Modeling and Validation of an Ultra-Compact Regenerative Liver Dialysis Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
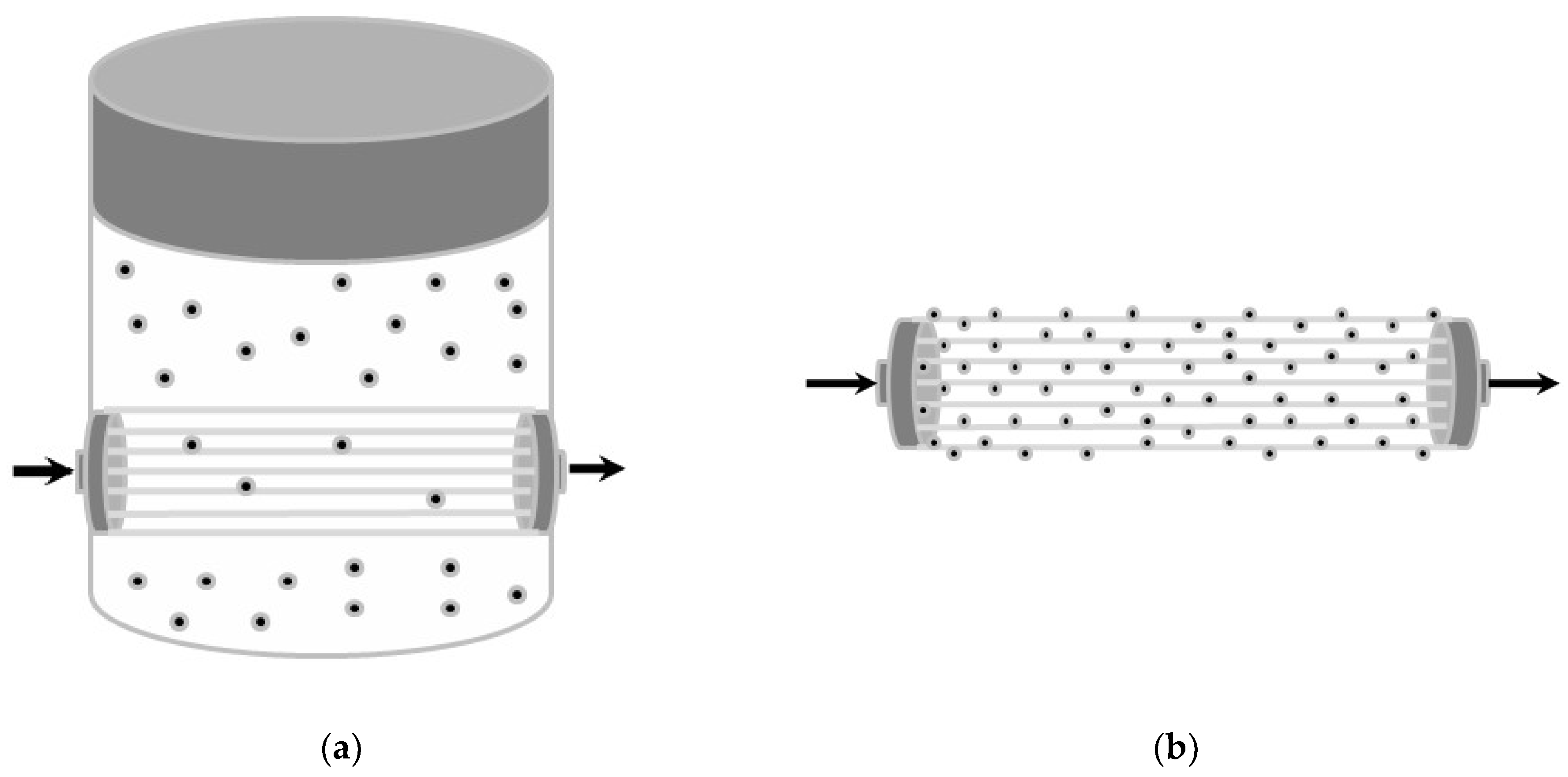
2.2.1. Experimental Setup Provided with Dialysis Fluid
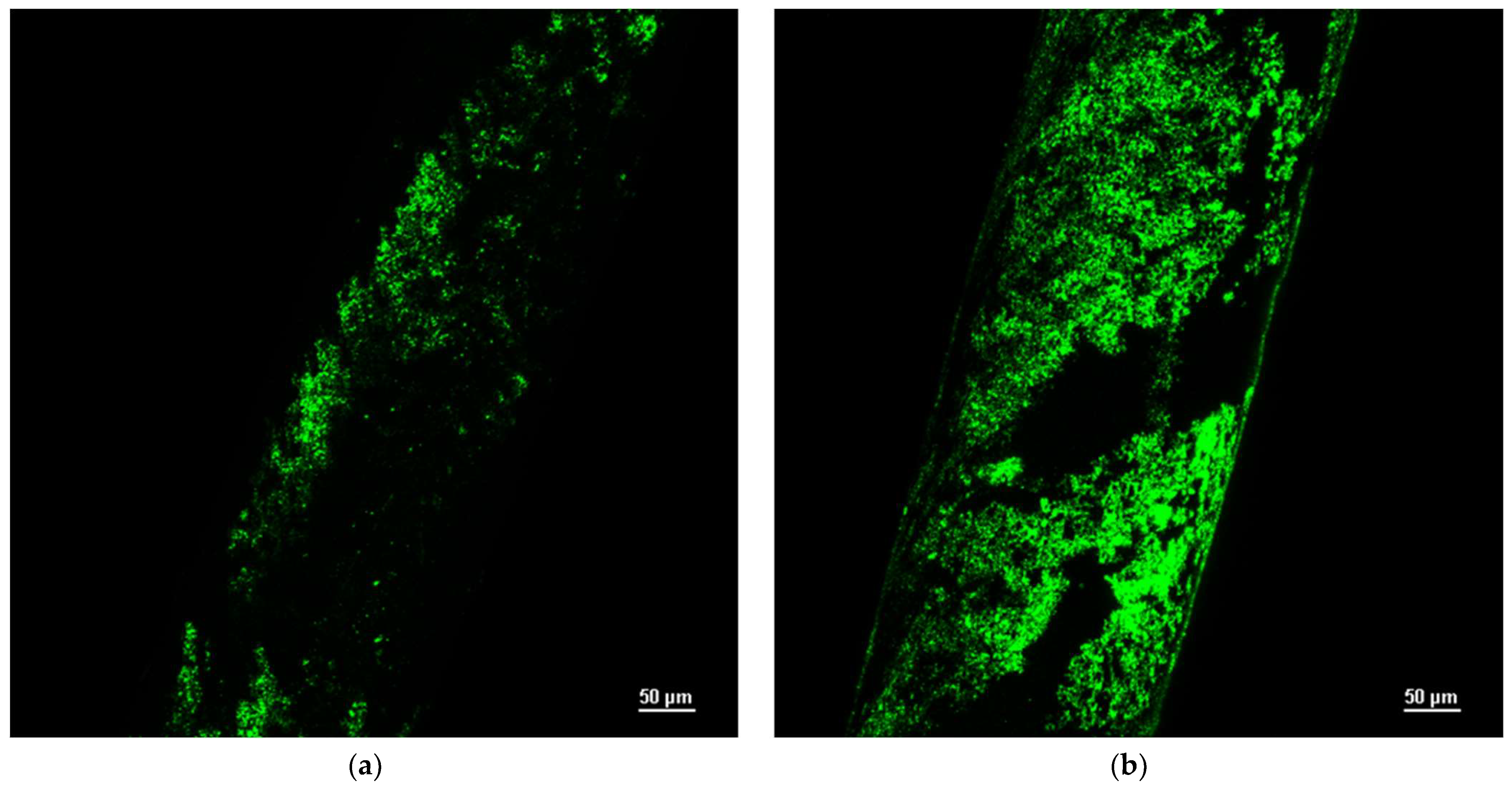
2.2.2. Experimental Setup Provided with Functionalized Hollow Fibers
- (a)
- Membrane functionalization by physical adsorption of the silica microspheres;
- (b)
- Membrane functionalization by chemical bonds with the silica microspheres.
3. Mathematical Modeling
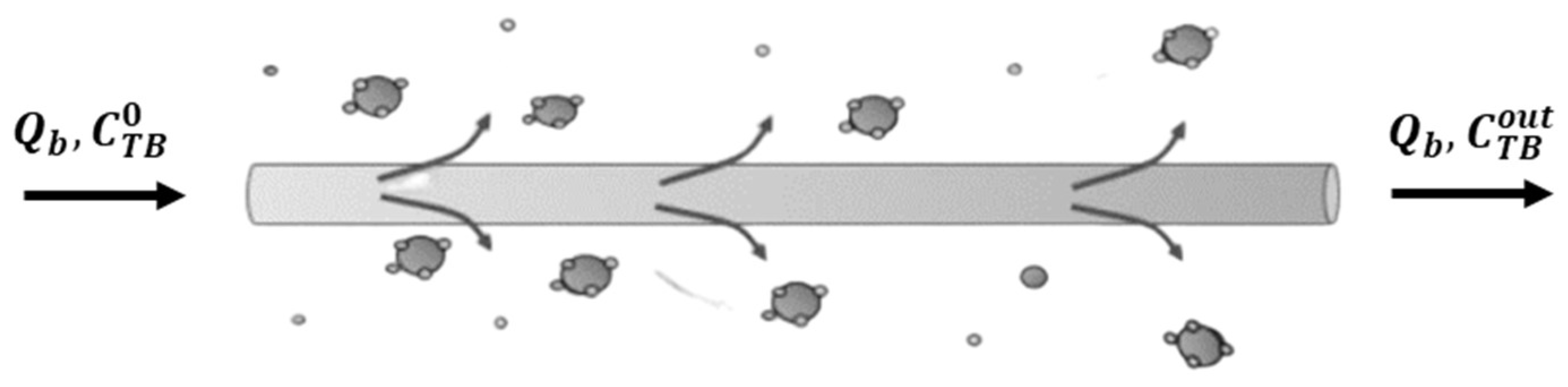
3.1. Model with Dialysis Fluid
- -
- Mass transfer across the membrane to the external compartment containing the dialysis fluid, which is assumed to be perfectly mixed;
- -
- Adsorption on silica microspheres.
3.2. Model without Dialysis Fluid
- -
- Mass transfer across the membrane;
- -
- Adsorbed-on-silica microspheres linked to the membrane surface.
4. Results and Discussion
- -
- Extended time for toxin removal from the blood;
- -
- Lower efficiency.
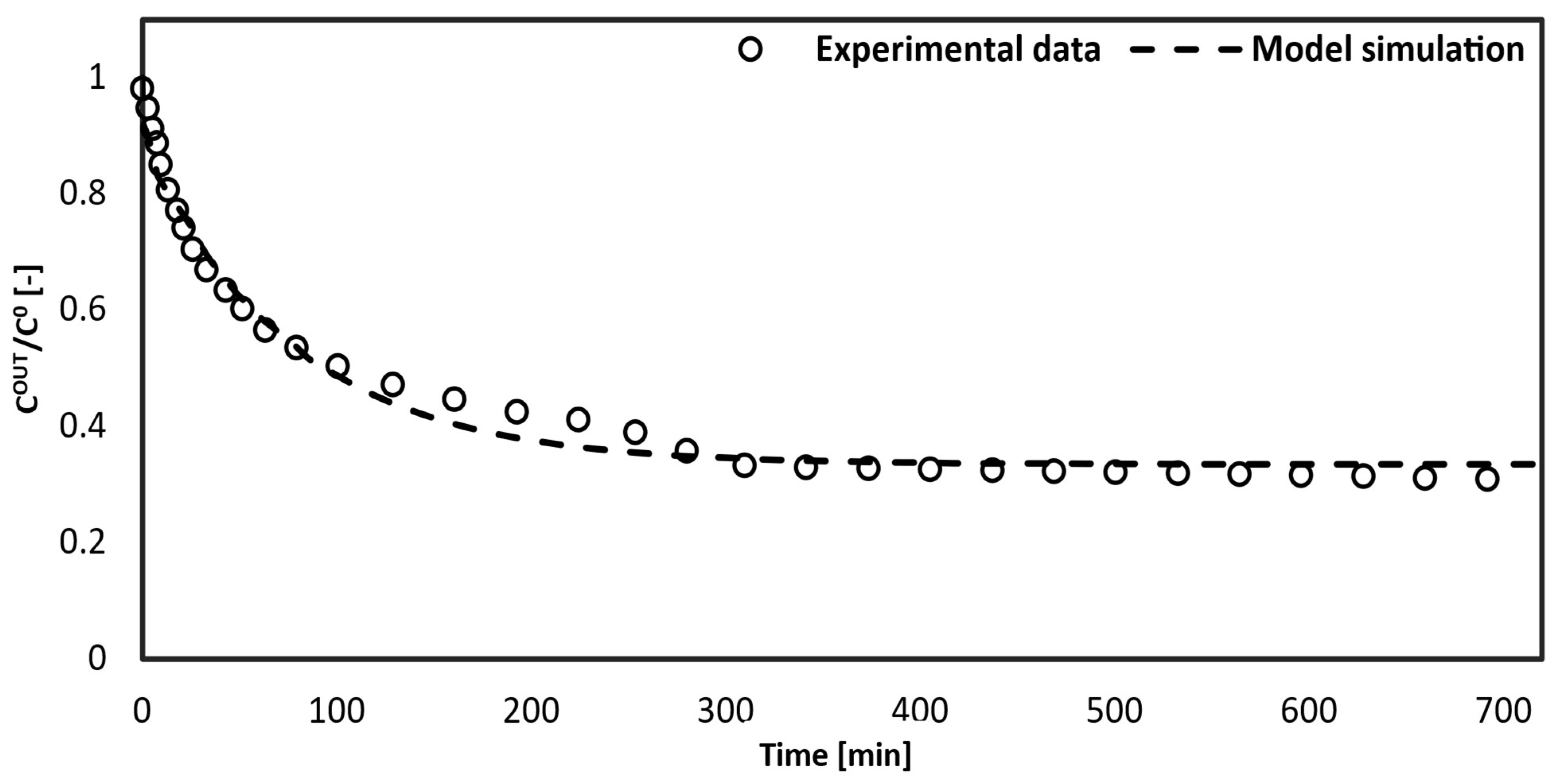
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Giancotti, A.; Monti, M.; Nevi, L.; Safarikia, S.; D’ambrosio, V.; Brunelli, R.; Pajno, C.; Corno, S.; Di Donato, V.; Musella, A.; et al. Functions and the Emerging Role of the Foetal Liver into Regenerative Medicine. Cells 2019, 8, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, A.; Annesini, M.C.; Piemonte, V.; Charcosset, C. Current Trends and Future Developments on (Bio-) Membranes: Membrane Applications in Artificial Organs and Tissue Engineering; Chapter 2; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Quaresima, S.; Mennini, G.; Manzia, T.M.; Avolio, A.W.; Angelico, R.; Spoletini, G.; Lai, Q. The liver transplant surgeon Mondays blues: An Italian perspective. Updat. Surg. 2022, 75, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.B.; Radosevich, D.M.; Bland, B.J.; Dunn, T.B.; Chinnakotla, S.; Sutherland, D.E.R.; Pruett, T.L.; Kandaswamy, R. Comparison of Recipient Outcomes Following Transplant From Local Versus Imported Pancreas Donors. Am. J. Transplant. 2012, 12, 447–457. [Google Scholar] [CrossRef]
- Caplan, A. Bioethics of Organ Transplantation. Cold Spring Harb. Perspect. Med. 2014, 4, a015685. [Google Scholar] [CrossRef] [Green Version]
- Tuerxun, K.; He, J.; Ibrahim, I.; Yusupu, Z.; Yasheng, A.; Xu, Q.; Tang, R.; Aikebaier, A.; Wu, Y.; Tuerdi, M.; et al. Bioartificial livers: A review of their design and manufacture. Biofabrication 2022, 14, 032003. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Cheng, J. Bioartificial liver support system: State of the art. J. Biomed. Eng. 2004, 21, 146–150. [Google Scholar]
- Dixit, V.; Gitnick, G. The bioartificial liver: State-of-the-art. Eur. J. Surg. Suppl. 1998, 164, 71–76. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Q.; Sun, Z.; Wang, C.; Cen, J.; Zhang, X.; Jin, Y.; Wu, B.; Yan, T.; Wang, Z.; et al. Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes. Cell Stem Cell 2023, 30, 617–631.e8. [Google Scholar] [CrossRef]
- Schmuck, R.B.; Nawrot, G.-H.; Fikatas, P.; Reutzel-Selke, A.; Pratschke, J.; Sauer, I.M. Single Pass Albumin Dialysis-A Dose-Finding Study to Define Optimal Albumin Concentration and Dialysate Flow. Artif. Organs 2017, 41, 153–161. [Google Scholar] [CrossRef]
- Muriel, P. Liver Pathophysiology, Chapter 2, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Demetriou, A.A. Hepatic assist devices. Panminerva Med. 2005, 47, 31–37. [Google Scholar]
- Tandon, R.; Froghi, S. Artificial liver support systems. J. Gastroenterol. Hepatol. 2021, 36, 1164–1179. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; David, C.; Marcu, A.; Olita, M.R.; Mihaila, M.; Tomescu, D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J. Clin. Med. 2023, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.J.G.; Bendjelid, K. Artificial liver support systems: What is new over the last decade? Ann. Intensiv. Care 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelli, S.; Annesini, M.; Morabito, V.; Cinti, P.; Pugliese, F.; Piemonte, V.; Turchetti, L.; Rossi, M.; Berloco, P. Cytokine Level Modifications: Molecular Adsorbent Recirculating System Versus Standard Medical Therapy. Transplant. Proc. 2009, 41, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Rossi, M.; Ferretti, G.; Pugliese, F.; Ruberto, F.; Lai, Q.; Novelli, S.; Piemonte, V.; Turchetti, L.; Morabito, V.; et al. Predictive Criteria for the Outcome of Patients with Acute Liver Failure Treated with the Albumin Dialysis Molecular Adsorbent Recirculating System. Ther. Apher. Dial. 2009, 13, 404–412. [Google Scholar] [CrossRef]
- Annesini, M.C.; Piemonte, V.; Turchetti, L. Artificial liver devices: A chemical engineering analysis. Asia-Pacific J. Chem. Eng. 2011, 6, 639–648. [Google Scholar] [CrossRef]
- Annesini, M.C.; Di Carlo, C.; Piemonte, V.; Turchetti, L. Bilirubin and Tryptophan Adsorption in Albumin-Containing Solutions: I. Equilibrium Isotherms on Activated Carbon. Biochem. Eng. J. 2008, 40, 205–210. [Google Scholar] [CrossRef]
- Stange, J.; Mitzner, S.R.; Risler, T.; Erley, C.M.; Lauchart, W.; Goehl, H.; Klammt, S.; Peszynski, P.; Freytag, J.; Hickstein, H.; et al. Molecular Adsorbent Recycling System (MARS): Clinical Results of a New Membrane-Based Blood Purification System for Bioartificial Liver Support. Artif. Organs 1999, 23, 319–330. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Jong, J.A.; Folkertsma, L.; Guo, Y.; Blüchel, C.; Verhaar, M.C.; Odijk, M.; Van Nostrum, C.F.; Hennink, W.E.; Gerritsen, K.G. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials 2020, 234, 119735. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Deng, X.; Zhang, Y.; Xiong, C. Development of Coconut Shell Activated Carbon-Tethered Urease for Degradation of Urea in a Packed Bed. ACS Sustain. Chem. Eng. 2014, 2, 433–439. [Google Scholar] [CrossRef]
- Davenport, A. A wearable dialysis device: The first step to continuous therapy. Nat. Rev. Nephrol. 2016, 12, 512–514. [Google Scholar] [CrossRef]
- Mendoza, J.M.; Arramreddy, R.; Schiller, B. Dialysate Sodium: Choosing the Optimal Hemodialysis Bath. Am. J. Kidney Dis. 2015, 66, 710–720. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Mihaila, S.M.; Jansen, J.; Wester, M.; Verhaar, M.C.; Joles, J.A.; Stamatialis, D.; Masereeuw, R.; Gerritsen, K.G.F. From portable dialysis to a bioengineered kidney. Expert Rev. Med. Devices 2018, 15, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Khawar, O.; McNamara, T.; Foster, S. In-vitro Dialysate Regeneration Using Dharma, the EasyDial Portable Hemodialysis Machine [abstract]. In ASN SA-PO758; Whiley: New York, NY, USA, 2017. [Google Scholar]
- Kim, J.H.; Kim, J.C.; Moon, J.-H.; Park, J.Y.; Lee, K.K.; Kang, E.; Kim, H.C.; Min, B.G.; Ronco, C. Development of a Cold Dialysate Regeneration System for Home Hemodialysis. Blood Purif. 2009, 28, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.-K.; Ishikawa, K.; Othman, R.; Yeoh, F.-Y. Nanoporous biomaterials for uremic toxin adsorption in artificial kidney systems: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.-S.; Zhang, H.-J.; Ke, J.-J.; Fu, Y.; Peng, Q.; Li, L.; Gao, Y.; Zhong, K.-B. A bioartificial transgenic porcine whole liver expressing human proteins alleviates acute liver failure in pigs. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Annesini, M.C.; Piemonte, V.; Turchetti, L. Adsorption of albumin-bound toxins on anion exchange resin: An equilibrium study. Asia-Pacific J. Chem. Eng. 2012, 7, 510–516. [Google Scholar] [CrossRef]
- Mazzeo, L.; Boscarino, T.; Bertino, A.; Falasconi, M.B.; Abruzzese, F.; Piemonte, V. Characterization of albumin-functionalized silica particles as novel solid adsorbent of bilirubin from albumin-containing solution. Chem. Eng. Trans. 2023; submitted. [Google Scholar]



| Technical Details | Value |
|---|---|
| Surface area | 1.4 |
| Length | 25 |
| Inner diameter hollow fiber | 180 |
| Wall thickness membrane | 35 |
| Number of fibers | 15,000 |
| Blood flow rate | 200–500 |
| Dialysate flow | 300–800 |
| Sieving coefficient, Albumin | <0.01 |
| Model Parameter | Description | Value (a) | Value (b) |
|---|---|---|---|
| L | Module length | 3.5–5 cm | 3.5–5 cm |
| Fiber number | |||
| Total concentration of blood toxin | |||
| Concentration of blood albumin | |||
| Concentration of dialysate albumin | - | ||
| Adsorbent particle mass | |||
| Dialysate volume | |||
| Blood flow rate | |||
| Particle solid density | |||
| Time |
| Module Parameter | Description | Value | Reference |
|---|---|---|---|
| Solid exchange coefficient | |||
| Equilibrium ratio | [32] | ||
| Global exchange coefficient |
| Module Parameter | Description | Value |
|---|---|---|
| Equilibrium ratio | ||
| Global exchange coefficient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscarino, T.; Mazzeo, L.; Abbruzzese, F.; Merone, M.; Piemonte, V. Modeling and Validation of an Ultra-Compact Regenerative Liver Dialysis Device. Bioengineering 2023, 10, 706. https://doi.org/10.3390/bioengineering10060706
Boscarino T, Mazzeo L, Abbruzzese F, Merone M, Piemonte V. Modeling and Validation of an Ultra-Compact Regenerative Liver Dialysis Device. Bioengineering. 2023; 10(6):706. https://doi.org/10.3390/bioengineering10060706
Chicago/Turabian StyleBoscarino, Tamara, Leone Mazzeo, Franca Abbruzzese, Mario Merone, and Vincenzo Piemonte. 2023. "Modeling and Validation of an Ultra-Compact Regenerative Liver Dialysis Device" Bioengineering 10, no. 6: 706. https://doi.org/10.3390/bioengineering10060706
APA StyleBoscarino, T., Mazzeo, L., Abbruzzese, F., Merone, M., & Piemonte, V. (2023). Modeling and Validation of an Ultra-Compact Regenerative Liver Dialysis Device. Bioengineering, 10(6), 706. https://doi.org/10.3390/bioengineering10060706










