Spatial Distribution of Inhibitory Innervations of Excitatory Pyramidal Cells by Major Interneuron Subtypes in the Auditory Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Viral Injection
2.3. Slice Preparation
2.4. Electrophysiology
2.5. Photostimulation
2.6. Data Analysis
2.7. Biocytin Staining
3. Results
3.1. Intrinsic Spiking Properties and ChR2-EYFP Expression in the Three Inhibitory Cell Types
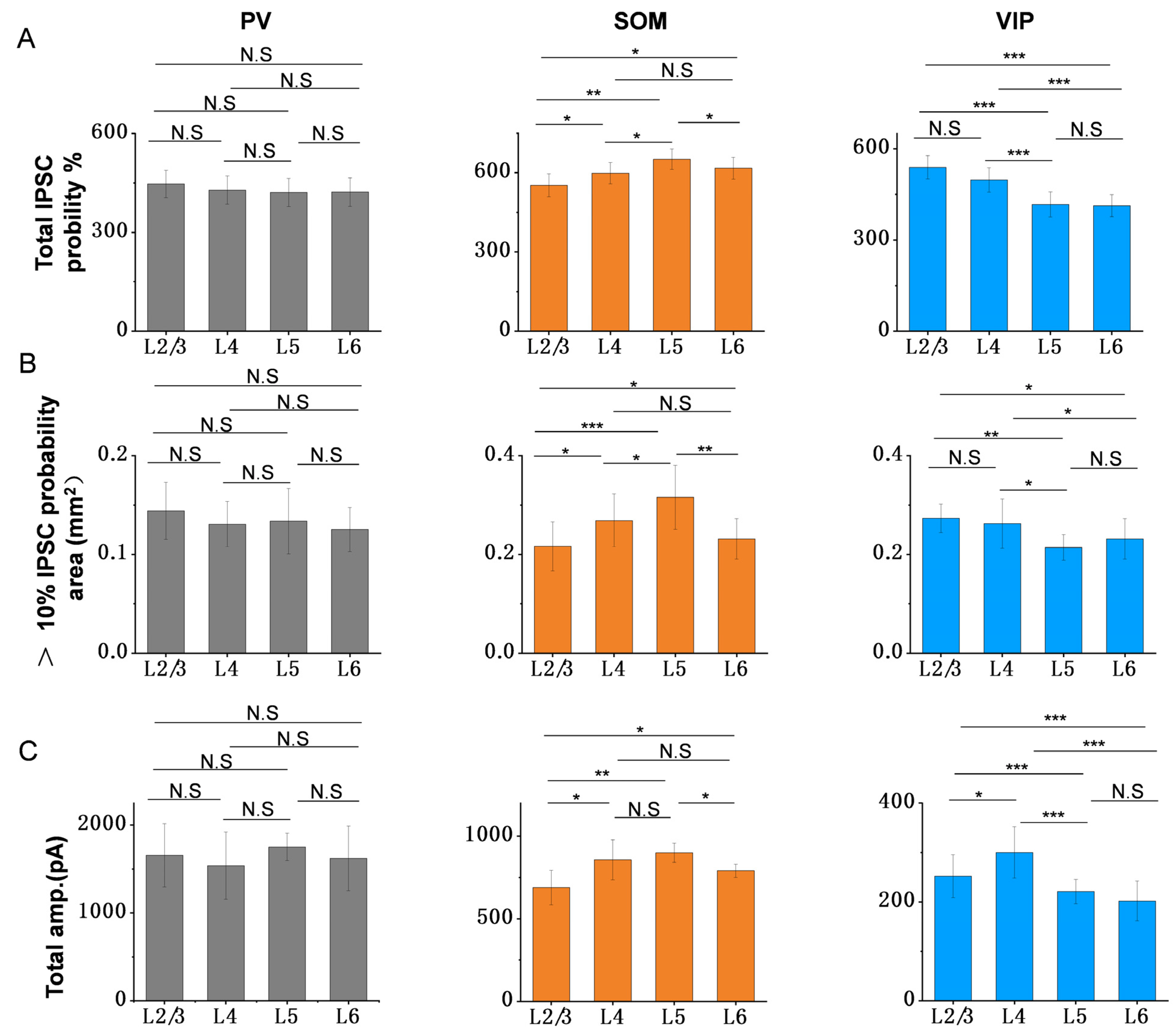
3.2. The Inhibitory Efficiency from Three Subtypes of Interneurons to PCs in Each Layer of ACx
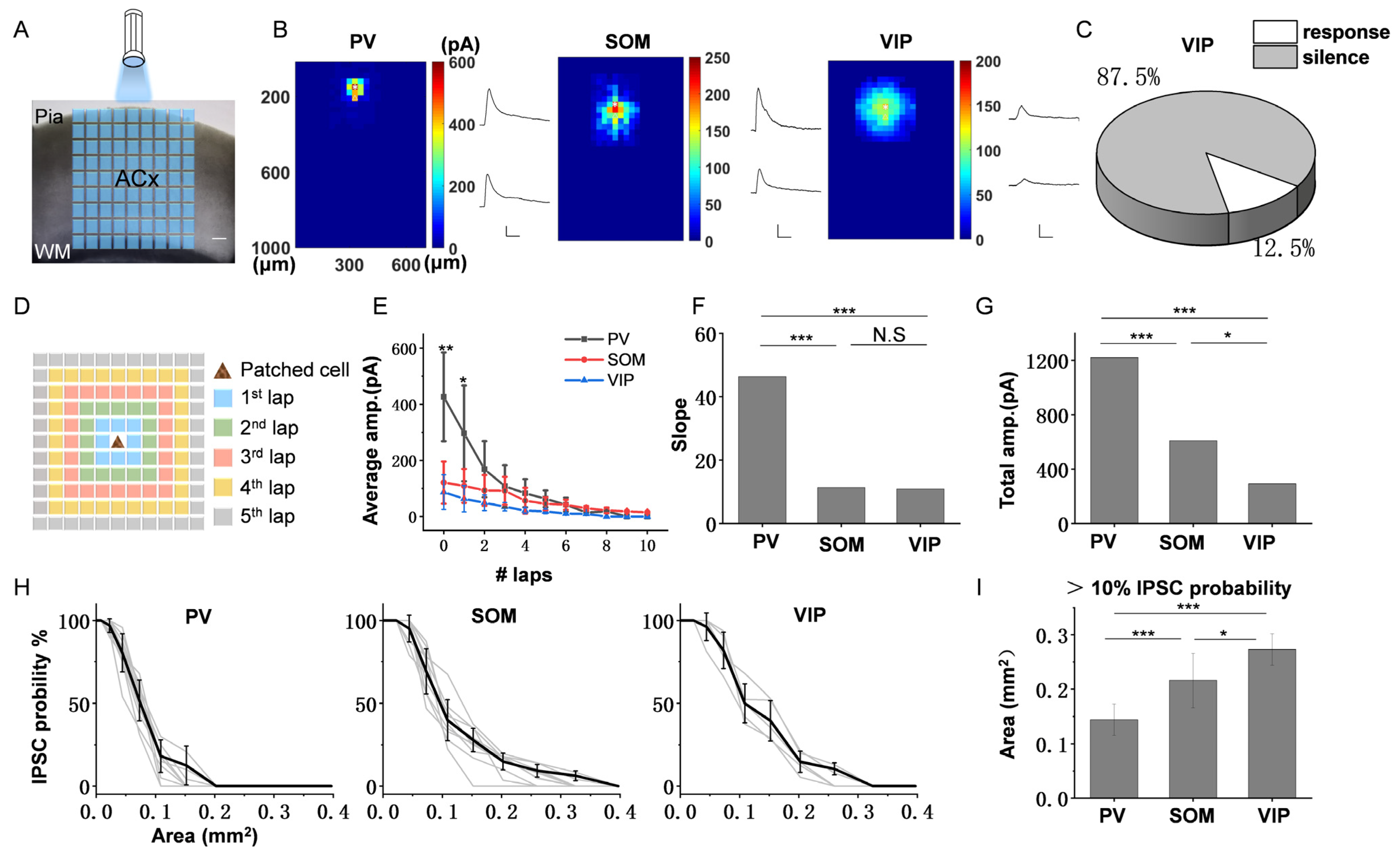
3.3. The Spatial Inhibitory Priority of the Three Subtypes of Interneurons in ACx
3.4. Summary of Inhibitory Efficiency and Spatial Inhibitory Priority of Different Interneurons to PCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Huang, J.; Zhuo, C.; Xu, Y.; Lin, X. Auditory verbal hallucination and the auditory network: From molecules to connectivity. Neuroscience 2019, 410, 59–67. [Google Scholar] [CrossRef]
- Zheng, Y.; Dixon, S.; MacPherson, K.; Smith, P.F. Glutamic acid decarboxylase levels in the cochlear nucleus of rats with acoustic trauma-induced chronic tinnitus. Neurosci. Lett. 2015, 586, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, B.D.; Rodrigues, P.V.; Salvi, R.J. Central gain control in tinnitus and hyperacusis. Front. Neurol. 2014, 5, 206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngodup, T.; Goetz, J.A.; McGuire, B.C.; Sun, W.; Lauer, A.M.; Xu-Friedman, M.A. Activity-dependent, homeostatic regulation of neurotransmitter release from auditory nerve fibers. Proc. Natl. Acad. Sci. USA 2015, 112, 6479–6484. [Google Scholar] [CrossRef][Green Version]
- Hayes, S.H.; Schormans, A.L.; Sigel, G.; Beh, K.; Herrmann, B.; Allman, B.L. Uncovering the contribution of enhanced central gain and altered cortical oscillations to tinnitus generation. Prog. Neurobiol. 2021, 196, 101893. [Google Scholar] [CrossRef] [PubMed]
- Basta, D.; Ernst, A. Effects of salicylate on spontaneous activity in inferior colliculus brain slices. Neurosci. Res. 2004, 50, 237–243. [Google Scholar] [CrossRef]
- Knipper, M.; Singer, W.; Schwabe, K.; Hagberg, G.E.; Hegner, Y.L.; Rüttiger, L.; Braun, C.; Land, R. Disturbed Balance of Inhibitory Signaling Links Hearing Loss and Cognition. Front. Neural Circuits 2021, 15, 785603. [Google Scholar] [CrossRef]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Roby, K.D.; Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010, 518, 389–404. [Google Scholar] [CrossRef][Green Version]
- Marek, R.; Jin, J.; Goode, T.D.; Giustino, T.F.; Wang, Q.; Acca, G.M.; Holehonnur, R.; Ploski, J.E.; Fitzgerald, P.J.; Lynagh, T.; et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat. Neurosci. 2018, 21, 384–392. [Google Scholar] [CrossRef][Green Version]
- Chen, G.; Zhang, Y.; Li, X.; Zhao, X.; Ye, Q.; Lin, Y.; Tao, H.W.; Rasch, M.J.; Zhang, X. Distinct Inhibitory Circuits Orchestrate Cortical beta and gamma Band Oscillations. Neuron 2017, 96, 1403–1418.e6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nanou, E.; Lee, A.; Catterall, W.A. Catterall, Control of Excitation/Inhibition Balance in a Hippocampal Circuit by Calcium Sensor Protein Regulation of Presynaptic Calcium Channels. J. Neurosci. 2018, 38, 4430–4440. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.M.; Murphy, C.; Uhlrich, D.J.; I Banks, M. PV+ Cells Enhance Temporal Population Codes but not Stimulus-Related Timing in Auditory Cortex. Cereb. Cortex 2019, 29, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Leitch, B. Chemogenetic Activation of Feed-Forward Inhibitory Parvalbumin-Expressing Interneurons in the Cortico-Thalamocortical Network During Absence Seizures. Front. Cell. Neurosci. 2021, 15, 688905. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Cantu, D.A.; Guilfoyle, J.; Chaudhari, G.R.; Newadkar, A.; Todisco, B.; De Alba, D.; Kourdougli, N.; Schmitt, L.M.; Pedapati, E.; et al. Author Correction: Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat. Neurosci. 2019, 22, 143. [Google Scholar] [CrossRef]
- Pirone, A.; Alexander, J.M.; Koenig, J.B.; Cook-Snyder, D.R.; Palnati, M.; Wickham, R.J.; Eden, L.; Shrestha, N.; Reijmers, L.; Biederer, T.; et al. Social Stimulus Causes Aberrant Activation of the Medial Prefrontal Cortex in a Mouse Model With Autism-Like Behaviors. Front. Synaptic Neurosci. 2018, 10, 35. [Google Scholar] [CrossRef][Green Version]
- Fanselow, E.E.; Richardson, K.A.; Connors, B.W. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J. Neurophysiol. 2008, 100, 2640–2652. [Google Scholar] [CrossRef][Green Version]
- Drexel, M.; Kirchmair, E.; Wieselthaler-Hölzl, A.; Preidt, A.P. Günther Sperk Somatostatin and neuropeptide Y neurons undergo different plasticity in parahippocampal regions in kainic acid-induced epilepsy. J. Neuropathol. Exp. Neurol. 2012, 71, 312–329. [Google Scholar] [CrossRef][Green Version]
- Luo, X.; Guet-McCreight, A.; Villette, V.; Francavilla, R.; Marino, B.; Chamberland, S.; Skinner, F.K.; Topolnik, L. Synaptic Mechanisms Underlying the Network State-Dependent Recruitment of VIP-Expressing Interneurons in the CA1 Hippocampus. Cereb. Cortex 2020, 30, 3667–3685. [Google Scholar] [CrossRef]
- Cunha-Reis, D.; Caulino-Rocha, A. VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy. Front. Cell. Neurosci. 2020, 14, 153. [Google Scholar] [CrossRef]
- Kato, H.K.; Asinof, S.K.; Isaacson, J.S. Network-Level Control of Frequency Tuning in Auditory Cortex. Neuron 2017, 95, 412–423.e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lakunina, A.A.; Nardoci, M.B.; Ahmadian, Y.; Jaramillo, S. Somatostatin-Expressing Interneurons in the Auditory Cortex Mediate Sustained Suppression by Spectral Surround. J. Neurosci. 2020, 40, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, I.; Wehr, M. Effects of Locomotion in Auditory Cortex Are Not Mediated by the VIP Network. Front. Neural Circuits 2021, 15, 618881. [Google Scholar] [CrossRef] [PubMed]
- Kätzel, D.; Zemelman, B.; Buetfering, C.; Woelfel, M.; Miesenboeck, G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat. Neurosci. 2011, 14, 100–107. [Google Scholar] [CrossRef][Green Version]
- Fino, E.; Yuste, R. Dense inhibitory connectivity in neocortex. Neuron 2011, 69, 1188–1203. [Google Scholar] [CrossRef][Green Version]
- Taniguchi, H.; He, M.; Wu, P.; Kim, S.; Paik, R.; Sugino, K.; Kvitsani, D.; Fu, Y.; Lu, J.; Lin, Y.; et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 2011, 71, 995–1013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chou, X.-L.; Fang, Q.; Yan, L.; Zhong, W.; Peng, B.; Li, H.; Wei, J.; Tao, H.W.; Zhang, L.I. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. eLife 2020, 9, e54157. [Google Scholar] [CrossRef]
- Ji, X.-Y.; Zingg, B.; Mesik, L.; Xiao, Z.; Zhang, L.I.; Tao, H.W. Thalamocortical Innervation Pattern in Mouse Auditory and Visual Cortex: Laminar and Cell-Type Specificity. Cereb. Cortex 2016, 26, 2612–2625. [Google Scholar] [CrossRef][Green Version]
- Fang, Q.; Chou, X.-L.; Peng, B.; Zhong, W.; Zhang, L.I.; Tao, H.W. A Differential Circuit via Retino-Colliculo-Pulvinar Pathway Enhances Feature Selectivity in Visual Cortex through Surround Suppression. Neuron 2020, 105, 355–369.e6. [Google Scholar] [CrossRef]
- Ibrahim, L.A.; Mesik, L.; Ji, X.-Y.; Fang, Q.; Li, H.-F.; Li, Y.-T.; Zingg, B.; Zhang, L.I.; Tao, H.W. Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron 2016, 89, 1031–1045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petreanu, L.; Mao, T.; Sternson, S.M.; Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 2009, 457, 1142–1145. [Google Scholar] [CrossRef][Green Version]
- Madisen, L.; Mao, T.; Koch, H.; Zhuo, J.-M.; Berenyi, A.; Fujisawa, S.; Hsu, Y.-W.; Garcia, A.J.; Gu, X.; Zanella, S.; et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 2012, 15, 793–802. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Gentet, L.J. Functional diversity of supragranular GABAergic neurons in the barrel cortex. Front. Neural Circuits 2012, 6, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feldmeyer, D.; Qi, G.; Emmenegger, V.; Staiger, J.F. Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex. Neuroscience 2018, 368, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, T.; Lee, M.Y.; Heeringa, A.N.; Beyer, L.A.; Swiderski, D.L.; Kanicki, A.C.; Kabara, L.L.; Dolan, D.F.; Shore, S.E.; Raphael, Y. Selective hair cell ablation and noise exposure lead to different patterns of changes in the cochlea and the cochlear nucleus. Neuroscience 2016, 332, 242–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyamae, T.; Chen, K.; Lewis, D.A.; Gonzalez-Burgos, G. Distinct Physiological Maturation of Parvalbumin-Positive Neuron Subtypes in Mouse Prefrontal Cortex. J. Neurosci. 2017, 37, 4883–4902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pi, H.-J.; Hangya, B.; Kvitsiani, D.; Sanders, J.I.; Huang, Z.J.; Kepecs, A. Cortical interneurons that specialize in disinhibitory control. Nature 2013, 503, 521–524. [Google Scholar] [CrossRef][Green Version]
- Pfeffer, C.K. Inhibitory neurons: Vip cells hit the brake on inhibition. Curr. Biol. 2014, 24, R18–R20. [Google Scholar] [CrossRef][Green Version]
- Masri, S.; Chan, N.; Marsh, T.; Zinsmaier, A.K.; Schaub, D.; Zhang, L.S.; Wang, W.; Bao, S. Chemogenetic Activation of Cortical Parvalbumin-Positive Interneurons Reverses Noise-Induced Impairments in Gap Detection. J. Neurosci. 2021, 41, 8848–8857. [Google Scholar] [CrossRef]
- Hattori, R.; Kuchibhotla, K.V.; Froemke, R.C.; Komiyama, T. Functions and dysfunctions of neocortical inhibitory neuron subtypes. Nat. Neurosci. 2017, 20, 1199–1208. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kubota, Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 1996, 16, 2701–2715. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Toledo-Rodriguez, M.; Gupta, A.; Wu, C.; Silberberg, G.; Luo, J.; Markram, H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 2004, 561, 65–90. [Google Scholar] [CrossRef]
- Halabisky, B.; Parada, I.; Buckmaster, P.S.; Prince, D.A. Excitatory input onto hilar somatostatin interneurons is increased in a chronic model of epilepsy. J. Neurophysiol. 2010, 104, 2214–2223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, W.; Williams, A.; Sun, Q.-Q. Circuit Mechanisms Underlying Epileptogenesis in a Mouse Model of Focal Cortical Malformation. Curr. Biol. 2021, 31, 334–345 e4. [Google Scholar] [CrossRef] [PubMed]
- Lamirault, L.; Guillou, J.-L.; Micheau, J.; Jaffard, R. Intrahippocampal injections of somatostatin dissociate acquisition from the flexible use of place responses. Eur. J. Neurosci. 2001, 14, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Nakagawasai, O.; Hozumi, S.; Tan-No, K.; Niijima, F.; Arai, Y.; Yasuhara, H.; Tadano, T. Immunohistochemical fluorescence intensity reduction of brain somatostatin in the impairment of learning and memory-related behaviour induced by olfactory bulbectomy. Behav. Brain Res. 2003, 142, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Prönneke, A.; Scheuer, B.; Wagener, R.J.; Möck, M.; Witte, M.; Staiger, J.F. Characterizing VIP Neurons in the Barrel Cortex of VIPcre/tdTomato Mice Reveals Layer-Specific Differences. Cereb. Cortex 2015, 25, 4854–4868. [Google Scholar] [CrossRef][Green Version]
- Pfeffer, C.K.; Xue, M.; He, M.; Huang, Z.J.; Scanziani, M. Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nat. Neurosci. 2013, 16, 1068–1076. [Google Scholar] [CrossRef]
- Staiger, J.F.; Masanneck, C.; Schleicher, A.; Zuschratter, W. Calbindin-containing interneurons are a target for VIP-immunoreactive synapses in rat primary somatosensory cortex. J. Comp. Neurol. 2004, 468, 179–189. [Google Scholar] [CrossRef]
- Dávid, C.; Schleicher, A.; Zuschratter, W.; Staiger, J.F. The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. Eur. J. Neurosci. 2007, 25, 2329–2340. [Google Scholar] [CrossRef]
- Ivanova, M.; Belcheva, S.; Belcheva, I.; Negrev, N.; Tashev, R. Lateralized hippocampal effects of vasoactive intestinal peptide on learning and memory in rats in a model of depression. Psychopharmacology 2012, 221, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M. Vasoactive intestinal peptide in neurodevelopmental disorders: Therapeutic potential. Curr. Pharm. Des. 2007, 13, 1079–1089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, C.M.; Ji, S.; Cai, H.; Maudsley, S.; Martin, B. Therapeutic potential of vasoactive intestinal peptide and its receptors in neurological disorders. CNS Neurol. Disord. Drug Targets 2010, 9, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.M.; Lim, M.A.; Cuasay, K.; Stone, M.M.; Seibert, K.M.; Spivak-Pohis, I.; Crawley, J.N.; Waschek, J.A.; Hill, J.M. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp. Neurol. 2008, 211, 67–84. [Google Scholar] [CrossRef][Green Version]
- Jardri, R.; Hugdahl, K.; Hughes, M.; Brunelin, J.; Waters, F.; Alderson-Day, B.; Smailes, D.; Sterzer, P.; Corlett, P.R.; Leptourgos, P.; et al. Are Hallucinations Due to an Imbalance Between Excitatory and Inhibitory Influences on the Brain? Schizophr Bull. 2016, 42, 1124–1134. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Sun, Q.-Q. Circuit-specific and neuronal subcellular-wide E-I balance in cortical pyramidal cells. Sci. Rep. 2018, 8, 3971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hjelmervik, H.; Craven, A.R.; Sinceviciute, I.; Johnsen, E.; Kompus, K.; Bless, J.J.; A Kroken, R.; Løberg, E.-M.; Ersland, L.; Grüner, R.; et al. Intra-Regional Glu-GABA vs Inter-Regional Glu-Glu Imbalance: A 1H-MRS Study of the Neurochemistry of Auditory Verbal Hallucinations in Schizophrenia. Schizophr Bull. 2020, 46, 633–642. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Kamigaki, T.; Do, J.P.H.; Chang, W.-C.; Jenvay, S.; Miyamichi, K.; Luo, L.; Dan, Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 2014, 345, 660–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Jonge, J.C.; Vinkers, C.H.; Hulshoff Pol, H.E.; Marsman, A. GABAergic Mechanisms in Schizophrenia: Linking Postmortem and In Vivo Studies. Front. Psychiatry 2017, 8, 118. [Google Scholar] [CrossRef][Green Version]
- Godoy, L.D.; Prizon, T.; Rossignoli, M.T.; Leite, J.P.; Liberato, J.L. Parvalbumin Role in Epilepsy and Psychiatric Comorbidities: From Mechanism to Intervention. Front. Integr. Neurosci. 2022, 16, 765324. [Google Scholar] [CrossRef] [PubMed]
- Krook-Magnuson, E.; Armstrong, C.; Oijala, M.; Soltesz, I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 2013, 4, 1376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paz, J.T.; Davidson, T.; Frechette, E.S.; Delord, B.; Parada, I.; Peng, K.; Deisseroth, K.; Huguenard, J.R. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2013, 16, 64–70. [Google Scholar] [CrossRef]
- Krook-Magnuson, E.; Armstrong, C.; Bui, A.; Lew, S.; Oijala, M.; Soltesz, I. In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol. 2015, 593, 2379–2388. [Google Scholar] [CrossRef][Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Zheng, W.; Ji, X. Spatial Distribution of Inhibitory Innervations of Excitatory Pyramidal Cells by Major Interneuron Subtypes in the Auditory Cortex. Bioengineering 2023, 10, 547. https://doi.org/10.3390/bioengineering10050547
Zhong W, Zheng W, Ji X. Spatial Distribution of Inhibitory Innervations of Excitatory Pyramidal Cells by Major Interneuron Subtypes in the Auditory Cortex. Bioengineering. 2023; 10(5):547. https://doi.org/10.3390/bioengineering10050547
Chicago/Turabian StyleZhong, Wen, Wenhong Zheng, and Xuying Ji. 2023. "Spatial Distribution of Inhibitory Innervations of Excitatory Pyramidal Cells by Major Interneuron Subtypes in the Auditory Cortex" Bioengineering 10, no. 5: 547. https://doi.org/10.3390/bioengineering10050547
APA StyleZhong, W., Zheng, W., & Ji, X. (2023). Spatial Distribution of Inhibitory Innervations of Excitatory Pyramidal Cells by Major Interneuron Subtypes in the Auditory Cortex. Bioengineering, 10(5), 547. https://doi.org/10.3390/bioengineering10050547




