Intraluminal Thrombus Characteristics in AAA Patients: Non-Invasive Diagnosis Using CFD
Abstract
:1. Introduction
2. Materials and Methods
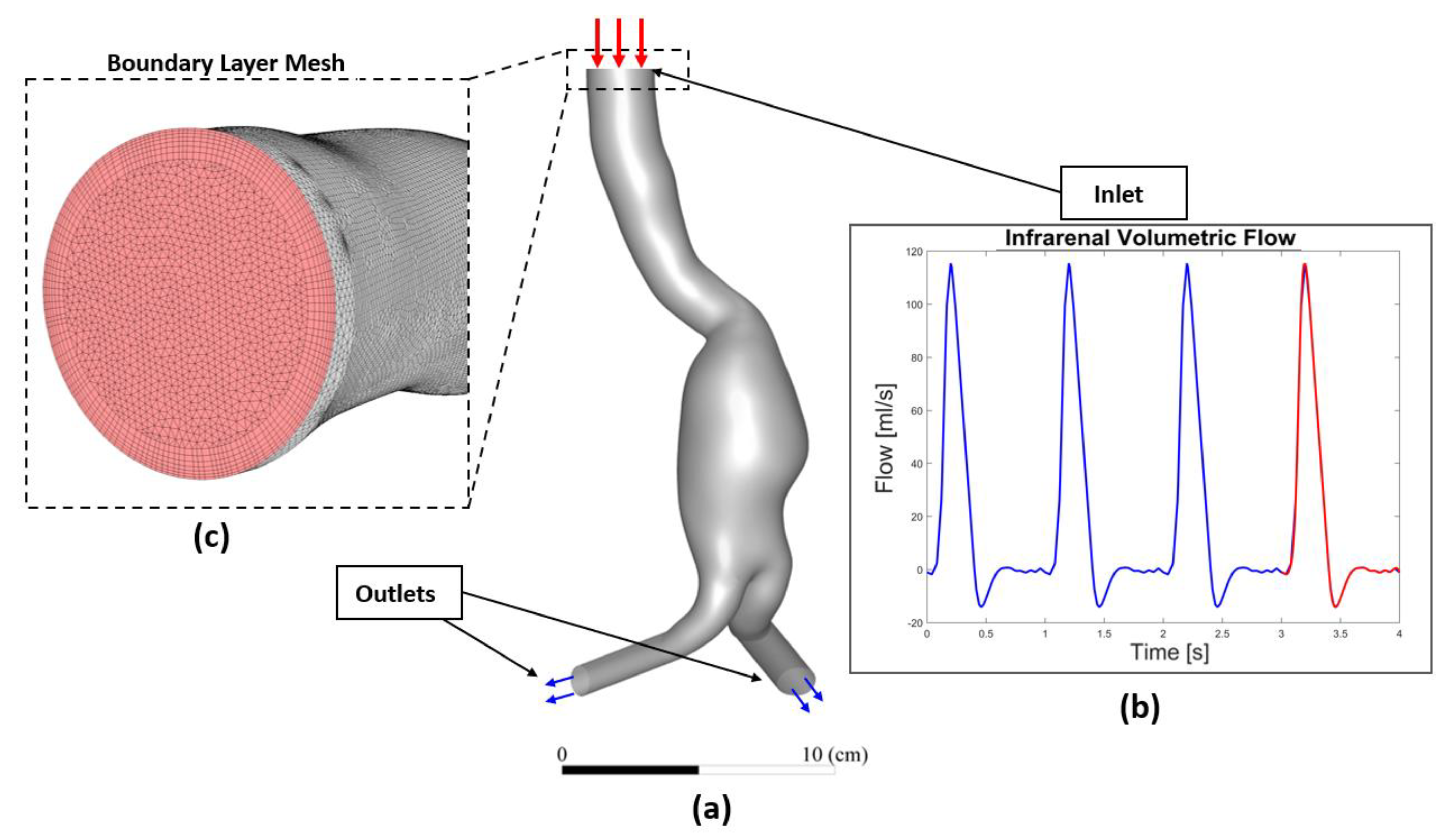
2.1. Workflow
2.2. Data Acquisition and Patient-Specific Geometries Reconstruction
2.3. Patient Specific CFD Simulation
2.4. Governing Equations
2.5. Wall Parameters’ Analysis
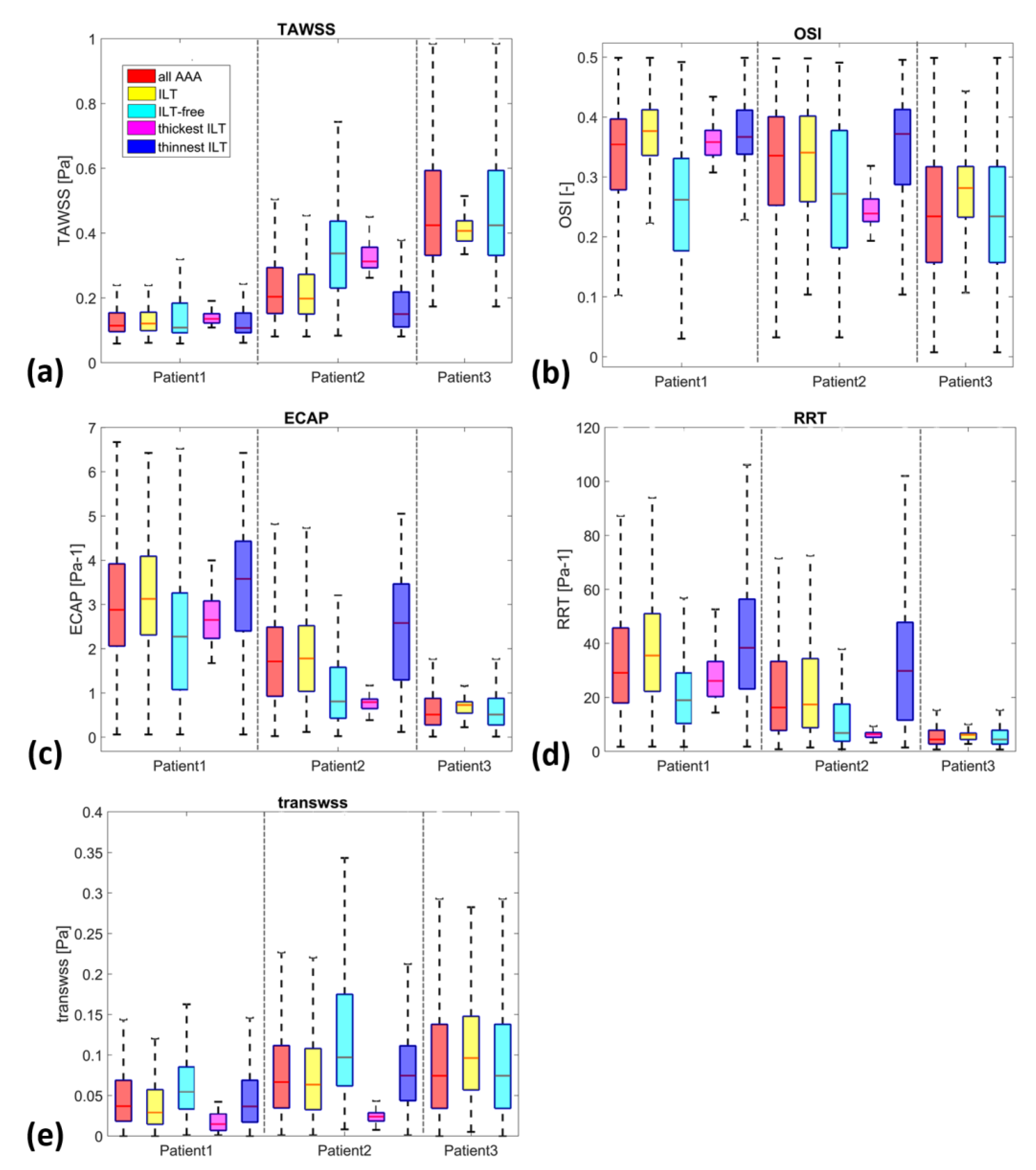
3. Results
3.1. Velocity Field and ILT Deposition
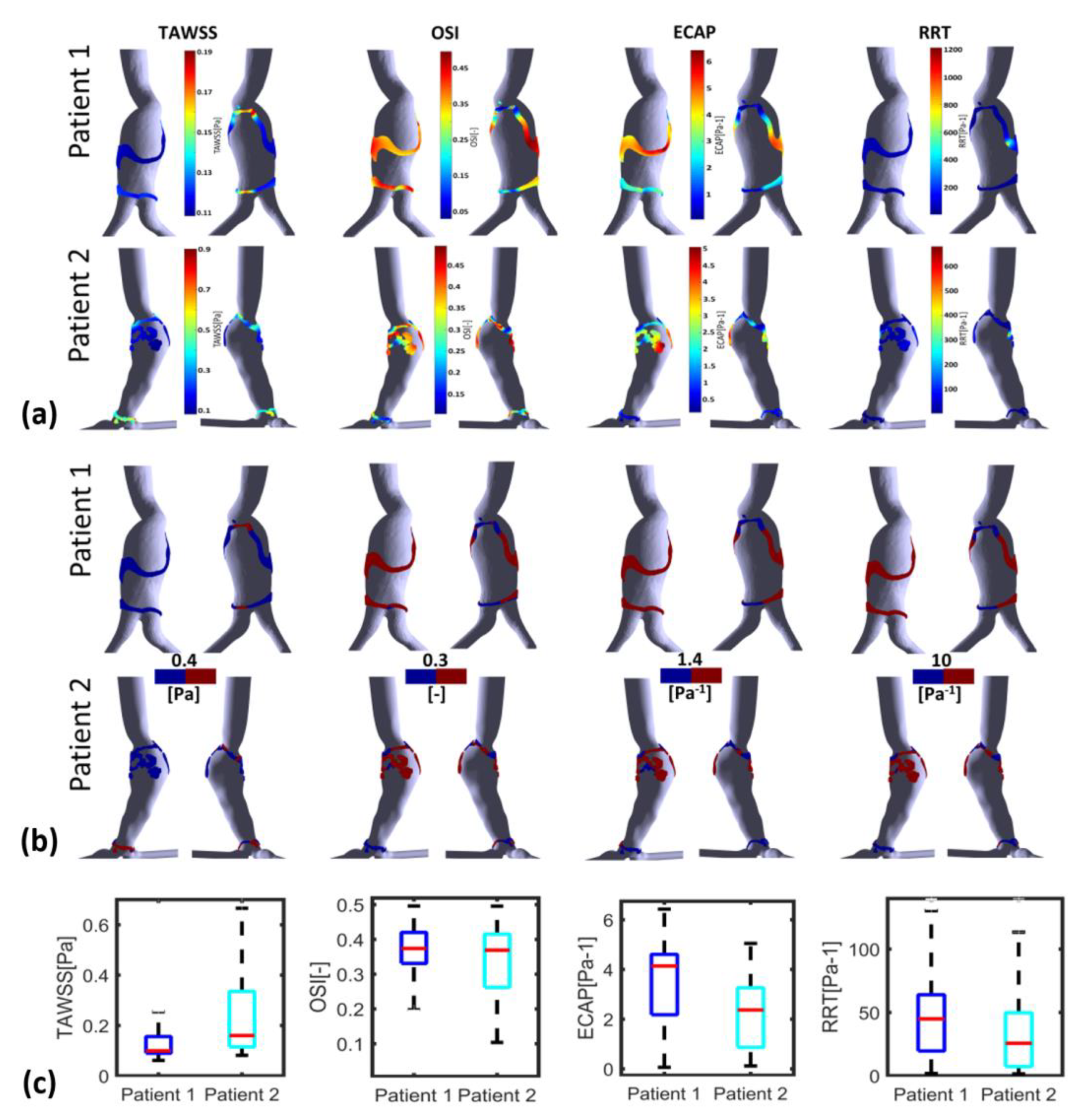
3.2. ILT Deposition and WSS Derivatives
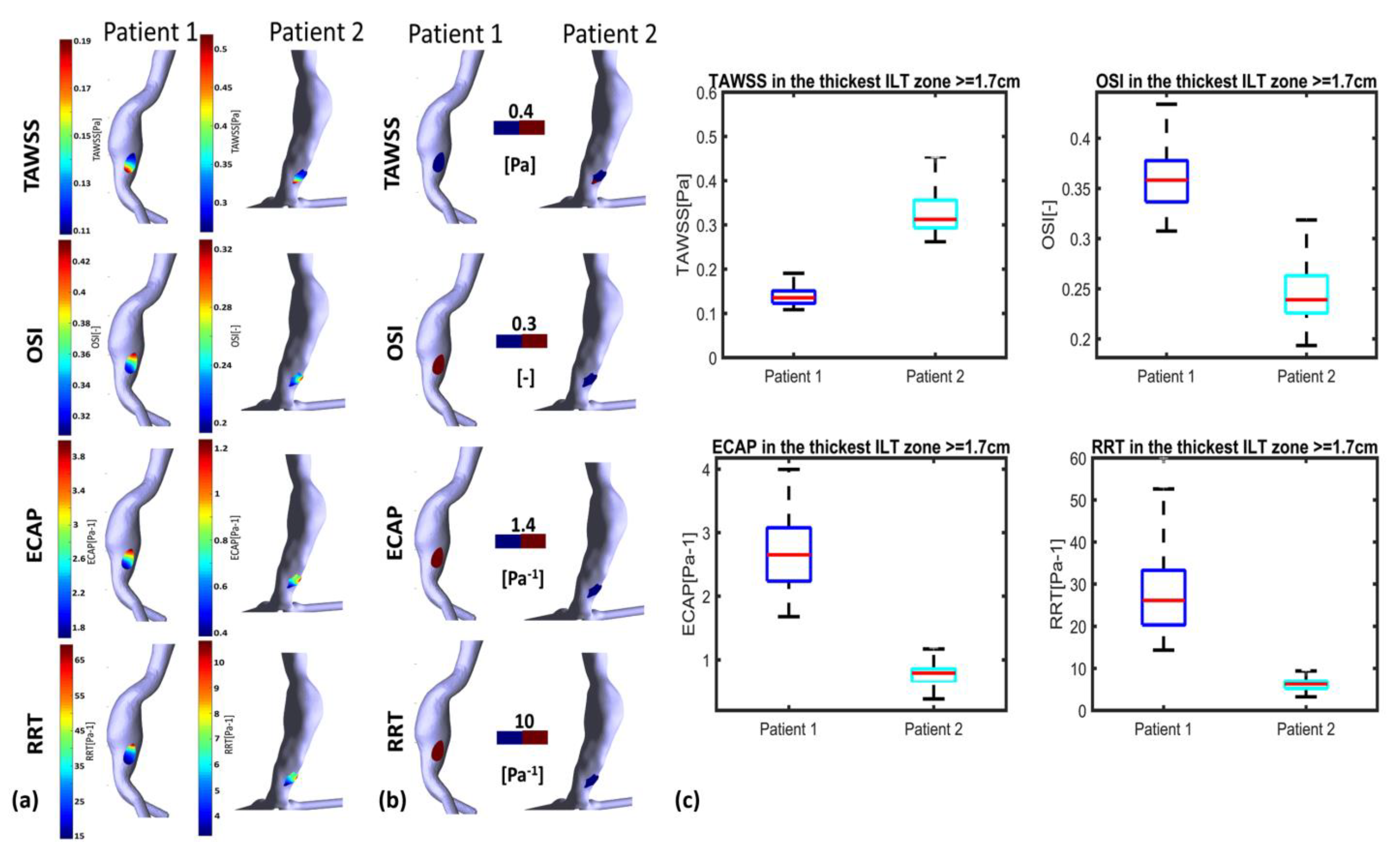
3.3. ILT-Thickness Regionalization as Possible Indicator of Thrombogenic Prone Regions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Mesh Sensitivity Analysis
| Grid 1 | Number of Cells (Millions) | WSS_Max (Pa) | GCI (%) | |
|---|---|---|---|---|
| Patient 1 | g1 Fine | 6.379 | 4.856 | GCI1-2 = 0.102 |
| g2 Medium | 3.799 | 4.872 | GCI2-3 = 0.327 | |
| g3 Coarse | 2.306 | 4.822 | ||
| Patient 2 | g1 Fine | 2.364 | 25.559 | GCI1-2 = 1.448 |
| g2 Medium | 3.877 | 25.132 | GCI2-3 = 0.651 | |
| g3 Coarse | 6.351 | 25.320 | ||
| Patient 3 | g1 Fine | 6.763 | 18.167 | GCI1-2 = 0.035 |
| g2 Medium | 3.885 | 17.630 | GCI2-3 = 0.209 | |
| g3 Coarse | 2.236 | 17.891 |
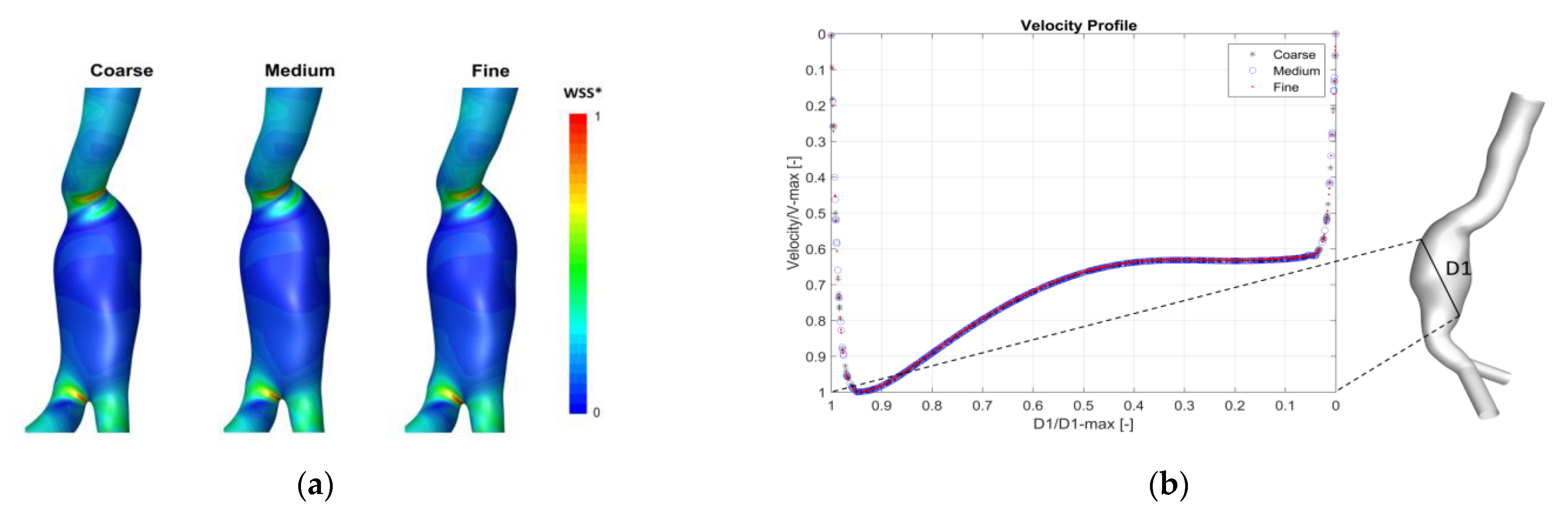
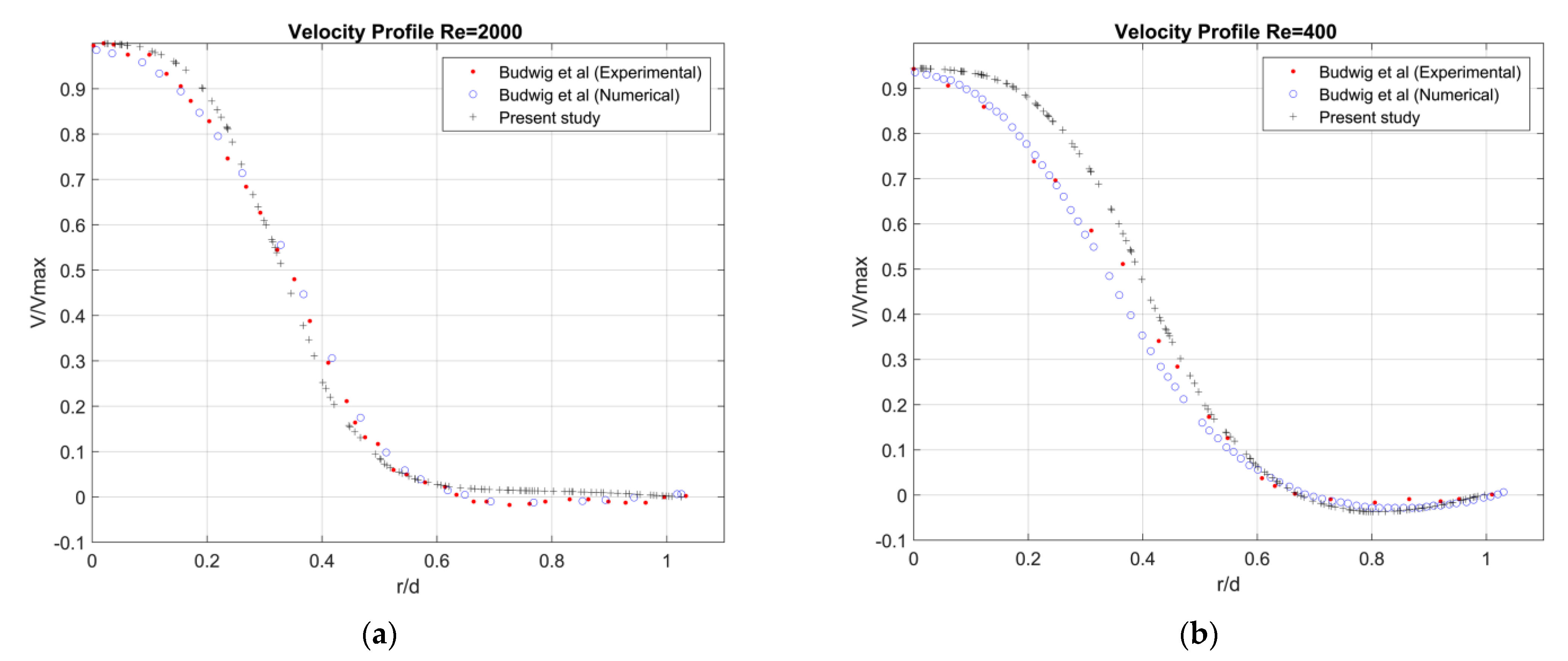
Appendix B. Validation
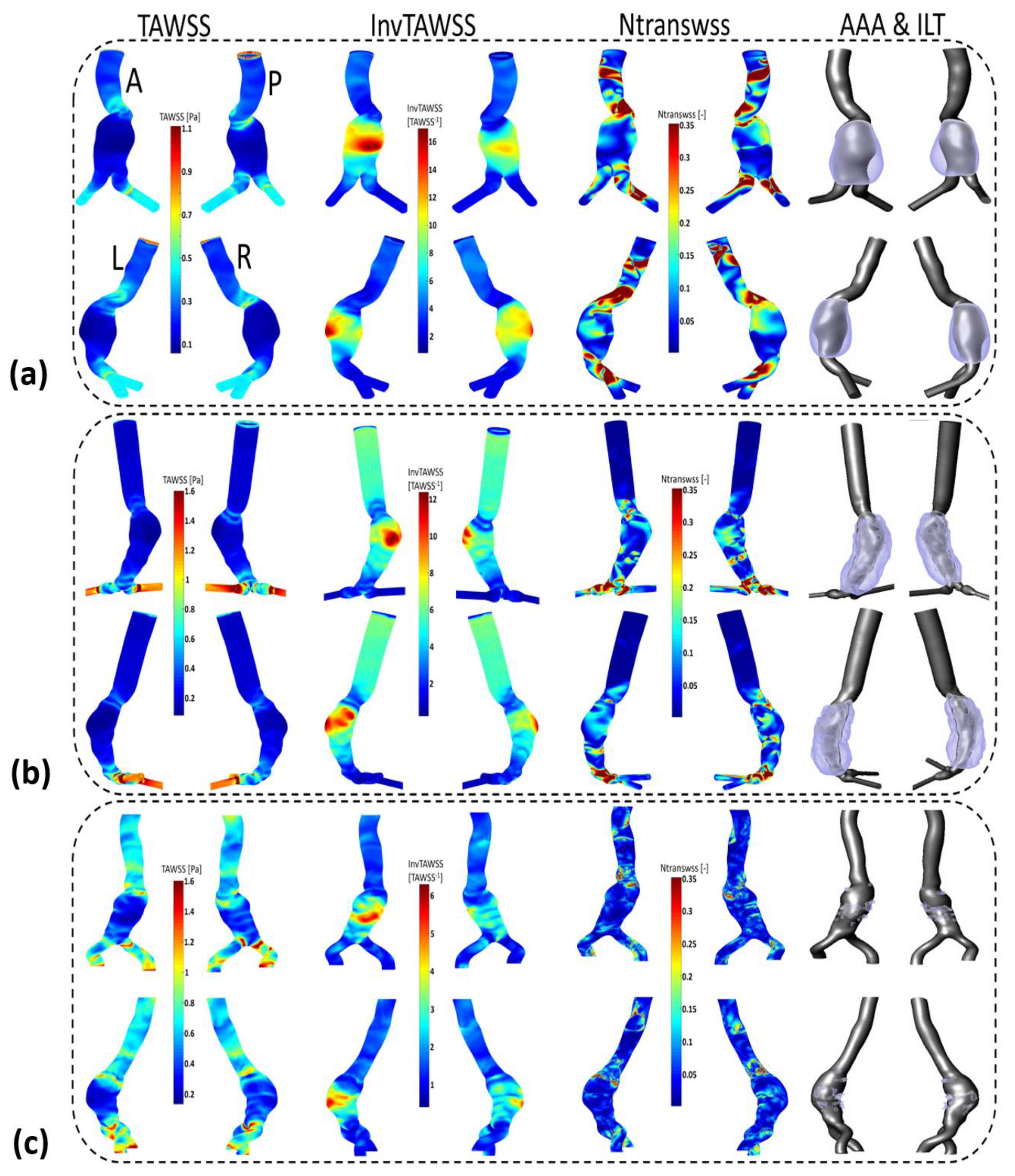
Appendix C

Appendix D
| AAA Areas | WSS Parameters | Flow Near the Wall to Which ECs Are Subjected to * | ILT Deposition | |||
|---|---|---|---|---|---|---|
| TAWSS | OSI | TransWSS | ||||
| P1 | Entrance of AAA | -High | -Low | -High | -Unidirectional disturbed flow  | -No ILT |
| AAA Sac | -Low | -Low -High | -High and (low in some regions) -Low -Proportionally High in some regions | -Unidirectional disturbed flow (non-disturbed flow) -Oscillating non disturbed flow  - Oscillating disturbed flow  | -No ILT -ILT  | |
| Downstream AAA sac | -High | -Low | -High | -Unidirectional disturbed flow  | -No ILT | |
| AAA Areas | WSS Parameters | Flow Near the Wall to Which ECs Are Subjected to * | ILT Deposition | |||
|---|---|---|---|---|---|---|
| TAWSS | OSI | TransWSS | ||||
| P2 | Entrance of AAA | -High | -Low | -Low -Proportionally High in some regions | -Unidirectional non disturbed flow -Unidirectional disturbed flow  | -No ILT |
| AAA Sac | -Low | -High | -Low -Proportionally High in some regions | -Oscillating non disturbed flow - Oscillating disturbed flow  | -ILT | |
| Downstream AAA sac | -High | -Low | -High | -Unidirectional disturbed flow  | -No ILT | |
| AAA Areas | WSS Parameters | Flow Near the Wall to Which ECs Are Subjected to * | ILT Deposition | |||
|---|---|---|---|---|---|---|
| TAWSS | OSI | TransWSS | ||||
| P3 | Entrance of AAA | -High -Low | -Anterior part low -Posterior part high | -High | -Unidirectional disturbed flow  -Oscillating disturbed flow  | -No ILT (anterior) A A-ILT (posterior) P  |
| AAA Sac | -Low -High | -High Dmax zone (belt from dome to posterior) -Low in the remaining regions | -Low | -Oscillating non disturbed flow  -Unidirectional non disturbed flow  | -ILT -No ILT | |
| Downstream AAA Sac | -High | -Low | -Low | -Unidirectional disturbed flow  | -No ILT | |
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Cosford, P.; Leng, G.C.; Thomas, J. Screening for abdominal aortic aneurysm. Cochrane Database Syst. Rev. 2007, 2, CD002945. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Esfahani, A.N.; Golab, E.; Golestanian, O.; Ashouri, N.; Sajadi, S.M.; Ghaemi, F.; Baleanu, D.; Karimipour, A. Effects of Brownian motions and thermophoresis diffusions on the hematocrit and LDL concentration/diameter of pulsatile non-Newtonian blood in abdominal aortic aneurysm. J. Non-Newton. Fluid Mech. 2021, 294, 104576. [Google Scholar] [CrossRef]
- Ullery, B.W.; Hallett, R.L.; Fleischmann, D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom. Imaging 2018, 43, 1032–1043. [Google Scholar] [CrossRef]
- Vorp, D.A.; Raghavan, M.; Webster, M.W. Mechanical wall stress in abdominal aortic aneurysm: Influence of diameter and asymmetry. J. Vasc. Surg. 1998, 27, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Schermerhorn, M. A 66-year-old man with an abdominal aortic aneurysm: Review of screening and treatment. JAMA 2009, 302, 2015–2022. [Google Scholar] [CrossRef]
- Bouferrouk, A.; Boutamine, S.; Mekarnia, A. Dépistage opportuniste de l’anévrisme de l’aorte abdominale lors d’une écho-cardiographie transthoracique chez des patients sélectionnés: Expérience d’un centre algérien. J. Mal. Vasc. 2015, 40, 312. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Doyle, B.J.; Callanan, A.; Burke, P.E.; Grace, P.A.; Walsh, M.T.; Vorp, D.A.; McGloughlin, T.M. Vessel asymmetry as an additional diagnostic tool in the assessment of abdominal aortic aneurysms. J. Vasc. Surg. 2009, 49, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Vorp, D.A. Biomechanics of abdominal aortic aneurysm. J. Biomech. 2007, 40, 1887–1902. [Google Scholar] [CrossRef] [Green Version]
- Karthikesalingam, A.; Vidal-Diez, A.; Holt, P.J.; Loftus, I.M.; Schermerhorn, M.L.; Soden, P.A.; Landon, B.E.; Thompson, M.M. Thresholds for Abdominal Aortic Aneurysm Repair in England and the United States. N. Engl. J. Med. 2016, 375, 2051–2059. [Google Scholar] [CrossRef]
- Forneris, A.; Marotti, F.B.; Satriano, A.; Moore, R.D.; Di Martino, E.S. A novel combined fluid dynamic and strain analysis approach identified abdominal aortic aneurysm rupture. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 172–176. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Anton, R.; Hung, M.-Y.; Menon, P.; Finol, E.; Pekkan, K. Effects of Intraluminal Thrombus on Patient-Specific Abdominal Aortic Aneurysm Hemodynamics via Stereoscopic Particle Image Velocity and Computational Fluid Dynamics Modeling. J. Biomech. Eng. 2014, 136, 031001–0310019. [Google Scholar] [CrossRef] [Green Version]
- Lozowy, R.J.; Kuhn, D.C.S.; Ducas, A.A.; Boyd, A.J. The Relationship Between Pulsatile Flow Impingement and Intraluminal Thrombus Deposition in Abdominal Aortic Aneurysms. Cardiovasc. Eng. Technol. 2016, 8, 57–69. [Google Scholar] [CrossRef]
- Chandra, S.; Raut, S.S.; Jana, A.; Biederman, R.W.; Doyle, M.; Muluk, S.C.; Finol, E.A. Fluid-Structure Interaction Modeling of Abdominal Aortic Aneurysms: The Impact of Patient-Specific Inflow Conditions and Fluid/Solid Coupling. J. Biomech. Eng. 2013, 135, 081001. [Google Scholar] [CrossRef] [Green Version]
- Colciago, C.M.; Deparis, S.; Domanin, M.; Riccobene, C.; Schenone, E.; Quarteroni, A. Analysis of morphological and hemodynamical indexes in abdominal aortic aneurysms as preliminary indicators of intraluminal thrombus deposition. Biomech. Model. Mechanobiol. 2019, 19, 1035–1053. [Google Scholar] [CrossRef]
- Zambrano, B.A.; Gharahi, H.; Lim, C.; Jaberi, F.A.; Choi, J.; Lee, W.; Baek, S. Association of Intraluminal Thrombus, Hemodynamic Forces, and Abdominal Aortic Aneurysm Expansion Using Longitudinal CT Images. Ann. Biomed. Eng. 2015, 44, 1502–1514. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Wang, Y.; Fan, Y.; Peng, L.; Liu, R.; Zhao, J.; Yuan, D.; Zheng, T. Role of intraluminal thrombus in abdominal aortic aneurysm ruptures: A hemodynamic point of view. Med. Phys. 2019, 46, 4263–4275. [Google Scholar] [CrossRef]
- Zambrano, B.A.; Gharahi, H.; Lim, C.Y.; Lee, W.; Baek, S. Association of vortical structures and hemodynamic parameters for regional thrombus accumulation in abdominal aortic aneurysms. Int. J. Numer. Methods Biomed. Eng. 2021, 38, e3555. [Google Scholar] [CrossRef]
- Doyle, B.J.; McGloughlin, T.M.; Kavanagh, E.G.; Hoskins, P.R. From detection to rupture: A serial computational fluid dynamics case study of a rapidly expanding, patient-specific, ruptured abdominal aortic aneurysm. In Computational Biomechanics for Medicine: Fundamental Science and Patient-Specific Applications; Springer: New York, NY, USA, 2014; pp. 53–68. [Google Scholar] [CrossRef]
- Kelsey, L.J.; Powell, J.T.; Norman, P.E.; Miller, K.; Doyle, B.J. A comparison of hemodynamic metrics and intraluminal thrombus burden in a common iliac artery aneurysm. Int. J. Numer. Methods Biomed. Eng. 2016, 33, e2821. [Google Scholar] [CrossRef] [Green Version]
- Arzani, A.; Suh, G.-Y.; Dalman, R.L.; Shadden, S.C. A longitudinal comparison of hemodynamics and intraluminal thrombus deposition in abdominal aortic aneurysms. Am. J. Physiol. Circ. Physiol. 2014, 307, H1786–H1795. [Google Scholar] [CrossRef] [PubMed]
- O’rourke, M.J.; McCullough, J.P.; Kelly, S. An investigation of the relationship between hemodynamics and thrombus deposition within patient-specific models of abdominal aortic aneurysm. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Di Achille, P.; Tellides, G.; Figueroa, C.A.; Humphrey, J.D. A haemodynamic predictor of intraluminal thrombus formation in abdominal aortic aneurysms. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014, 470, 20140163. [Google Scholar] [CrossRef] [Green Version]
- Updegrove, A.; Wilson, N.M.; Merkow, J.; Lan, H.; Marsden, A.L.; Shadden, S.C. SimVascular: An Open Source Pipeline for Cardiovascular Simulation. Ann. Biomed. Eng. 2016, 45, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Wood, N. Aspects of Fluid Dynamics Applied to the Larger Arteries. J. Theor. Biol. 1999, 199, 137–161. [Google Scholar] [CrossRef]
- Di Martino, E.S.; Bohra, A.; Geest, J.P.V.; Gupta, N.; Makaroun, M.S.; Vorp, D.A. Biomechanical properties of ruptured versus electively repaired abdominal aortic aneurysm wall tissue. J. Vasc. Surg. 2006, 43, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, M.L.; Kratzberg, J.; de Tolosa, E.M.C.; Hanaoka, M.M.; Walker, P.; da Silva, E.S. Regional distribution of wall thickness and failure properties of human abdominal aortic aneurysm. J. Biomech. 2006, 39, 3010–3016. [Google Scholar] [CrossRef]
- Roache, P.J. Verification and Validation in Computational Science and Engineering; Hermosa: Albuquerque, NM, USA, 1998; Volume 895, p. 895. [Google Scholar]
- Wang, Y.; Luo, K.; Qiao, Y.; Fan, J. An integrated fluid-chemical model toward modeling the thrombus formation in an idealized model of aortic dissection. Comput. Biol. Med. 2021, 136, 104709. [Google Scholar] [CrossRef]
- Perinajová, R.; Juffermans, J.F.; Westenberg, J.J.; van der Palen, R.L.; Boogaard, P.J.V.D.; Lamb, H.J.; Kenjereš, S. Geometrically induced wall shear stress variability in CFD-MRI coupled simulations of blood flow in the thoracic aortas. Comput. Biol. Med. 2021, 133, 104385. [Google Scholar] [CrossRef]
- Armour, C.H.; Guo, B.; Saitta, S.; Pirola, S.; Liu, Y.; Dong, Z.; Xu, X.Y. Evaluation and verification of patient-specific modelling of type B aortic dissection. Comput. Biol. Med. 2021, 140, 105053. [Google Scholar] [CrossRef]
- Tzirakis, K.; Kamarianakis, Y.; Kontopodis, N.; Ioannou, C.V. The Effect of Blood Rheology and Inlet Boundary Conditions on Realistic Abdominal Aortic Aneurysms under Pulsatile Flow Conditions. Bioengineering 2023, 10, 272. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; Song, H.; Zhang, H.; Chen, L.; Guo, W. Analysis of Postoperative Remodeling Characteristics after Modular Inner Branched Stent-Graft Treatment of Aortic Arch Pathologies Using Computational Fluid Dynamics. Bioengineering 2023, 10, 164. [Google Scholar] [CrossRef]
- Belkacemi, D.; Al-Rawi, M.; Abbes, M.T.; Laribi, B. Flow Behaviour and Wall Shear Stress Derivatives in Abdominal Aortic Aneurysm Models: A Detailed CFD Analysis into Asymmetry Effect. CFD Lett. 2022, 14, 60–74. [Google Scholar] [CrossRef]
- Joly, F.; Soulez, G.; Garcia, D.; Lessard, S.; Kauffmann, C. Flow stagnation volume and abdominal aortic aneurysm growth: Insights from patient-specific computational flow dynamics of Lagrangian-coherent structures. Comput. Biol. Med. 2018, 92, 98–109. [Google Scholar] [CrossRef]
- Budwig, R.; Elger, D.; Hooper, H.; Slippy, J. Steady Flow in Abdominal Aortic Aneurysm Models. J. Biomech. Eng. 1993, 115, 418–423. [Google Scholar] [CrossRef]
- Les, A.S.; Yeung, J.J.; Schultz, G.M.; Herfkens, R.J.; Dalman, R.L.; Taylor, C.A. Supraceliac and Infrarenal Aortic Flow in Patients with Abdominal Aortic Aneurysms: Mean Flows, Waveforms, and Allometric Scaling Relationships. Cardiovasc. Eng. Technol. 2010, 1, 39–51. [Google Scholar] [CrossRef]
- McClarty, D.B.; Kuhn, D.C.; Boyd, A.J. Hemodynamic Changes in an Actively Rupturing Abdominal Aortic Aneurysm. J. Vasc. Res. 2021, 58, 172–179. [Google Scholar] [CrossRef]
- Morbiducci, U.; Gallo, D.; Massai, D.; Consolo, F.; Ponzini, R.; Antiga, L.; Bignardi, C.; Deriu, M.A.; Redaelli, A. Outflow Conditions for Image-Based Hemodynamic Models of the Carotid Bifurcation: Implications for Indicators of Abnormal Flow. J. Biomech. Eng. 2010, 132, 091005. [Google Scholar] [CrossRef]
- Kaewchoothong, N.; Algabri, Y.A.; Assawalertsakul, T.; Nuntadusit, C.; Chatpun, S. Computational Study of Abdominal Aortic Aneurysms with Severely Angulated Neck Based on Transient Hemodynamics Using an Idealized Model. Appl. Sci. 2022, 12, 2113. [Google Scholar] [CrossRef]
- Yasuda, K.; Armstrong, R.C.; Cohen, R.E. Shear flow properties of concentrated solutions of linear and star branched polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Gijsen, F.; van de Vosse, F.; Janssen, J. Wall shear stress in backward-facing step flow of a red blood cell suspension. Biorheology 1998, 35, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Gharahi, H.; Zambrano, B.A.; Zhu, D.C.; DeMarco, J.K.; Baek, S. Computational fluid dynamic simulation of human carotid artery bifurcation based on anatomy and volumetric blood flow rate measured with magnetic resonance imaging. Int. J. Adv. Eng. Sci. Appl. Math. 2016, 8, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Tzirakis, K.; Kamarianakis, Y.; Kontopodis, N.; Ioannou, C.V. Classification of Blood Rheological Models through an Idealized Symmetrical Bifurcation. Symmetry 2023, 15, 630. [Google Scholar] [CrossRef]
- Weddell, J.C.; Kwack, J.; Imoukhuede, P.I.; Masud, A. Hemodynamic Analysis in an Idealized Artery Tree: Differences in Wall Shear Stress between Newtonian and Non-Newtonian Blood Models. PLoS ONE 2015, 10, e0124575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.I.; Kensey, K.R. Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: Steady flows. Biorheology 1991, 28, 241–262. [Google Scholar] [CrossRef]
- He, X.; Ku, D.N. Pulsatile Flow in the Human Left Coronary Artery Bifurcation: Average Conditions. J. Biomech. Eng. 1996, 118, 74–82. [Google Scholar] [CrossRef]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Computation in the rabbit aorta of a new metric—The transverse wall shear stress—To quantify the multidirectional character of disturbed blood flow. J. Biomech. 2013, 46, 2651–2658. [Google Scholar] [CrossRef] [Green Version]
- Himburg, H.A.; Grzybowski, D.; Hazel, A.; LaMack, J.; Li, X.-M.; Friedman, M.H. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am. J. Physiol. Circ. Physiol. 2004, 286, H1916–H1922. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawi, M.; Al-Jumaily, A.M.; Belkacemi, D. Non-invasive diagnostics of blockage growth in the descending aorta-computational approach. Med. Biol. Eng. Comput. 2022, 60, 3265–3279. [Google Scholar] [CrossRef]
- Al-Rawi, M.; Al-Jumaily, A.; Belkacemi, D. Do Long Aorta Branches Impact on the Rheological Properties? In ASME International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers: Columbus, OH, USA, 2021; Volume 85598, p. V005T05A032. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Tzirakis, K.; Kamarianakis, Y.; Metaxa, E.; Kontopodis, N.; Ioannou, C.V.; Papaharilaou, Y. A robust approach for exploring hemodynamics and thrombus growth associations in abdominal aortic aneurysms. Med. Biol. Eng. Comput. 2017, 55, 1493–1506. [Google Scholar] [CrossRef]
- Suess, T.; Anderson, J.; Danielson, L.; Pohlson, K.; Remund, T.; Blears, E.; Gent, S.; Kelly, P. Examination of near-wall hemodynamic parameters in the renal bridging stent of various stent graft configurations for repairing visceral branched aortic aneurysms. J. Vasc. Surg. 2015, 64, 788–796. [Google Scholar] [CrossRef] [Green Version]
- Tedaldi, E.; Montanari, C.; Aycock, K.; Sturla, F.; Redaelli, A.; Manning, K.B. An experimental and computational study of the inferior vena cava hemodynamics under respiratory-induced collapse of the infrarenal IVC. Med. Eng. Phys. 2018, 54, 44–55. [Google Scholar] [CrossRef]


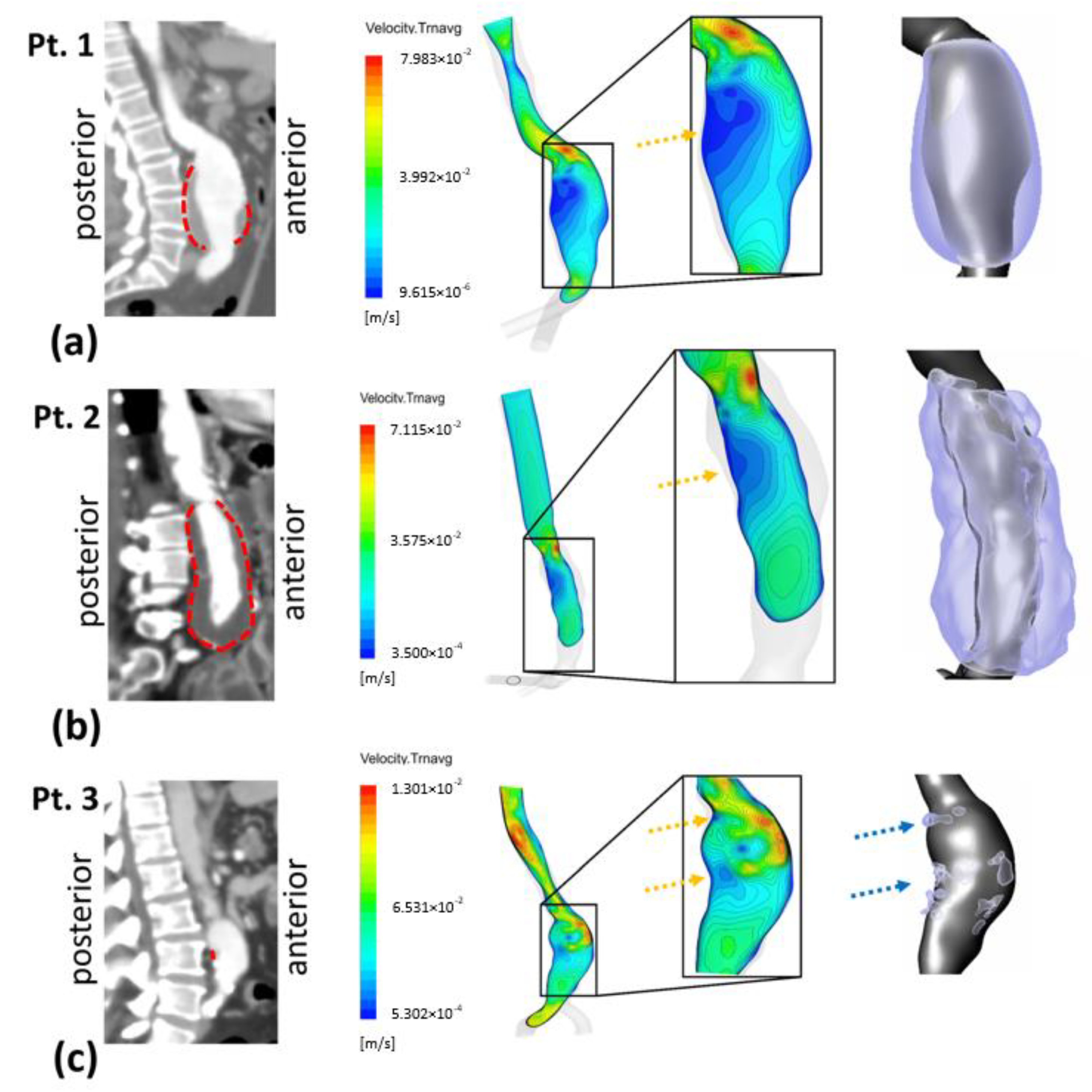


| Patient | Age (Years) | Gender | Maximum Lumen AAA Diameter (mm) | Inlet AAA Diameter (mm) | Right Iliac Diameter (mm) | Left Iliac Diameter (mm) | Length of AAA (mm) | Max ILT Thickness (mm) | ILT Thickness/ILT Distribution |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 70 | male | 56.7 | 25.2 | 15.9 | 17.2 | 118.1 | 19.9 | thick-thin/partially in AAA sac |
| P2 | 76 | male | 45.2 | 23.6 | 16.5 | 14.9 | 146.5 | 18.5 | thick-thin/entire in AAA sac |
| P3 | 60 | male | 32.4 | 19.6 | 13.4 | 12.9 | 86.3 | 1.6 | thin/small part of AAA sac |
| Patient | Number of Cells (Millions) | Number of Nodes (Millions) |
|---|---|---|
| P1 | 3.799 | 0.948 |
| P2 | 3.877 | 0.985 |
| P3 | 3.885 | 1.024 |
| Patient | ILT Region | TAWSS vs. ILT Thickness | OSI vs. ILT Thickness | TransWSS vs. ILT Thickness | ECAP vs. ILT Thickness | RRT vs. ILT Thickness |
|---|---|---|---|---|---|---|
| P1 | All ILT | 0.1951 | 0.0671 | −0.2560 | −0.1569 | −0.0741 |
| P2 | All ILT | 0.4026 | −0.1991 | −0.1662 | −0.3811 | −0.3245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkacemi, D.; Tahar Abbes, M.; Al-Rawi, M.; Al-Jumaily, A.M.; Bachene, S.; Laribi, B. Intraluminal Thrombus Characteristics in AAA Patients: Non-Invasive Diagnosis Using CFD. Bioengineering 2023, 10, 540. https://doi.org/10.3390/bioengineering10050540
Belkacemi D, Tahar Abbes M, Al-Rawi M, Al-Jumaily AM, Bachene S, Laribi B. Intraluminal Thrombus Characteristics in AAA Patients: Non-Invasive Diagnosis Using CFD. Bioengineering. 2023; 10(5):540. https://doi.org/10.3390/bioengineering10050540
Chicago/Turabian StyleBelkacemi, Djelloul, Miloud Tahar Abbes, Mohammad Al-Rawi, Ahmed M. Al-Jumaily, Sofiane Bachene, and Boualem Laribi. 2023. "Intraluminal Thrombus Characteristics in AAA Patients: Non-Invasive Diagnosis Using CFD" Bioengineering 10, no. 5: 540. https://doi.org/10.3390/bioengineering10050540






