The Impact of Nutrient Limitation and Harvest Method on the Wet Preservation of Chlorella vulgaris Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chlorella Biomass Cultivation, Harvest, and Storage
2.2. Lipid Analysis
2.3. Ethanol Analysis
2.4. Organic Acid Analysis
2.5. Statistics
3. Results
3.1. Algae Growth and Harvest
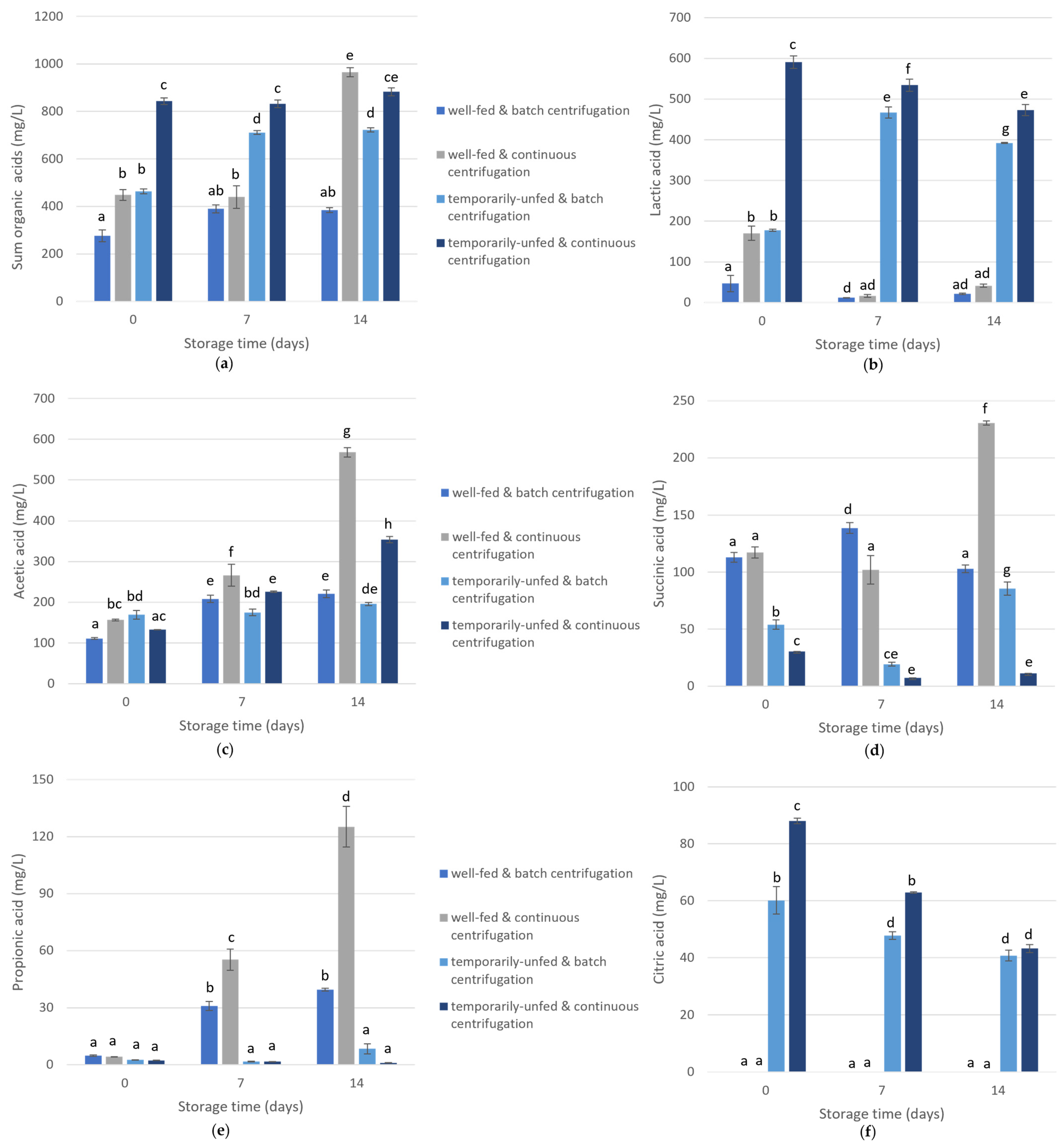
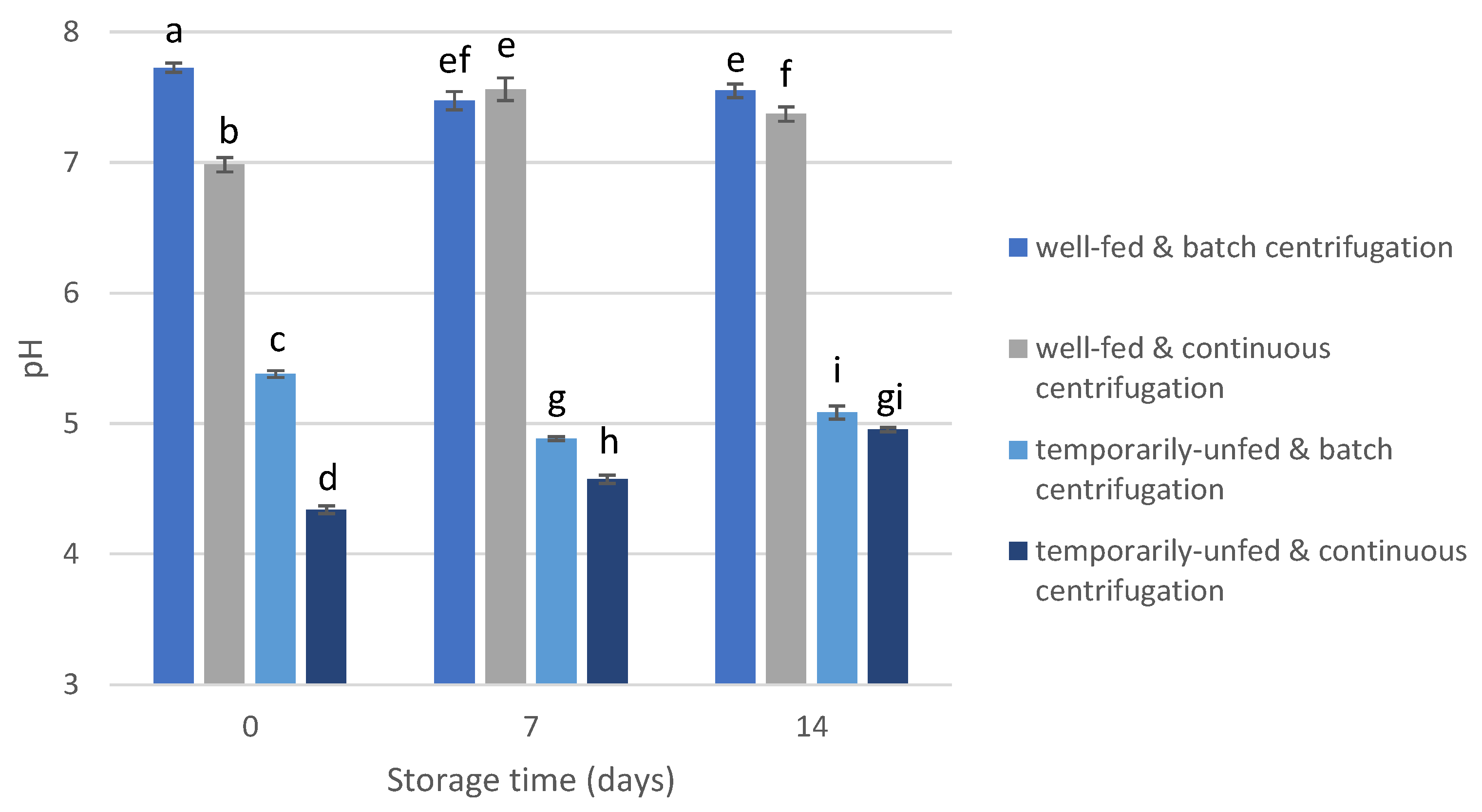
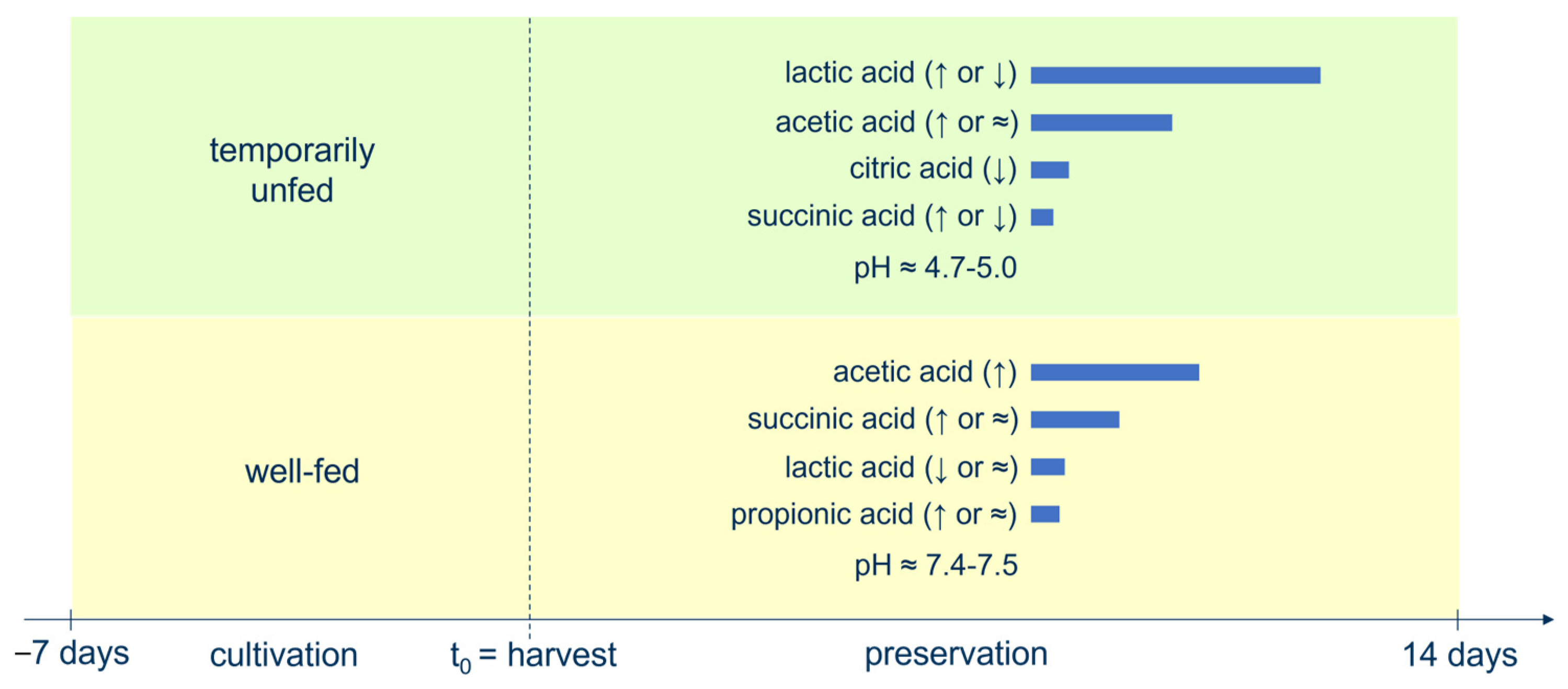
3.2. Short-Chain Fatty Acid and pH Levels
3.3. Lipid Analysis
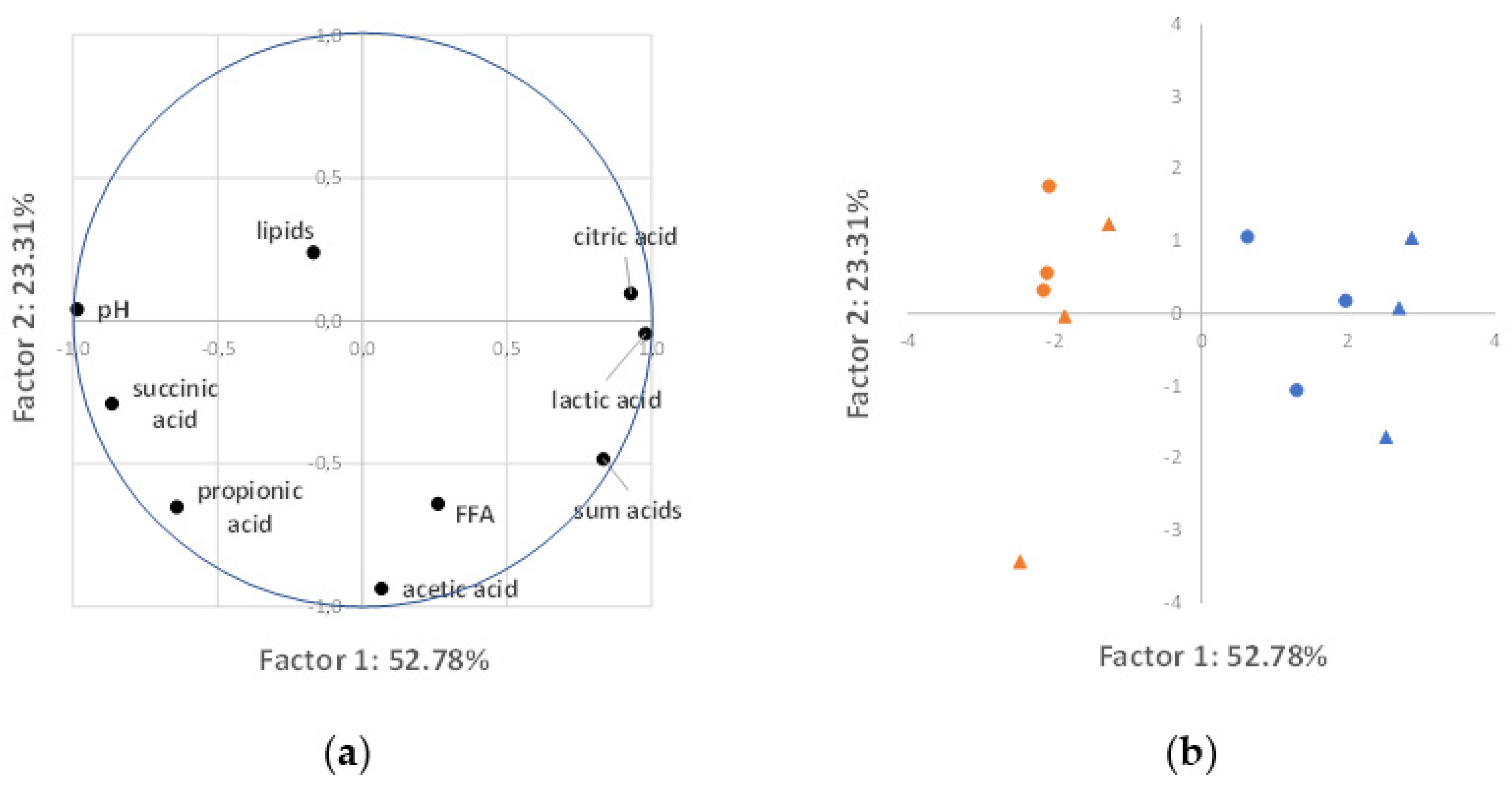
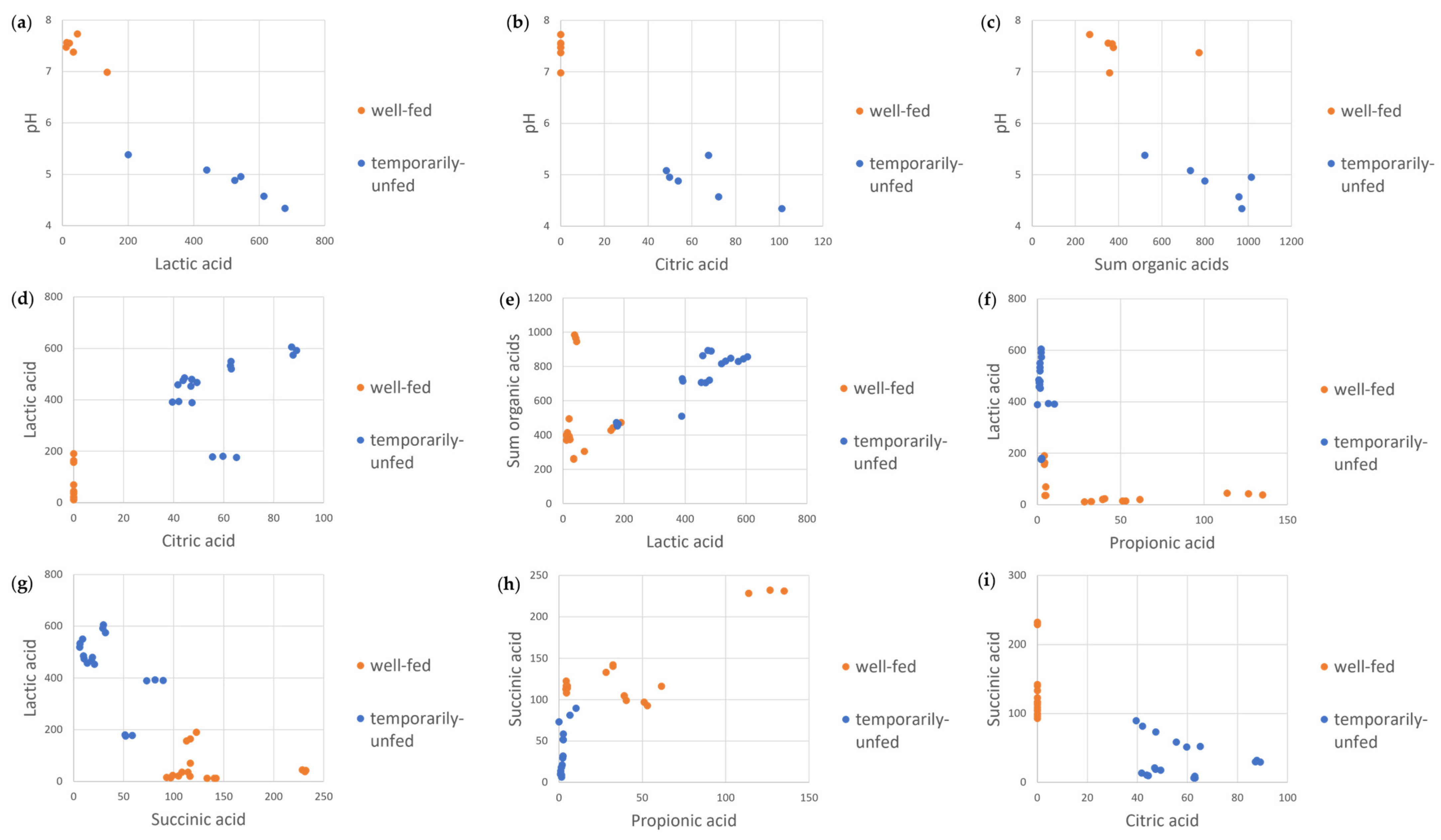
3.4. Correlation Analysis
4. Discussion
4.1. The Impact of Nutritional Status at Harvest
4.2. The Impact of Harvest Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vazquez Calderon, F.; Sanchez Lopez, J. Overview of the Algae Industry in Europe; Publications office of the European Union: Luxembourg, 2022; pp. 1–67. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, Hopes, and the Way Forward for Microalgal Biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Algae Products Market. Available online: https://www.alliedmarketresearch.com/algae-products-market (accessed on 10 February 2023).
- Olaizola, M.; Grewe, C. Commercial Microalgal Cultivation Systems. In Grand Challenges in Algae Biotechnology; Hallmann, A., Rampelotto, P.H., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 3–34. [Google Scholar]
- Enzing, C.; Nooijen, A.; Eggink, G.; Springer, J.; Wijffels, R.H. Algae and Genetic Modification Research, Production and Risks; Technopolis Group: Amsterdam, The Netherlands, 2012; pp. 1–59. [Google Scholar]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae Based Production of Single-Cell Protein. Curr. Opin. Biotechnol. 2022, 75, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Balduyck, L.; Goiris, K.; Bruneel, C.; Muylaert, K.; Foubert, I. Stability of Valuable Components during Wet and Dry Storage. In Handbook of Marine Microalgae; Kim, S.K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 81–91. [Google Scholar]
- Balduyck, L.; Stock, T.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Muylaert, K.; Foubert, I. Integrity of the Microalgal Cell Plays a Major Role in the Lipolytic Stability during Wet Storage. Algal. Res. 2017, 25, 516–524. [Google Scholar] [CrossRef]
- Balduyck, L.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Van Durme, J.; Muylaert, K.; Foubert, I. Lipolysis in T-Isochrysis lutea during Wet Storage at Different Temperatures. Algal. Res. 2016, 18, 281–287. [Google Scholar] [CrossRef]
- Mulgund, A. Increasing Lipid Accumulation in Microalgae through Environmental Manipulation, Metabolic and Genetic Engineering: A Review in the Energy NEXUS Framework. Energy Nexus 2022, 5, 100054. [Google Scholar] [CrossRef]
- Schreiber, C.; Behrendt, D.; Huber, G.; Pfaff, C.; Widzgowski, J.; Ackermann, B.; Müller, A.; Zachleder, V.; Moudříková, Š.; Mojzeš, P.; et al. Growth of Algal Biomass in Laboratory and in Large-Scale Algal Photobioreactors in the Temperate Climate of Western Germany. Bioresour. Technol. 2017, 234, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Kangani, C.O.; Kelley, D.E.; DeLany, J.P. New Method for GC/FID and GC–C-IRMS Analysis of Plasma Free Fatty Acid Concentration and Isotopic Enrichment. J. Chromatogr. B 2008, 873, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.; Krzywinski, M.; Altman, N. Principal Component Analysis. Nat. Methods 2017, 14, 641–642. [Google Scholar] [CrossRef]
- Catalanotti, C.; Yang, W.; Posewitz, M.C.; Grossman, A.R. Fermentation Metabolism and Its Evolution in Algae. Front. Plant. Sci. 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Martinko, J.M. Brock Biology of Microorganisms, 11th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006; pp. 1–1056. [Google Scholar]
- Kosourov, S.; Seibert, M.; Ghirardi, M.L. Effects of Extracellular PH on the Metabolic Pathways in Sulfur-Deprived, H2-Producing Chlamydomonas reinhardtii Cultures. Plant Cell Physiol. 2003, 44, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Steinert, G.; de Vree, J.; Meijer, S.; Heryanto, C.; Bosma, R.; Wijffels, R.H.; Barbosa, M.J.; Smidt, H.; Sipkema, D. Bacterial Diversity in Different Outdoor Pilot Plant Photobioreactor Types during Production of the Microalga Nannochloropsis sp. CCAP211/78. Appl. Microbiol. Biotechnol. 2022, 106, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zheng, Y. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Oginni, O.; Wahlen, B.; Wendt, L.; Walton, M.; Dempster, T.; Gerken, H. Effects of Inoculation with Lactic Acid Bacteria on the Preservation of Nannochloropsis Gaditana Biomass in Wet Anaerobic Storage and Its Impact on Biomass Quality. Fermentation 2022, 8, 159. [Google Scholar] [CrossRef]
- Verspreet, J.; Kreps, S.; Bastiaens, L. Evaluation of Microbial Load, Formation of Odorous Metabolites and Lipid Stability during Wet Preservation of Nannochloropsis Gaditana Concentrates. Appl. Sci. 2020, 10, 3419. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Dalheim, L.; Svenning, J.B.; Eilertsen, H.C.; Vasskog, T.; Olsen, R.L. Stability of Lipids during Wet Storage of the Marine Diatom Porosira glacialis under Semi-Preserved Conditions at 4 and 20 °C. J. Appl. Phycol 2021, 33, 385–395. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verspreet, J.; Kuchendorf, C.M.; Ackermann, B.; Bastiaens, L. The Impact of Nutrient Limitation and Harvest Method on the Wet Preservation of Chlorella vulgaris Biomass. Bioengineering 2023, 10, 600. https://doi.org/10.3390/bioengineering10050600
Verspreet J, Kuchendorf CM, Ackermann B, Bastiaens L. The Impact of Nutrient Limitation and Harvest Method on the Wet Preservation of Chlorella vulgaris Biomass. Bioengineering. 2023; 10(5):600. https://doi.org/10.3390/bioengineering10050600
Chicago/Turabian StyleVerspreet, Joran, Christina M. Kuchendorf, Bärbel Ackermann, and Leen Bastiaens. 2023. "The Impact of Nutrient Limitation and Harvest Method on the Wet Preservation of Chlorella vulgaris Biomass" Bioengineering 10, no. 5: 600. https://doi.org/10.3390/bioengineering10050600






