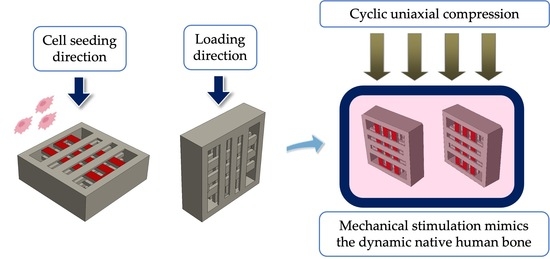
Effect of Uniaxial Compression Frequency on Osteogenic Cell Responses in Dynamic 3D Cultures
Abstract
1. Introduction
2. Materials and Methods
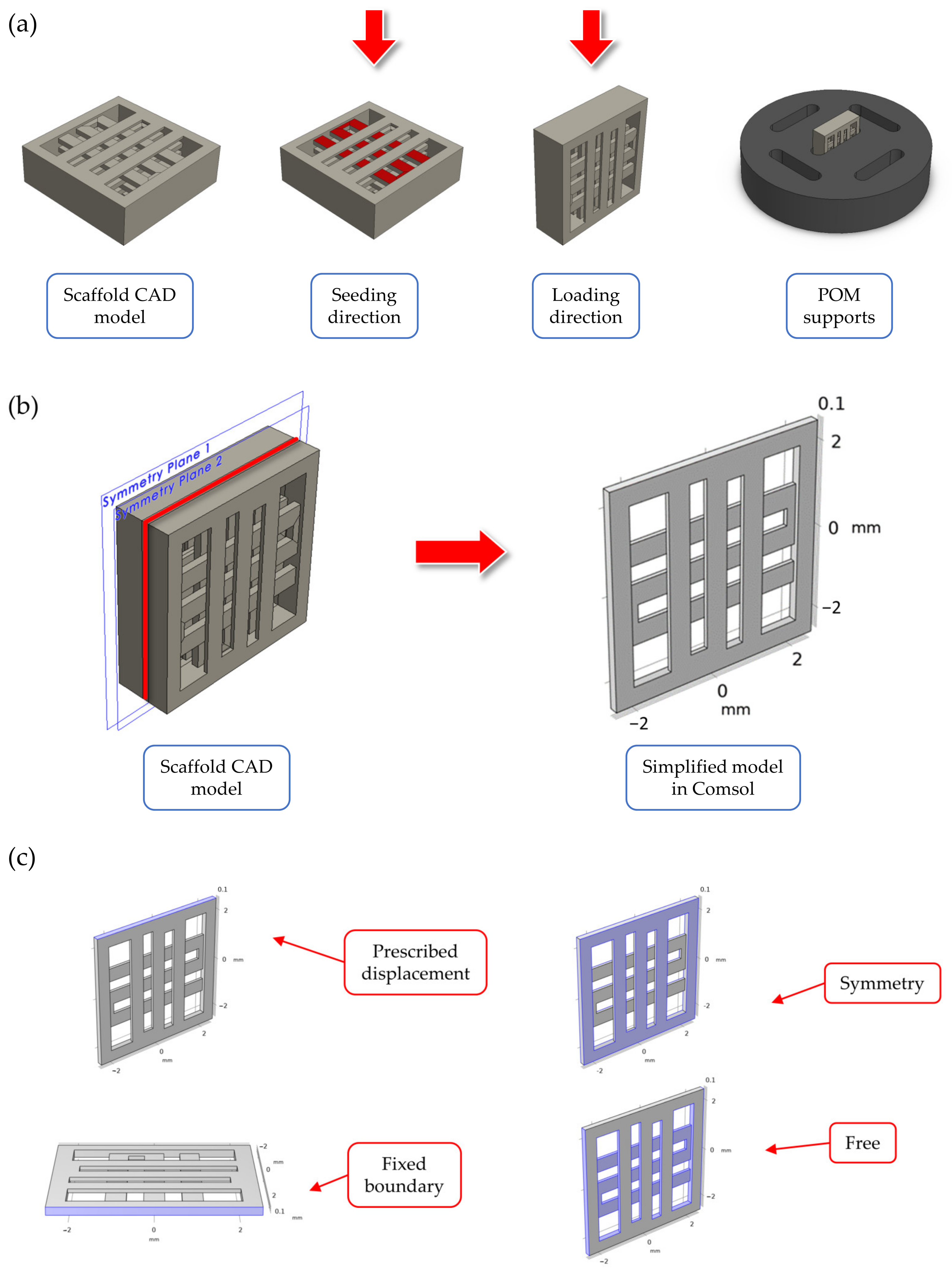
2.1. Overview of the Scaffold Design
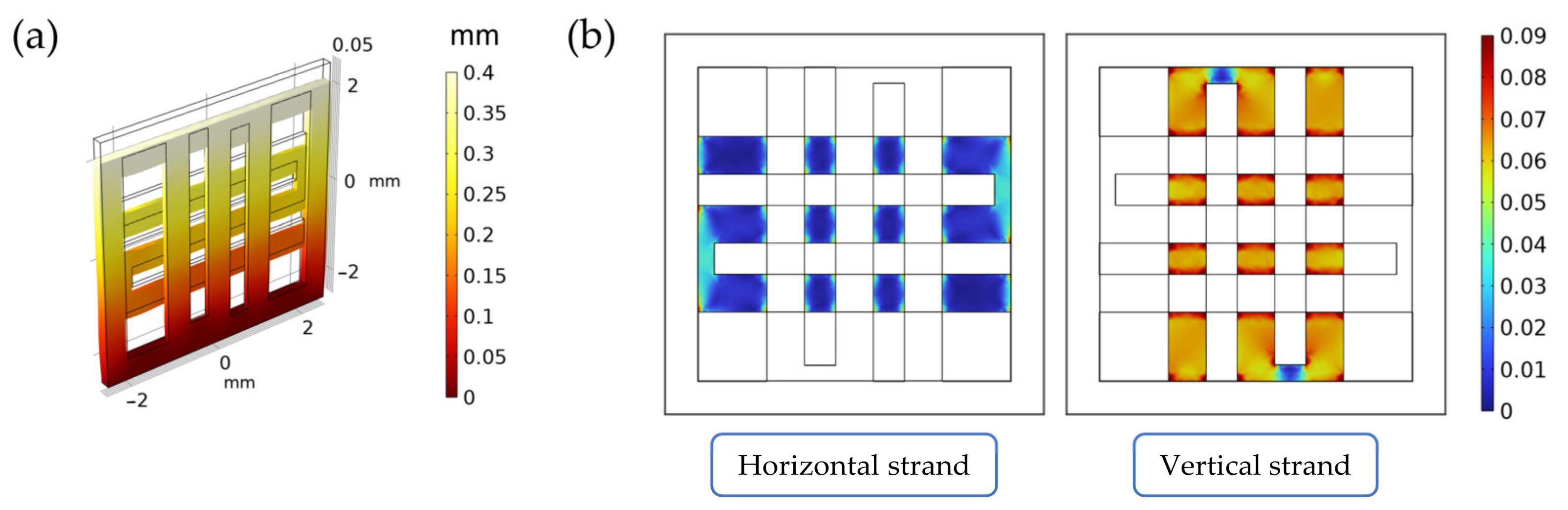
2.2. Finite Element Simulations of the Scaffold during Mechanical Compression
2.3. Scaffolds Fabrication
2.4. Seeding of MC3T3-E1 onto the Polymeric Scaffolds
2.5. Mechanical Stimulation Protocol
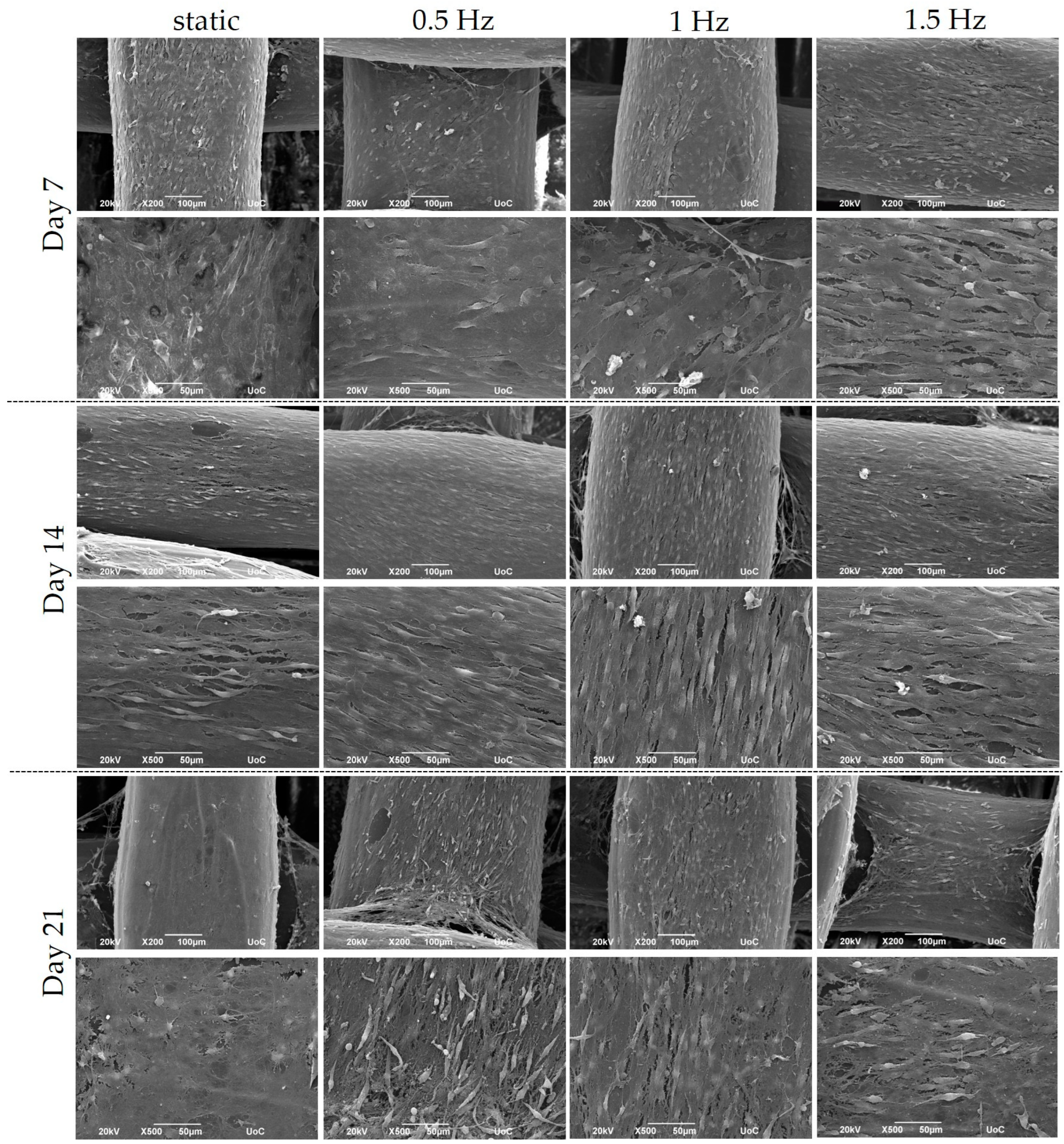
2.6. Cell Morphology by Scanning Electron Microscopy (SEM)
2.7. Pre-Osteoblastic Cell Viability Evaluation
2.8. Alkaline Phosphatase Activity Assessment
2.9. Calcium Secretion Measurement
2.10. Collagen Secretion by the Pre-Osteoblasts
2.11. Statistical Analysis
3. Results
3.1. Finite Element Simulations of the Scaffold during Mechanical Compression
3.2. Pre-Osteoblastic Cell Morphology
3.3. Cell Viability Assessment within the Scaffolds
3.4. Evaluation of the Differentiation Potential of the Cell-Seeded Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skerry, T.M. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch. Biochem. Biophys. 2008, 473, 117–123. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Liedert, A.; Ignatius, A. Mechanobiology of bone remodeling and fracture healing in the aged organism. Innov. Surg. Sci. 2016, 1, 57–63. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Sittichokechaiwut, A.; Edwards, J.H.; Scutt, A.M.; Reilly, G.C. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur. Cells Mater. 2010, 20, 45–57. [Google Scholar] [CrossRef]
- Binderman, I.; Zor, U.; Kaye, A.M.; Shimshoni, Z.; Harell, A.; Sömjen, D. The transduction of mechanical force into biochemical events in bone cells may involve activation of phospholipase a2. Calcif. Tissue Int. 1988, 42, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Harell, A.; Dekel, S.; Binderman, I. Biochemical effect of mechanical stress on cultured bone cells. Calcif. Tissue Res. 1977, 22, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. 1 biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Woodhead Publishing: Sawston, UK, 2019; pp. 1–37. [Google Scholar]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.; Luo, H.; Qin, K.; Hu, D.; Wang, E.; Li, A.; et al. Stem cell-friendly scaffold biomaterials: Applications for bone tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Afrose, M.F.; Masood, S.H.; Iovenitti, P.; Nikzad, M.; Sbarski, I. Effects of part build orientations on fatigue behaviour of fdm-processed pla material. Prog. Addit. Manuf. 2016, 1, 21–28. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (pcl) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2012, 34, 123–140. [Google Scholar]
- Espalin, D.; Arcaute, K.; Rodríguez, D.; Medina, F.; Posner, M.A.; Wicker, R.B. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010, 16, 164–173. [Google Scholar] [CrossRef]
- Gunatillake, T.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Bonatti, A.F.; Chiesa, I.; Micalizzi, S.; Vozzi, G.; De Maria, C. Bioprinting for bone tissue engineering. Minerva Orthop. 2021, 72, 376–394. [Google Scholar] [CrossRef]
- Fodran, E.; Koch, M.; Menon, U. Mechanical and dimensional characteristics of fused deposition modeling build styles. In Proceedings of the 1996 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 12–14 August 1996. [Google Scholar]
- Mallick, K.K.; Cox, S.C. Biomaterial scaffolds for tissue engineering. Front. Biosci. 2013, 5, 341–360. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Cheon, K.-H.; Park, C.; Jang, T.-S.; Kim, H.-E.; Jung, H.-D. Fabrication of poly(lactic acid)/ti composite scaffolds with enhanced mechanical properties and biocompatibility via fused filament fabrication (fff)–based 3d printing. Addit. Manuf. 2019, 30, 100883. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Juarez, T.M.; Helmke, C.D.; Gustin, M.C.; Mikos, A.G. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials 2001, 22, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Mullender, M.; El Haj, A.J.; Yang, Y.; van Duin, M.A.; Burger, E.H.; Klein-Nulend, J. Mechanotransduction of bone cells in vitro: Mechanobiology of bone tissue. Med. Biol. Eng. Comput. 2004, 42, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; McCulloch, C.A. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 2007, 9, 1–34. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; McNamara, L.M. Chapter 6—bone mechanobiology in health and disease. In Mechanobiology in Health and Disease; Verbruggen, S.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 157–214. [Google Scholar]
- Mauney, J.R.; Sjostorm, S.; Blumberg, J.; Horan, R.; O’Leary, J.P.; Vunjak-Novakovic, G.; Volloch, V.; Kaplan, D.L. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-d partially demineralized bone scaffolds in vitro. Calcif. Tissue Int. 2004, 74, 458–468. [Google Scholar] [CrossRef]
- Rubin, J.; Rubin, C.; Jacobs, C.R. Molecular pathways mediating mechanical signaling in bone. Gene 2006, 367, 1–16. [Google Scholar] [CrossRef]
- Jørgensen, N.R.; Henriksen, Z.; Brot, C.; Eriksen, E.F.; Sørensen, O.H.; Civitelli, R.; Steinberg, T.H. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J. Bone Miner. Res. 2000, 15, 1024–1032. [Google Scholar] [CrossRef]
- Batra, N.N.; Li, Y.J.; Yellowley, C.E.; You, L.; Malone, A.M.; Kim, C.H.; Jacobs, C.R. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J. Biomech. 2005, 38, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.L.; Haut, T.R.; Yellowley, C.E.; Donahue, H.J.; Jacobs, C.R. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J. Biomech. 2003, 36, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.R.; Yellowley, C.E.; Davis, B.R.; Zhou, Z.; Cimbala, J.M.; Donahue, H.J. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 1998, 31, 969–976. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Roelofsen, J.; Klein-Nulend, J.; Burger, E.H. Mechanical stimulation by intermittent hydrostatic compression promotes bone-specific gene expression in vitro. J. Biomech. 1995, 28, 1493–1503. [Google Scholar] [CrossRef]
- Kapur, S.; Baylink, D.J.; Lau, K.H. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone 2003, 32, 241–251. [Google Scholar] [CrossRef]
- Sato, K.; Adachi, T.; Matsuo, M.; Tomita, Y. Quantitative evaluation of threshold fiber strain that induces reorganization of cytoskeletal actin fiber structure in osteoblastic cells. J. Biomech. 2005, 38, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Takahashi, T.; Tsujisawa, T.; Ariyoshi, W.; Nishihara, T. Mechanical stress-mediated runx2 activation is dependent on ras/erk1/2 mapk signaling in osteoblasts. J. Cell. Biochem. 2007, 101, 1266–1277. [Google Scholar] [CrossRef]
- Brighton, C.T.; Sennett, B.J.; Farmer, J.C.; Iannotti, J.P.; Hansen, C.A.; Williams, J.L.; Williamson, J. The inositol phosphate pathway as a mediator in the proliferative response of rat calvarial bone cells to cyclical biaxial mechanical strain. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1992, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Sato, S.; Saito, S.; Suzuki, Y.; Brunette, D.M. Mechanical stretching increases the number of cultured bone cells synthesizing DNA and alters their pattern of protein synthesis. Calcif. Tissue Int. 1985, 37, 431–436. [Google Scholar] [CrossRef]
- Yeh, C.-K.; Rodan, G.A. Tensile forces enhance prostaglandin e synthesis in osteoblastic cells grown on collagen ribbons. Calcif. Tissue Int. 1984, 36, S67–S71. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Putnam, A.J.; Kulik, T.J.; Mooney, D.J. Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol. Bioeng. 1998, 57, 46–54. [Google Scholar] [CrossRef]
- Granet, C.; Laroche, N.; Vico, L.; Alexandre, C.; Lafage-Proust, M.H. Rotating-wall vessels, promising bioreactors for osteoblastic cell culture: Comparison with other 3d conditions. Med. Biol. Eng. Comput. 1998, 36, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Sikavitsas, V.I.; Behravesh, E.; Reis, R.L.; Mikos, A.G. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J. Biomed. Mater. Res. Part A 2003, 67, 87–95. [Google Scholar] [CrossRef]
- Babaliari, E.; Petekidis, G.; Chatzinikolaidou, M. A precisely flow-controlled microfluidic system for enhanced pre-osteoblastic cell response for bone tissue engineering. Bioengineering 2018, 5, 66. [Google Scholar] [CrossRef]
- Milan, J.L.; Planell, J.A.; Lacroix, D. Computational modelling of the mechanical environment of osteogenesis within a polylactic acid-calcium phosphate glass scaffold. Biomaterials 2009, 30, 4219–4226. [Google Scholar] [CrossRef]
- Frost, H.M. Perspectives: Bone’s mechanical usage windows. Bone Miner. 1992, 19, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone formation by applied dynamic loads. J. Bone Jt. Surgery Am. Vol. 1984, 66, 397–402. [Google Scholar] [CrossRef]
- Eickhoff, J.A.; Molczyk, L.; Gallagher, J.C.; De Jong, S. Influence of isotonic, isometric and isokinetic muscle strength on bone mineral density of the spine and femur in young women. Bone Miner. 1993, 20, 201–209. [Google Scholar] [CrossRef]
- Rubin, C.T.; McLeod, K.J. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin. Orthop. Relat. Res. 1994, 165–174. [Google Scholar] [CrossRef]
- Kaspar, D.; Seidl, W.; Neidlinger-Wilke, C.; Beck, A.; Claes, L.; Ignatius, A. Proliferation of human-derived osteoblast-like cells depends on the cycle number and frequency of uniaxial strain. J. Biomech. 2002, 35, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T.; Strafford, B.; Gross, S.B.; Leatherwood, D.F.; Williams, J.L.; Pollack, S.R. The proliferative and synthetic response of isolated calvarial bone cells of rats to cyclic biaxial mechanical strain. J. Bone Jt. Surgery Am. Vol. 1991, 73, 320–331. [Google Scholar] [CrossRef]
- Naseem, R.; Montalbano, G.; German, M.J.; Ferreira, A.M.; Gentile, P.; Dalgarno, K. Influence of pcl and phbv on plla thermal and mechanical properties in binary and ternary polymer blends. Molecules 2022, 27, 7633. [Google Scholar] [CrossRef]
- Kontogianni, G.-I.; Bonatti, A.F.; De Maria, C.; Naseem, R.; Melo, P.; Coelho, C.; Vozzi, G.; Dalgarno, K.; Quadros, P.; Vitale-Brovarone, C.; et al. Promotion of in vitro osteogenic activity by melt extrusion-based plla/pcl/phbv scaffolds enriched with nano-hydroxyapatite and strontium substituted nano-hydroxyapatite. Polymers 2023, 15, 1052. [Google Scholar] [CrossRef]
- Hendrikson, W.J.; Deegan, A.J.; Yang, Y.; van Blitterswijk, C.A.; Verdonschot, N.; Moroni, L.; Rouwkema, J. Influence of additive manufactured scaffold architecture on the distribution of surface strains and fluid flow shear stresses and expected osteochondral cell differentiation. Front. Bioeng. Biotechnol. 2017, 5, 6. [Google Scholar] [CrossRef]
- Bonatti, A.F.; Fortunato, G.M.; De Maria, C.; Vozzi, G.J.B. Bioprinting technologies: An overview. In Bioprinting; Academic Press: Cambridge, MA, USA, 2022; pp. 19–49. [Google Scholar]
- Hadjicharalambous, C.; Mygdali, E.; Prymak, O.; Buyakov, A.; Kulkov, S.; Chatzinikolaidou, M. Proliferation and osteogenic response of mc3t3-e1 pre-osteoblastic cells on porous zirconia ceramics stabilized with magnesia or yttria. J. Biomed. Mater. Research. Part A 2015, 103, 3612–3624. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Kozlova, D.; Sokolova, V.; Epple, M.; Chatzinikolaidou, M. Calcium phosphate nanoparticles carrying bmp-7 plasmid DNA induce an osteogenic response in mc3t3-e1 pre-osteoblasts. J. Biomed. Mater. Research. Part A 2015, 103, 3834–3842. [Google Scholar] [CrossRef]
- Loukelis, K.; Papadogianni, D.; Chatzinikolaidou, M. Kappa-carrageenan/chitosan/gelatin scaffolds enriched with potassium chloride for bone tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1720–1730. [Google Scholar] [CrossRef]
- Garnero, P.; Delmas, P.D. Bone turnover markers. In Encyclopedia of Endocrine Diseases; Martini, L., Ed.; Elsevier: New York, NY, USA, 2004; pp. 401–413. [Google Scholar]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Karsdal, M.A. Chapter 1—type i collagen. In Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–11. [Google Scholar]
- Blair, H.C.; Robinson, L.J.; Huang, C.L.; Sun, L.; Friedman, P.A.; Schlesinger, P.H.; Zaidi, M. Calcium and bone disease. BioFactors 2011, 37, 159–167. [Google Scholar] [CrossRef]
- Carter, A.; Popowski, K.; Cheng, K.; Greenbaum, A.; Ligler, F.S.; Moatti, A. Enhancement of bone regeneration through the converse piezoelectric effect, a novel approach for applying mechanical stimulation. Bioelectricity 2021, 3, 255–271. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. Piezoelectric effects in collagen. Jpn. J. Appl. Phys. 1964, 3, 117. [Google Scholar] [CrossRef]
- Lang, T.; LeBlanc, A.; Evans, H.; Lu, Y.; Genant, H.; Yu, A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Tripuwabhrut, P.; Mustafa, M.; Gjerde, C.G.; Brudvik, P.; Mustafa, K. Effect of compressive force on human osteoblast-like cells and bone remodelling: An in vitro study. Arch. Oral Biol. 2013, 58, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, A.; Fujiwara, N.; Nakano, T. Continuous cyclic stretch induces osteoblast alignment and formation of anisotropic collagen fiber matrix. Acta Biomater. 2013, 9, 7227–7235. [Google Scholar] [CrossRef]
- Kearney, E.M.; Farrell, E.; Prendergast, P.J.; Campbell, V.A. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann. Biomed. Eng. 2010, 38, 1767–1779. [Google Scholar] [CrossRef]
- Sawatjui, N.; Limpaiboon, T.; Schrobback, K.; Klein, T. Biomimetic scaffolds and dynamic compression enhance the properties of chondrocyte- and msc-based tissue-engineered cartilage. J. Tissue Eng. Regen. Med. 2018, 12, 1220–1229. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Teoh, S.H.; Teo, E.Y.; Khoon Chong, M.S.; Shin, C.W.; Tien, F.T.; Choolani, M.A.; Chan, J.K. A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 2010, 31, 8684–8695. [Google Scholar] [CrossRef]
- Luo, L.; Thorpe, S.D.; Buckley, C.T.; Kelly, D.J. The effects of dynamic compression on the development of cartilage grafts engineered using bone marrow and infrapatellar fat pad derived stem cells. Biomed. Mater. 2015, 10, 055011. [Google Scholar] [CrossRef]
- Owan, I.; Burr, D.B.; Turner, C.H.; Qiu, J.; Tu, Y.; Onyia, J.E.; Duncan, R.L. Mechanotransduction in bone: Osteoblasts are more responsive to fluid forces than mechanical strain. Am. J. Physiol. 1997, 273, C810–C815. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, S.J.; Lee, H.S.; Moon, W.; Cho, D.W. Effects of combined mechanical stimulation on the proliferation and differentiation of pre-osteoblasts. Exp. Mol. Med. 2011, 43, 367–373. [Google Scholar] [CrossRef]
- Wiesmann, A.; Bühring, H.J.; Mentrup, C.; Wiesmann, H.P. Decreased cd90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.J.; Banes, A.J.; Jordan, R.D. The effects of mechanical strain on osteoblasts in vitro. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1990, 48, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, D.; Seidl, W.; Neidlinger-Wilke, C.; Ignatius, A.; Claes, L. Dynamic cell stretching increases human osteoblast proliferation and cicp synthesis but decreases osteocalcin synthesis and alkaline phosphatase activity. J. Biomech. 2000, 33, 45–51. [Google Scholar] [CrossRef]
- Jagodzinski, M.; Breitbart, A.; Wehmeier, M.; Hesse, E.; Haasper, C.; Krettek, C.; Zeichen, J.; Hankemeier, S. Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture. J. Biomech. 2008, 41, 1885–1891. [Google Scholar] [CrossRef]
- Schreivogel, S.; Kuchibhotla, V.; Knaus, P.; Duda, G.N.; Petersen, A. Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J. Tissue Eng. Regen. Med. 2019, 13, 1992–2008. [Google Scholar] [CrossRef]
- Ji, J.; Sun, W.; Wang, W.; Munyombwe, T.; Yang, X.B. The effect of mechanical loading on osteogenesis of human dental pulp stromal cells in a novel in vitro model. Cell Tissue Res. 2014, 358, 123–133. [Google Scholar] [CrossRef] [PubMed]


| Material Property | Symbol | Unit of Measure | Value |
|---|---|---|---|
| Young modulus | E | MPa | 32.2 |
| Poisson ratio | N | - | 0.3 |
| Density | Ρ | kg/m3 | 1200 |
| Mesh Setting | Average Size [mm] | Min Size [mm] | Max Size [mm] | Model DoF |
|---|---|---|---|---|
| Finer | 0.23 | 0.10 | 0.34 | ≈42 k |
| Printing Speed [mm/s] | Extrusion Temperature [°C] | Bed Temperature [°C] | Layer Height [mm] |
|---|---|---|---|
| 10 | 210 | 80 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontogianni, G.-I.; Loukelis, K.; Bonatti, A.F.; Batoni, E.; De Maria, C.; Naseem, R.; Dalgarno, K.; Vozzi, G.; MacManus, D.B.; Mondal, S.; et al. Effect of Uniaxial Compression Frequency on Osteogenic Cell Responses in Dynamic 3D Cultures. Bioengineering 2023, 10, 532. https://doi.org/10.3390/bioengineering10050532
Kontogianni G-I, Loukelis K, Bonatti AF, Batoni E, De Maria C, Naseem R, Dalgarno K, Vozzi G, MacManus DB, Mondal S, et al. Effect of Uniaxial Compression Frequency on Osteogenic Cell Responses in Dynamic 3D Cultures. Bioengineering. 2023; 10(5):532. https://doi.org/10.3390/bioengineering10050532
Chicago/Turabian StyleKontogianni, Georgia-Ioanna, Konstantinos Loukelis, Amedeo Franco Bonatti, Elisa Batoni, Carmelo De Maria, Raasti Naseem, Kenneth Dalgarno, Giovanni Vozzi, David B. MacManus, Subrata Mondal, and et al. 2023. "Effect of Uniaxial Compression Frequency on Osteogenic Cell Responses in Dynamic 3D Cultures" Bioengineering 10, no. 5: 532. https://doi.org/10.3390/bioengineering10050532
APA StyleKontogianni, G.-I., Loukelis, K., Bonatti, A. F., Batoni, E., De Maria, C., Naseem, R., Dalgarno, K., Vozzi, G., MacManus, D. B., Mondal, S., Dunne, N., Vitale-Brovarone, C., & Chatzinikolaidou, M. (2023). Effect of Uniaxial Compression Frequency on Osteogenic Cell Responses in Dynamic 3D Cultures. Bioengineering, 10(5), 532. https://doi.org/10.3390/bioengineering10050532