Multifunctional Biosensing Platform Based on Nickel-Modified Laser-Induced Graphene
Abstract
:1. Introduction
2. Results and Discussion
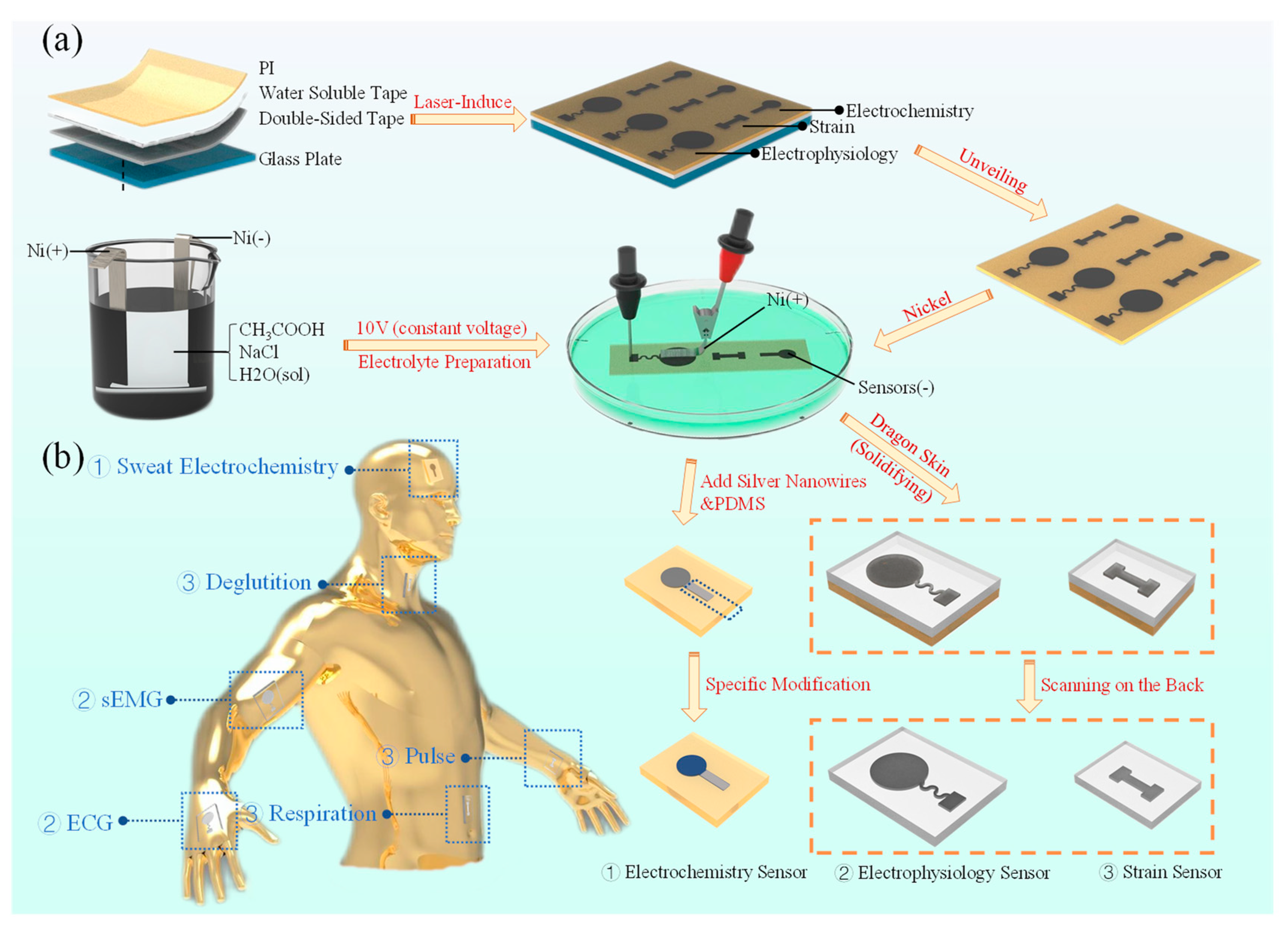
2.1. Preparation of LIG–Ni Flexible Sensor Manufacturing
2.2. Electromechanical Characterization
2.3. LIG–Ni Application for Electrophysiological Monitoring
2.4. LIG–Ni Application for Strain Monitoring
2.5. LIG–Ni Application for Electrochemical Monitoring
3. Conclusions
4. Methods
4.1. Board and LIG Preparation
4.2. Electrolyte Preparation
4.3. LIG Plated Ni Layer
4.4. Fabrication of LIG–Ni Electrophysiological Sensor
4.5. Fabrication of LIG–Ni Strain Sensors
4.6. Fabrication of LIG–Ni Electrochemical Sensor
4.6.1. Fabrication of LIG–Ni Glucose Sensor
4.6.2. Fabrication of LIG–Ni pH Sensor
4.6.3. Fabrication of LIG–Ni Na+ Sensor
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, S.; Hu, S.; Song, W.; Gu, M.; Liu, J.; Song, J.; Liu, Z.; Li, Z.; Huang, K.; Wu, Y.; et al. An ultralight, flexible, and biocompatible all-fiber motion sensor for artificial intelligence wearable electronics. NPJ Flex. Electron. 2022, 6, 27. [Google Scholar] [CrossRef]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.-S.; et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, Q.; Wang, Z.; Liu, Q.; Zhang, B.; He, D.; Wu, Z.; Mu, S. Highly sensitive wearable sensor based on a flexible multi-layer graphene film antenna. Sci. Bull. 2018, 63, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, H.; Wu, P.; Huang, W.; Gao, W.; Fang, F.; Cai, N.; Chen, R.; Zhu, Z. Wearable Flexible Strain Sensor Based on Three-Dimensional Wavy Laser-Induced Graphene and Silicone Rubber. Sensors 2020, 20, 4266. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, S.; Zhao, S.; Yang, H. Design and Manufacture of Data Gloves for Rehabilitation Training and Gesture Recognition Based on Flexible Sensors. J. Healthc. Eng. 2021, 2021, 6359403. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent Advances in Sensing Applications of Graphene Assemblies and Their Composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Yuan, Z.; Hou, L.; Bariya, M.; Nyein, H.Y.Y.; Tai, L.-C.; Ji, W.; Li, L.; Javey, A. A multi-modal sweat sensing patch for cross-verification of sweat rate, total ionic charge, and Na+ concentration. Lab Chip 2019, 19, 3179–3189. [Google Scholar] [CrossRef]
- Bai, W.; Irie, M.; Liu, Z.; Luan, H.; Franklin, D.; Nandoliya, K.; Guo, H.; Zang, H.; Weng, Y.; Lu, D.; et al. Bioresorbable Multilayer Photonic Cavities as Temporary Implants for Tether-Free Measurements of Regional Tissue Temperatures. BME Front. 2021, 2021, 8653218. [Google Scholar] [CrossRef]
- Dubatovka, A.; Buhmann, J.M. Automatic Detection of Atrial Fibrillation from Single-Lead ECG Using Deep Learning of the Cardiac Cycle. BME Front. 2022, 2022, 9813062. [Google Scholar] [CrossRef]
- Bounik, R.; Cardes, F.; Ulusan, H.; Modena, M.M.; Hierlemann, A. Impedance Imaging of Cells and Tissues: Design and Applications. BME Front. 2022, 2022, 9857485. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef] [PubMed]
- Tjon, K.C.E.; Yuan, J. Impedance characterization of silver/silver chloride micro-electrodes for bio-sensing applications. Electrochim. Acta 2019, 320, 134638. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.; Yu, J.; Zhang, S.; He, L.; Wu, S.; Liu, C.; Deng, Y. Facile Fabrication of Robust and Reusable PDMS Supported Graphene Dry Electrodes for Wearable Electrocardiogram Monitoring. Adv. Mater. Technol. 2021, 6, 2100262. [Google Scholar] [CrossRef]
- Alfonso, F.S.; Zhou, Y.; Liu, E.; McGuire, A.F.; Yang, Y.; Kantarci, H.; Li, D.; Copenhaver, E.; Zuchero, J.B.; Müller, H.; et al. Label-free optical detection of bioelectric potentials using electrochromic thin films. Proc. Natl. Acad. Sci. USA 2020, 117, 17260–17268. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, E.; Yang, Y.; Alfonso, F.S.; Ahmed, B.; Nakasone, K.; Forró, C.; Müller, H.; Cui, B. Dual-Color Optical Recording of Bioelectric Potentials by Polymer Electrochromism. J. Am. Chem. Soc. 2022, 144, 23505–23515. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Liu, Y.; Liu, L.; Xu, Q.; Liu, H.; Wang, W.; Chen, L. Ultra-Stretchable Monofilament Flexible Sensor with Low Hysteresis and Linearity based on MWCNTs/Ecoflex Composite Materials. Macromol. Mater. Eng. 2021, 306, 2100113. [Google Scholar] [CrossRef]
- Tian, Y.; He, P.; Yang, B.; Yi, Z.; Lu, L.; Liu, J. A Flexible Piezoelectric Strain Sensor Array with Laser-Patterned Serpentine Interconnects. IEEE Sens. J. 2020, 20, 8463–8468. [Google Scholar] [CrossRef]
- Tognazzi, A.; Rocco, D.; Gandolfi, M.; Locatelli, A.; Carletti, L.; De Angelis, C. High Quality Factor Silicon Membrane Metasurface for Intensity-Based Refractive Index Sensing. Optics 2021, 2, 193–199. [Google Scholar] [CrossRef]
- Chen, H.; Mei, Z.; Qi, K.; Wang, Y.; Chen, R. A wearable enzyme-free glucose sensor based on nickel nanoparticles decorated laser-induced graphene. J. Electroanal. Chem. 2022, 920, 116585. [Google Scholar] [CrossRef]
- Antikhovich, I.V.; Chernik, A.A.; Zharskii, I.M.; Bolvako, A.K. Electrodeposition of a nickel coating from a low-temperature acetate-chloride nickel-plating electrolyte. Russ. J. Electrochem. 2015, 51, 281–285. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-induced graphene non-enzymatic glucose sensors for on-body measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef]
- Geng, S.-K.; Zheng, Y.; Li, S.-Q.; Su, H.; Zhao, X.; Hu, J.; Shu, H.-B.; Jaroniec, M.; Chen, P.; Liu, Q.-H.; et al. Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst. Nat. Energy 2021, 6, 904–912. [Google Scholar] [CrossRef]
- Zhang, S.; Chhetry, A.; Zahed, A.; Sharma, S.; Park, C.; Yoon, S.; Park, J.Y. On-skin ultrathin and stretchable multifunctional sensor for smart healthcare wearables. NPJ Flex. Electron. 2022, 6, 11. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Z. Research progress on the effects of nickel on hormone secretion in the endocrine axis and on target organs. Ecotoxicol. Environ. Saf. 2021, 213, 112034. [Google Scholar] [CrossRef]
- Affanni, A. Wireless Sensors System for Stress Detection by Means of ECG and EDA Acquisition. Sensors 2020, 20, 2026. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yao, Y.; Montes-García, V.; Stoeckel, M.; Von Holst, M.; Ciesielski, A.; Samorì, P. Highly Sensitive Strain Sensors Based on Molecules–Gold Nanoparticles Networks for High-Resolution Human Pulse Analysis. Small 2021, 17, e2007593. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.; Becerra, L.L.; Hsu, P.-Y.; Kaipu, V.; Mercier, P.P.; Cheng, C.-K.; Lipomi, D.J. Epidermal Graphene Sensors and Machine Learning for Estimating Swallowed Volume. ACS Appl. Nano Mater. 2021, 4, 8126–8134. [Google Scholar] [CrossRef]
- Sindhu, B.; Kothuru, A.; Sahatiya, P.; Goel, S.; Nandi, S. Laser-Induced Graphene Printed Wearable Flexible Antenna-Based Strain Sensor for Wireless Human Motion Monitoring. IEEE Trans. Electron Devices 2021, 68, 3189–3194. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef]
- Strakosas, X.; Selberg, J.; Pansodtee, P.; Yonas, N.; Manapongpun, P.; Teodorescu, M.; Rolandi, M. A non-enzymatic glucose sensor enabled by bioelectronic pH control. Sci. Rep. 2019, 9, 10844. [Google Scholar] [CrossRef]
- He, W.; Huang, Y.; Wu, J. Enzyme-Free Glucose Biosensors Based on MoS2 Nanocomposites. Nanoscale Res. Lett. 2020, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Peng, Y. Ginkgo Leaf Inspired Fabrication of Micro/Nanostructures and Demonstration of Flexible Enzyme-Free Glucose Sensors. Sensors 2022, 22, 7507. [Google Scholar] [CrossRef]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, N.; Lai, X.; Wu, J.; Wu, L.; Zhao, X.; Feng, L. Paper-Based Sandwich-Structured Wearable Sensor with Sebum Filtering for Continuous Detection of Sweat pH. ACS Sens. 2023, 8, 176–186. [Google Scholar] [CrossRef]
- Berggren, M.; Malliaras, G.G. How conducting polymer electrodes operate. Science 2019, 364, 233–234. [Google Scholar] [CrossRef]
- Yeung, K.K.; Li, J.; Huang, T.; Hosseini, I.I.; Al Mahdi, R.; Alam, M.; Sun, H.; Mahshid, S.; Yang, J.; Ye, T.T.; et al. Utilizing Gradient Porous Graphene Substrate as the Solid-Contact Layer To Enhance Wearable Electrochemical Sweat Sensor Sensitivity. Nano Lett. 2022, 22, 6647–6654. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Tai, L.-C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.; Harris, J.K.; Ratcliff, E. Predicting limits of detection in real-time sweat-based human performance monitoring. In Smart Biomedical and Physiological Sensor Technology XVI; SPIE: Bellingham, WA, USA, 2019; Volume 11020. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2018, 31, 239–245. [Google Scholar] [CrossRef]
- Alizadeh, A.; Burns, A.; Lenigk, R.; Gettings, R.; Ashe, J.; Porter, A.; McCaul, M.; Barrett, R.; Diamond, D.; White, P.; et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip 2018, 18, 2632–2641. [Google Scholar] [CrossRef]
- Garjonyte, R.; Malinauskas, A. Amperometric glucose biosensors based on Prussian Blue– and polyaniline–glucose oxidase modified electrodes. Biosens. Bioelectron. 2000, 15, 445–451. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Zhang, Y.; Bao, B.; Hu, X.; Li, J.; Wu, H.; Yang, K.; Zhang, S.; Yang, H.; Guo, K. Multifunctional Biosensing Platform Based on Nickel-Modified Laser-Induced Graphene. Bioengineering 2023, 10, 620. https://doi.org/10.3390/bioengineering10050620
Tong Y, Zhang Y, Bao B, Hu X, Li J, Wu H, Yang K, Zhang S, Yang H, Guo K. Multifunctional Biosensing Platform Based on Nickel-Modified Laser-Induced Graphene. Bioengineering. 2023; 10(5):620. https://doi.org/10.3390/bioengineering10050620
Chicago/Turabian StyleTong, Yao, Yingying Zhang, Benkun Bao, Xuhui Hu, Jiuqiang Li, Han Wu, Kerong Yang, Senhao Zhang, Hongbo Yang, and Kai Guo. 2023. "Multifunctional Biosensing Platform Based on Nickel-Modified Laser-Induced Graphene" Bioengineering 10, no. 5: 620. https://doi.org/10.3390/bioengineering10050620







