Using Hydroxyapatite Nanoparticles as the Building Blocks. J. Mater. Chem. 2008, 18, 4079–4084. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Sparabombe, S.; Tiriduzzi, P.; Bambini, F.; Putignano, A. A 3-Day Randomized Clinical Trial to Investigate the Desensitizing Properties of Three Dentifrices. J. Periodontol. 2013, 84, e65–e73. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Giuliodori, F.; Lorenzini, A.; Putignano, A. A Double-Blind Randomized-Controlled Trial Comparing the Desensitizing Efficacy of a New Dentifrice Containing Carbonate/Hydroxyapatite Nanocrystals and a Sodium Fluoride/Potassium Nitrate Dentifrice. J. Clin. Periodontol. 2010, 37, 510–517. [Google Scholar] [CrossRef]
- Manchery, N.; John, J.; Nagappan, N.; Subbiah, G.K.; Premnath, P. Remineralization Potential of Dentifrice Containing Nanohydroxyapatite on Artificial Carious Lesions of Enamel: A Comparative in Vitro Study. Dent. Res. J. 2019, 16, 310–317. [Google Scholar] [CrossRef]
- Orilisi, G.; Monterubbianesi, R.; Notarstefano, V.; Tosco, V.; Vitiello, F.; Giuliani, G.; Putignano, A.; Orsini, G. New Insights from Raman MicroSpectroscopy and Scanning Electron Microscopy on the Microstructure and Chemical Composition of Vestibular and Lingual Surfaces in Permanent and Deciduous Human Teeth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119966. [Google Scholar] [CrossRef]
- Favaro, J.C.; Detomini, T.R.; Maia, L.P.; Poli, R.C.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Anticaries Agent Based on Silver Nanoparticles and Fluoride: Characterization and Biological and Remineralizing Effects-An In Vitro Study. Int. J. Dent. 2022, 2022, 9483589. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Tosco, V.; Bellezze, T.; Giuliani, G.; Özcan, M.; Putignano, A.; Orsini, G. A Comparative Evaluation of Nanohydroxyapatite-Enriched Hydrogen Peroxide Home Bleaching System on Color, Hardness and Microstructure of Dental Enamel. Materials 2021, 14, 3072. [Google Scholar] [CrossRef]
- Vitiello, F.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Gatto, M.L.; Sparabombe, S.; Memé, L.; Mengucci, P.; Putignano, A.; Orsini, G. Remineralization Efficacy of Four Remineralizing Agents on Artificial Enamel Lesions: SEM-EDS Investigation. Materials 2022, 15, 4398. [Google Scholar] [CrossRef]
- Giulio, A.B.; Matteo, Z.; Serena, I.P.; Silvia, M.; Luigi, C. In Vitro Evaluation of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Effect on Stripped Enamel Surfaces. A SEM Investigation. J. Dent. 2009, 37, 228–232. [Google Scholar] [CrossRef]
- Motohashi, J.; Furukawa, S.; Shimoda, S.; Tsurumoto, A. Transition of Fluoride into Tooth Substance from Sustained Fluoride-Releasing Sealant—In Vitro Evaluation. J. Hard Tissue Biol. 2010, 19, 195–202. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, J.W.; Kim, K.M.; Kwon, J.S. Prevention of Secondary Caries Using Resin-Based Pit and Fissure Sealants Containing Hydrated Calcium Silicate. Polymers 2020, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.S.; Kargul, B. Comparative Evaluation of the Spectral-Domain Optical Coherence Tomography and Microhardness for Remineralization of Enamel Caries Lesions. Dent. Mater. J. 2021, 40, 1115–1121. [Google Scholar] [CrossRef]
- Shetty, S.; Hegde, M.N.; Bopanna, T.P. Enamel Remineralization Assessment after Treatment with Three Different Remineralizing Agents Using Surface Microhardness: An in Vitro Study. J. Conserv. Dent. 2014, 17, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139–144. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. Remineralization, the Natural Caries Repair Process--the Need for New Approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; De Ataide, I.D.N.; Fernandes, M.; Lambor, R. Assessment of Enamel Remineralisation After Treatment with Four Different Remineralising Agents: A Scanning Electron Microscopy (SEM) Study. J. Clin. Diagn. Res. 2017, 11, ZC136–ZC141. [Google Scholar] [CrossRef]
- Bhavsar, B.; Vijo, M.; Sharma, P.; Patnaik, T.; Alam, M.K.; Patil, S. Comparative Assessment of Enamel Remineralisation on the Surface Microhardness of Demineralized Enamel—An in Vitro Study. PeerJ 2022, 10, e14098. [Google Scholar] [CrossRef]
- Fernando, J.R.; Walker, G.D.; Park, T.K.-S.; Shen, P.; Yuan, Y.; Reynolds, C.; Reynolds, E.C. Comparison of Calcium-Based Technologies to Remineralise Enamel Subsurface Lesions Using Microradiography and Microhardness. Sci. Rep. 2022, 12, 9888. [Google Scholar] [CrossRef]
- Campanella, V.; Gallusi, G.; Nardi, R.; Mea, A.; Di Taranto, V.; Montemurro, E.; Marzo, G.; Libonati, A. Dentinal Substrate Variability and Bonding Effectiveness: SEM Investigation. J. Biol. Regul. Homeost. Agents 2020, 34, 49–54, DENTAL SUPPLEMENT. [Google Scholar]
- De Carvalho Filho, A.C.B.; Sanches, R.P.; Martin, A.A.; Do Espírito Santo, A.M.; Soares, L.E.S. Energy Dispersive X-Ray Spectrometry Study of the Protective Effects of Fluoride Varnish and Gel on Enamel Erosion. Microsc. Res. Tech. 2011, 74, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Bohns, F.R.; Degrazia, F.W.; de Souza Balbinot, G.; Leitune, V.C.B.; Samuel, S.M.W.; García-Esparza, M.A.; Sauro, S.; Collares, F.M. Boron Nitride Nanotubes as Filler for Resin-Based Dental Sealants. Sci. Rep. 2019, 9, 7710. [Google Scholar] [CrossRef] [PubMed]
- Salman, N.; El-Tekeya, M.; Bakry, N.; Soliman, S. Remineralization Effect of Fluoride Varnish Containing Casein Phosphopeptide Amorphous Calcium Phosphate on Caries-Like Lesions in Primary Teeth (In Vitro Study). Alex. Dent. J. 2019, 44, 13–16. [Google Scholar] [CrossRef]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B.T. Remineralization of Early Caries by a Nano-Hydroxyapatite Dentifrice. J. Clin. Dent. 2011, 22, 139–143. [Google Scholar] [PubMed]
- Featherstone, J.D.; Zero, D.T. An in Situ Model for Simultaneous Assessment of Inhibition of Demineralization and Enhancement of Remineralization. J. Dent. Res. 1992, 71, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, R.; Kumar, A.; Indira, R.; Srinivasan, M.R.; Ramachandran, S. Remineralization of Early Enamel Lesions Using Casein Phosphopeptide Amorphous Calcium Phosphate: An Ex-Vivo Study. Contemp. Clin. Dent. 2010, 1, 210–213. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Mehrabkhani, M.; Ahrari, F.; Parisay, I.; Jahantigh, M. The Effects of Three Remineralizing Agents on Regression of White Spot Lesions in Children: A Two-Week, Single-Blind, Randomized Clinical Trial. J. Clin. Exp. Dent. 2017, 9, e641–e648. [Google Scholar] [CrossRef]
- Patil, N.; Choudhari, S.; Kulkarni, S.; Joshi, S.R. Comparative Evaluation of Remineralizing Potential of Three Agents on Artificially Demineralized Human Enamel: An in Vitro Study. J. Conserv. Dent. 2013, 16, 116–120. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Jonathan, R.; Benin, P.; Kuumar, A. Evaluation to Determine the Caries Remineralization Potential of Three Dentifrices: An in Vitro Study. J. Conserv. Dent. 2013, 16, 375–379. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Wang, Y.; Yin, W.; Antoun, J.S.; Farella, M.; Mei, L. Long-Term Remineralizing Effect of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) on Early Caries Lesions in Vivo: A Systematic Review. J. Dent. 2014, 42, 769–777. [Google Scholar] [CrossRef]
- Akküç, S.; Duruk, G.; Keleş, A. Remineralization Effect of Three Different Agents on Initial Caries and Erosive Lesions: A Micro-Computed Tomography and Scanning Electron Microscopy Analysis. BMC Oral Health 2023, 23, 106. [Google Scholar] [CrossRef]
- Devadiga, D.; Shetty, P.; Hegde, M.N.; Reddy, U. Bioactive Remineralization of Dentin Surface with Calcium Phosphate-Based Agents: An in Vitro Analysis. J. Conserv. Dent. 2022, 25, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, K.; Coutinho, T.; Silva, E.; Pereira, A.; Tostes, M. Evaluation of CPP-ACP and Fluoride on Inhibition of Human Enamel Demineralisation: Cross-Sectional Hardness and MicroCT Studies. Oral Health Prev. Dent. 2017, 15, 549–555. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Né, Y.G.; Souza-Monteiro, D.; Frazão, D.R.; Alvarenga, M.O.P.; Aragão, W.A.B.; Fagundes, N.F.; de Souza-Rodrigues, R.D.; Lima, R.R. Treatment for Dental Erosion: A Systematic Review of in Vitro Studies. PeerJ 2022, 10, e13864. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Buzalaf, M.a.R.; Duangthip, D.; Anttonen, V.; Ganss, C.; João-Souza, S.H.; Baumann, T.; Carvalho, T.S. The Use of Fluoride for the Prevention of Dental Erosion and Erosive Tooth Wear in Children and Adolescents. Eur. Arch. Paediatr. Dent. 2019, 20, 517–527. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Pasciuta, R.; Grusovin, M.G.; Mancini, N.; Burioni, R. Evaluation of Resistance against Bacterial Microleakage of a New Conical Implant-Abutment Connection versus Conventional Connections: An in Vitro Study. New Microbiol. 2016, 39, 49–56. [Google Scholar]
- Anderson, C.J.; Kugel, G.; Zou, Y.; Ferrari, M.; Gerlach, R. A Randomized, Controlled, Two-Month Pilot Trial of Stannous Fluoride Dentifrice versus Sodium Fluoride Dentifrice after Oxalate Treatment for Dentinal Hypersensitivity. Clin. Oral Investig. 2020, 24, 4043–4049. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic Hydroxyapatite Paste for Molar-Incisor Hypomineralization: A Randomized Clinical Trial. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Simon, L.S.; Dash, J.K.; Deppika, U.; Philip, S.; Sarangi, S. Management of Post Orthodontic White Spot Lesions Using Resin Infiltration and CPP-ACP Materials—A Clinical Study. J. Clin. Pediatr. Dent. 2022, 46, 70–74. [Google Scholar] [CrossRef]
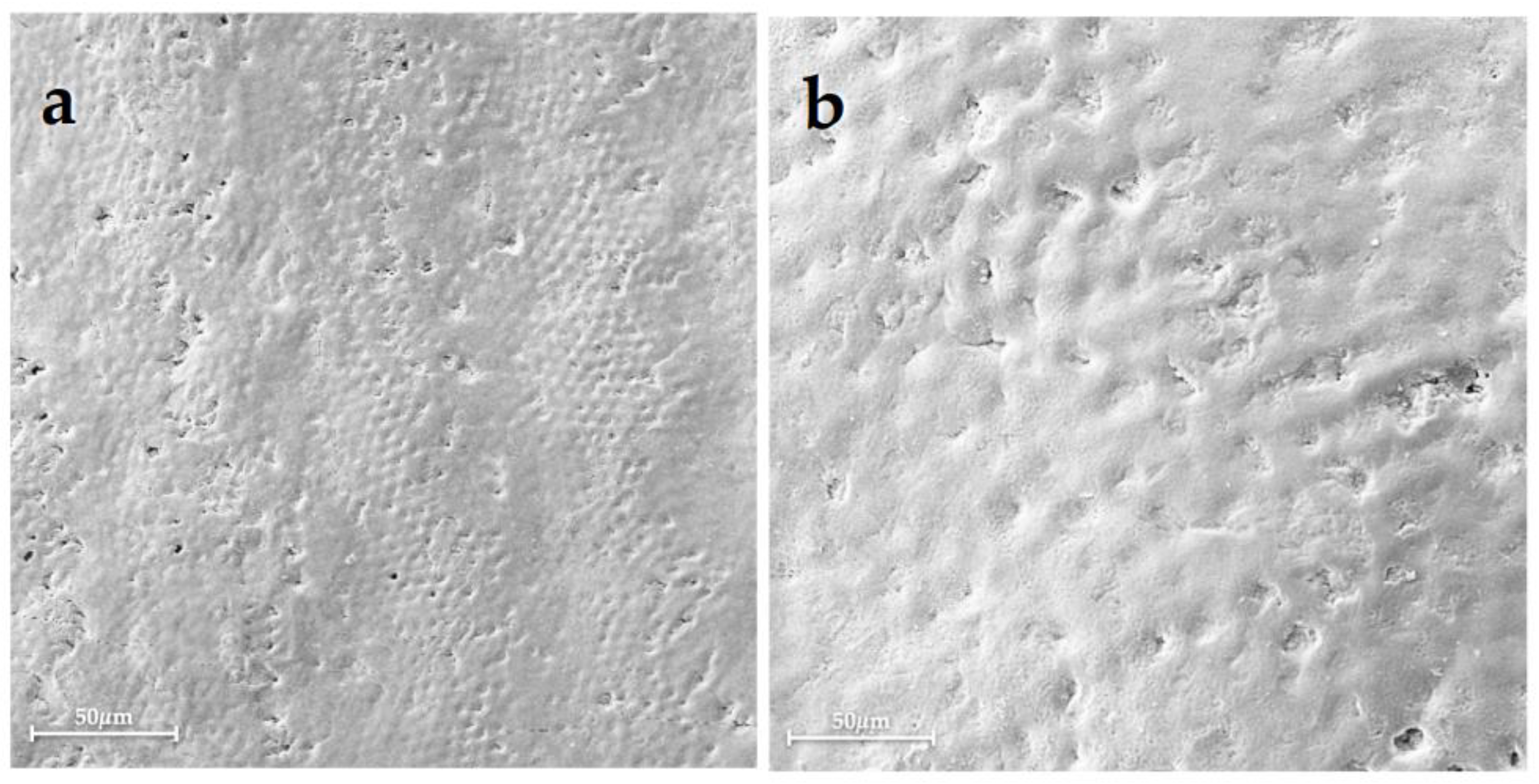
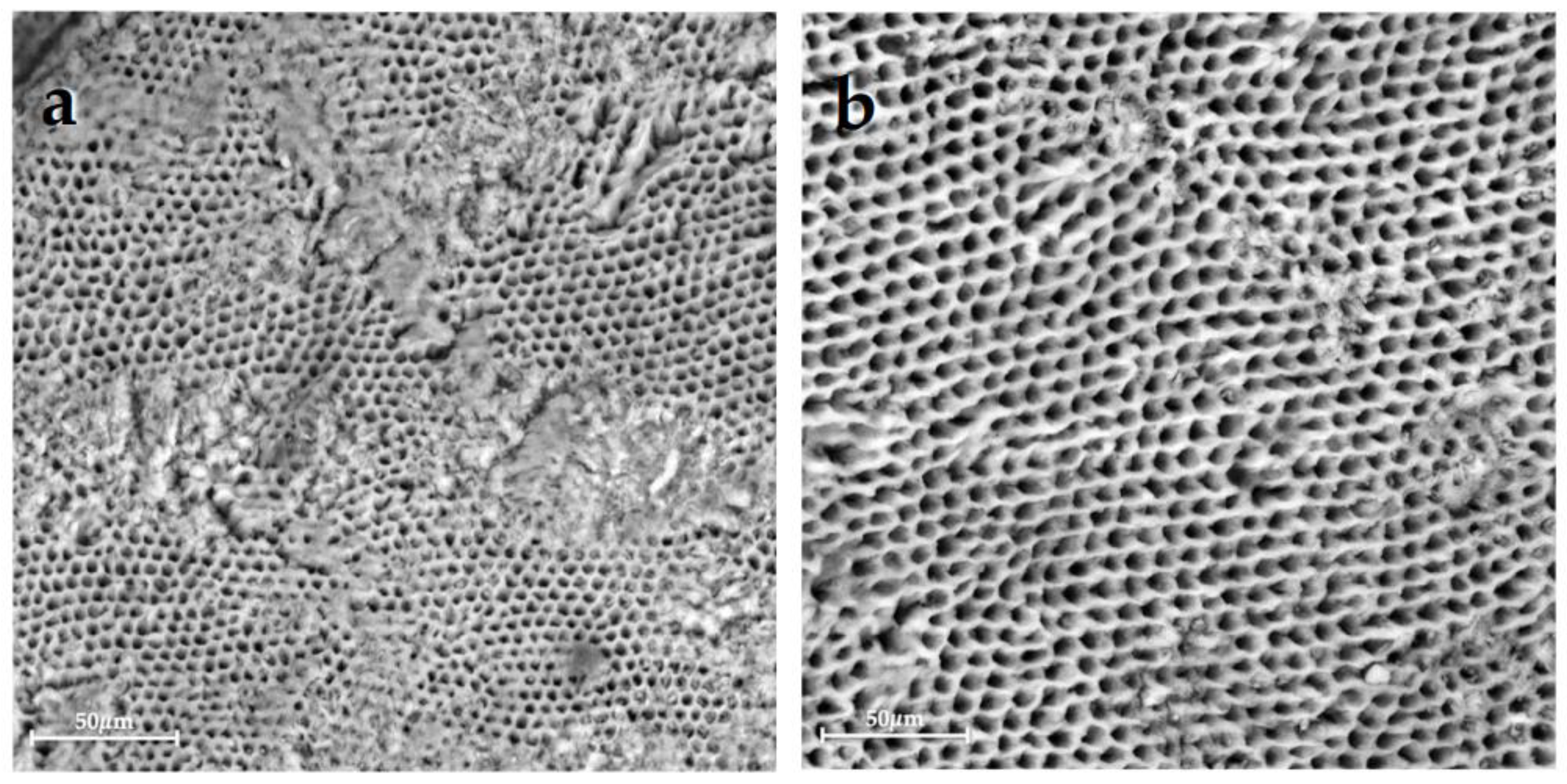
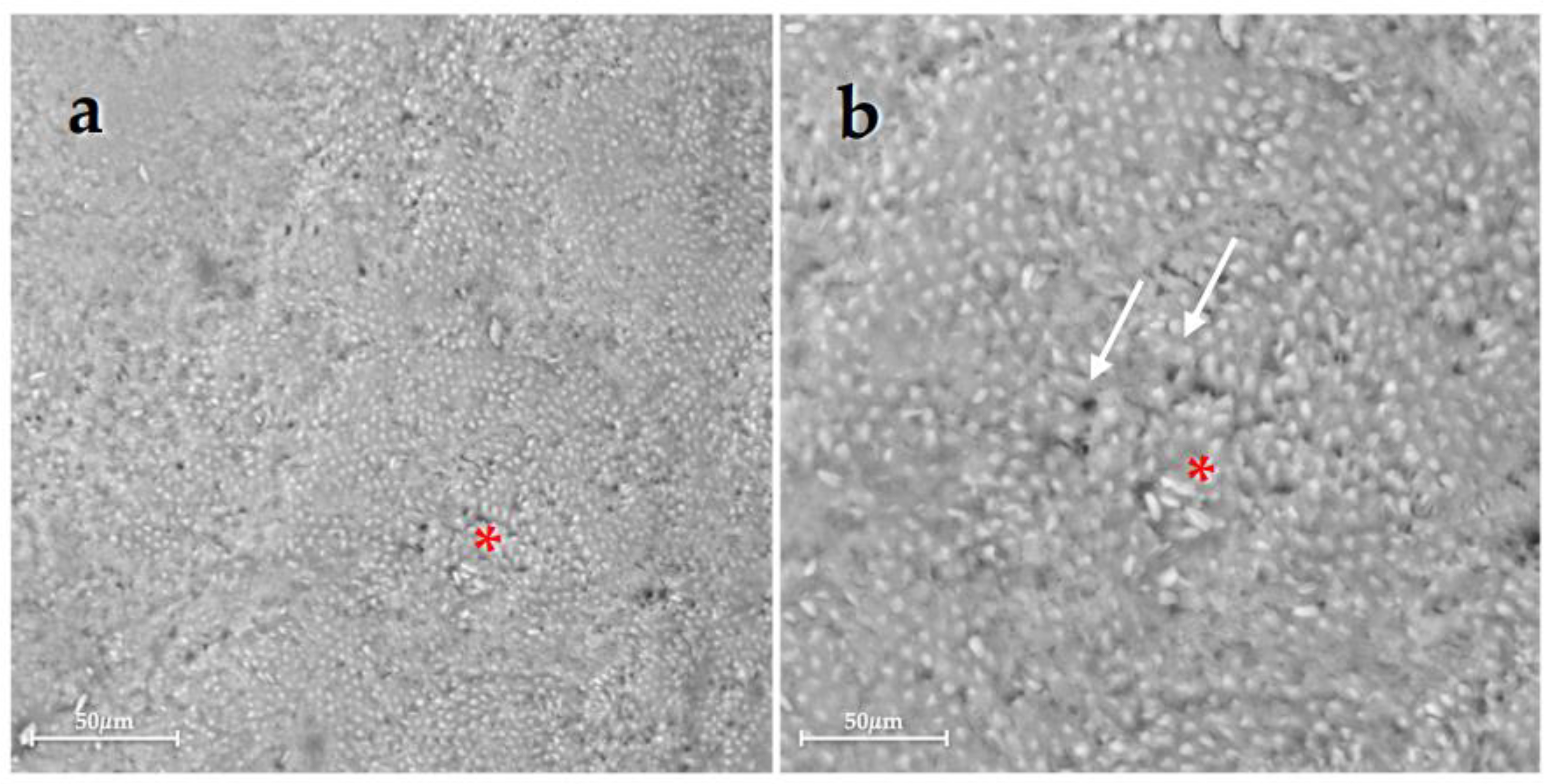
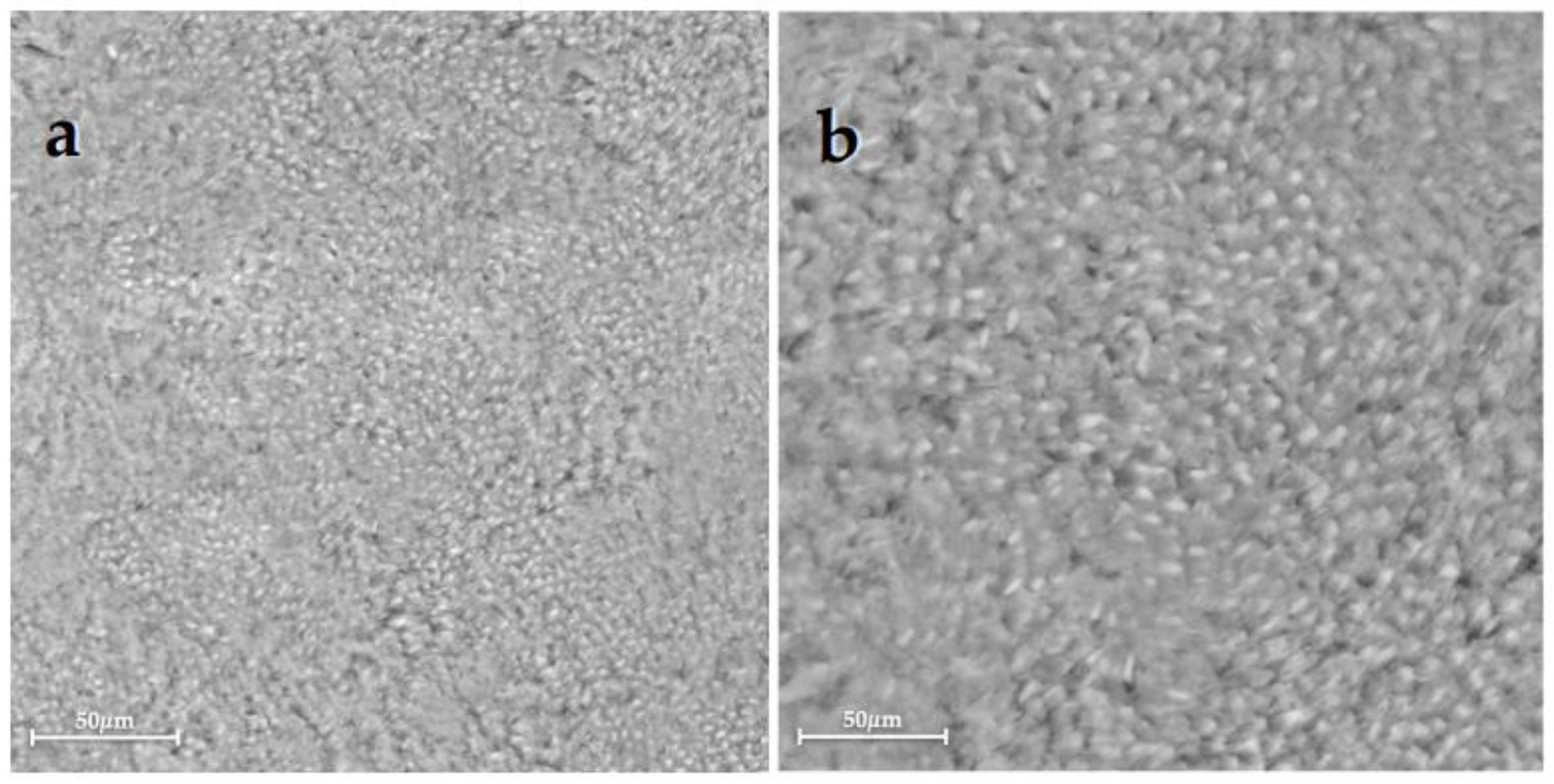
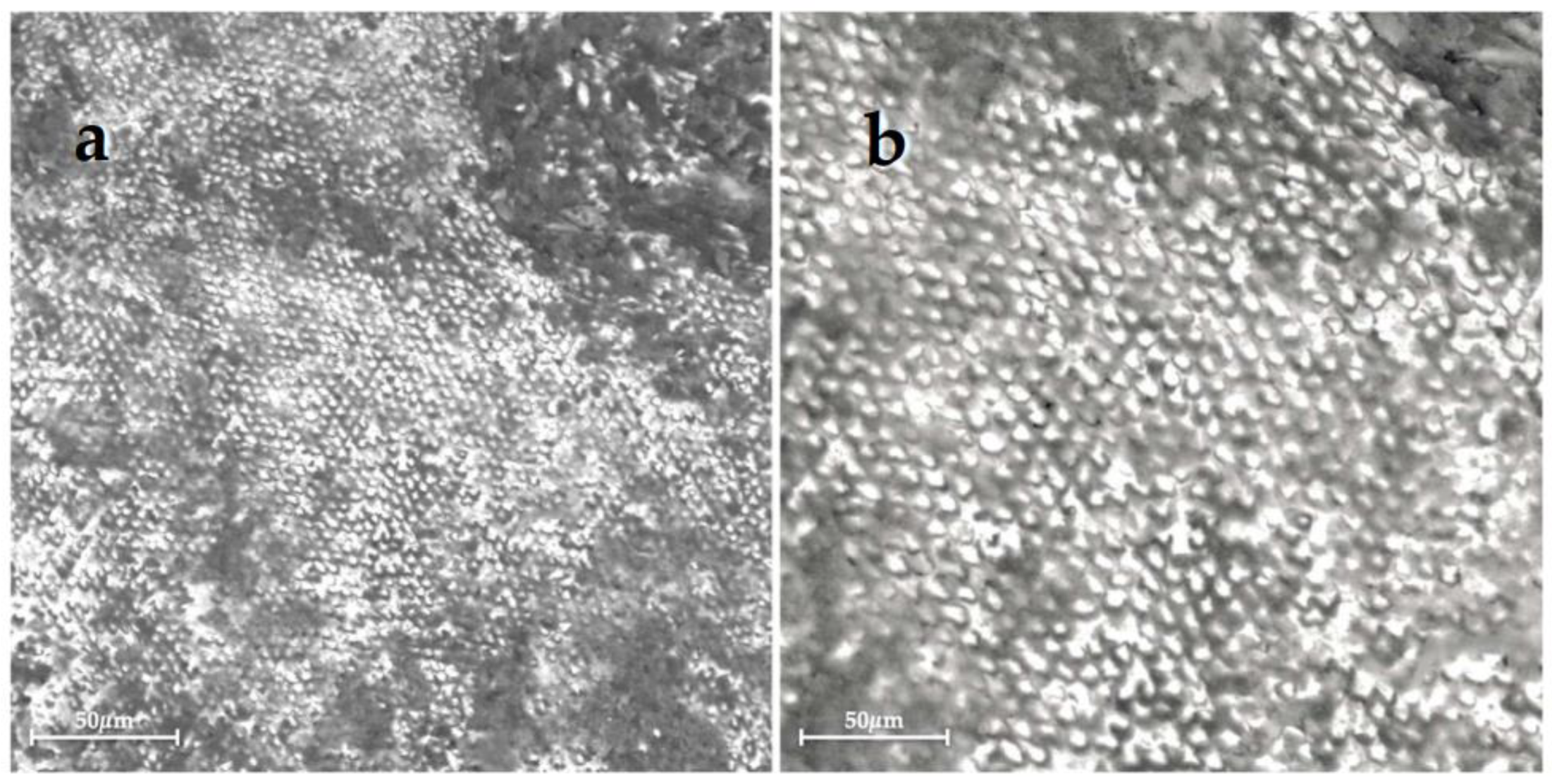
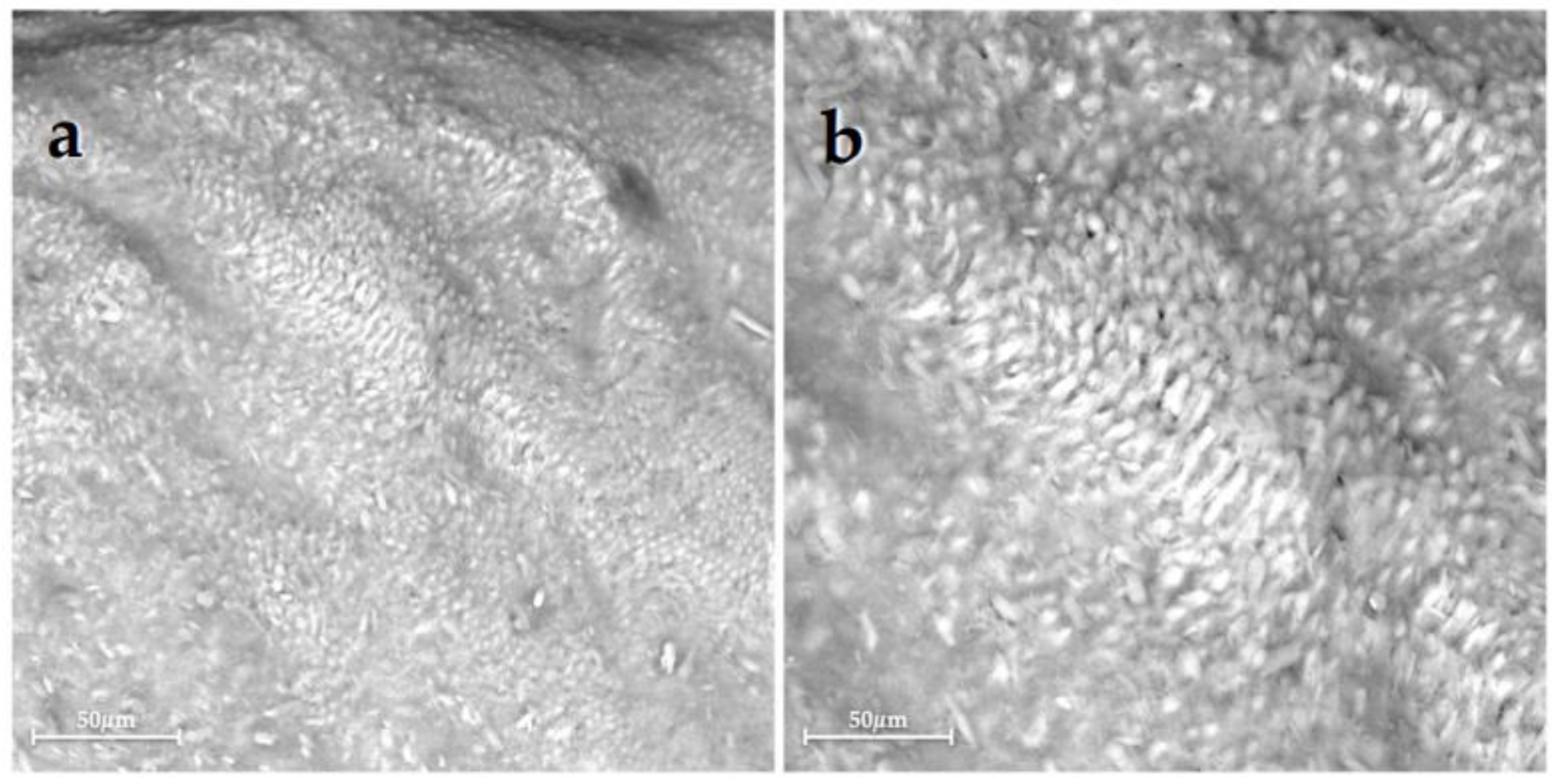
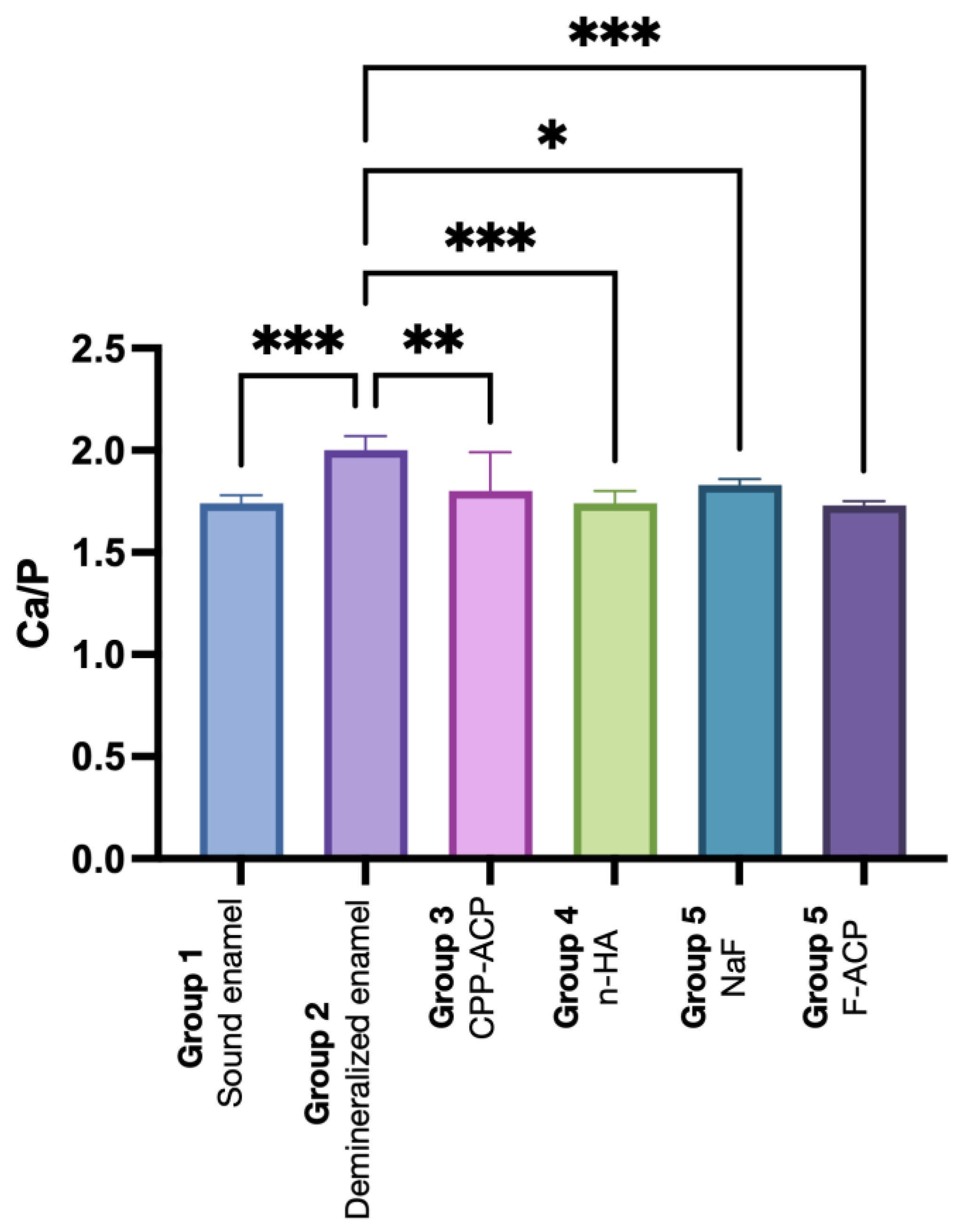
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Ca | 63.5 ± 0.5 a | 66.7 ± 0.7 b | 64 ± 2 a | 63.5 ± 0.8 a | 64.7 ± 0.4 a | 63.5 ± 0.3 a |
| P | 36.5 ± 0.5 a | 33.4 ± 0.7 a | 36 ± 2 a | 36.5 ± 0.8 a | 35.3 ± 0.4 a | 36.6 ± 0.3 a |
| Ca/P | 1.74 ± 0.04 a | 2.00 ± 0.07 b | 1.80 ± 0.02 a | 1.74 ± 0.06 a | 1.83 ± 0.03 a | 1.73 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosco, V.; Vitiello, F.; Monterubbianesi, R.; Gatto, M.L.; Orilisi, G.; Mengucci, P.; Putignano, A.; Orsini, G. Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An In Vitro Study. Bioengineering 2023, 10, 462. https://doi.org/10.3390/bioengineering10040462
Tosco V, Vitiello F, Monterubbianesi R, Gatto ML, Orilisi G, Mengucci P, Putignano A, Orsini G. Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An In Vitro Study. Bioengineering. 2023; 10(4):462. https://doi.org/10.3390/bioengineering10040462
Chicago/Turabian StyleTosco, Vincenzo, Flavia Vitiello, Riccardo Monterubbianesi, Maria Laura Gatto, Giulia Orilisi, Paolo Mengucci, Angelo Putignano, and Giovanna Orsini. 2023. "Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An In Vitro Study" Bioengineering 10, no. 4: 462. https://doi.org/10.3390/bioengineering10040462
APA StyleTosco, V., Vitiello, F., Monterubbianesi, R., Gatto, M. L., Orilisi, G., Mengucci, P., Putignano, A., & Orsini, G. (2023). Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An In Vitro Study. Bioengineering, 10(4), 462. https://doi.org/10.3390/bioengineering10040462














