Feasibility Study of 3D FACT and IVIM Sequences in the Evaluation of Female Osteoporosis
Abstract
1. Introduction
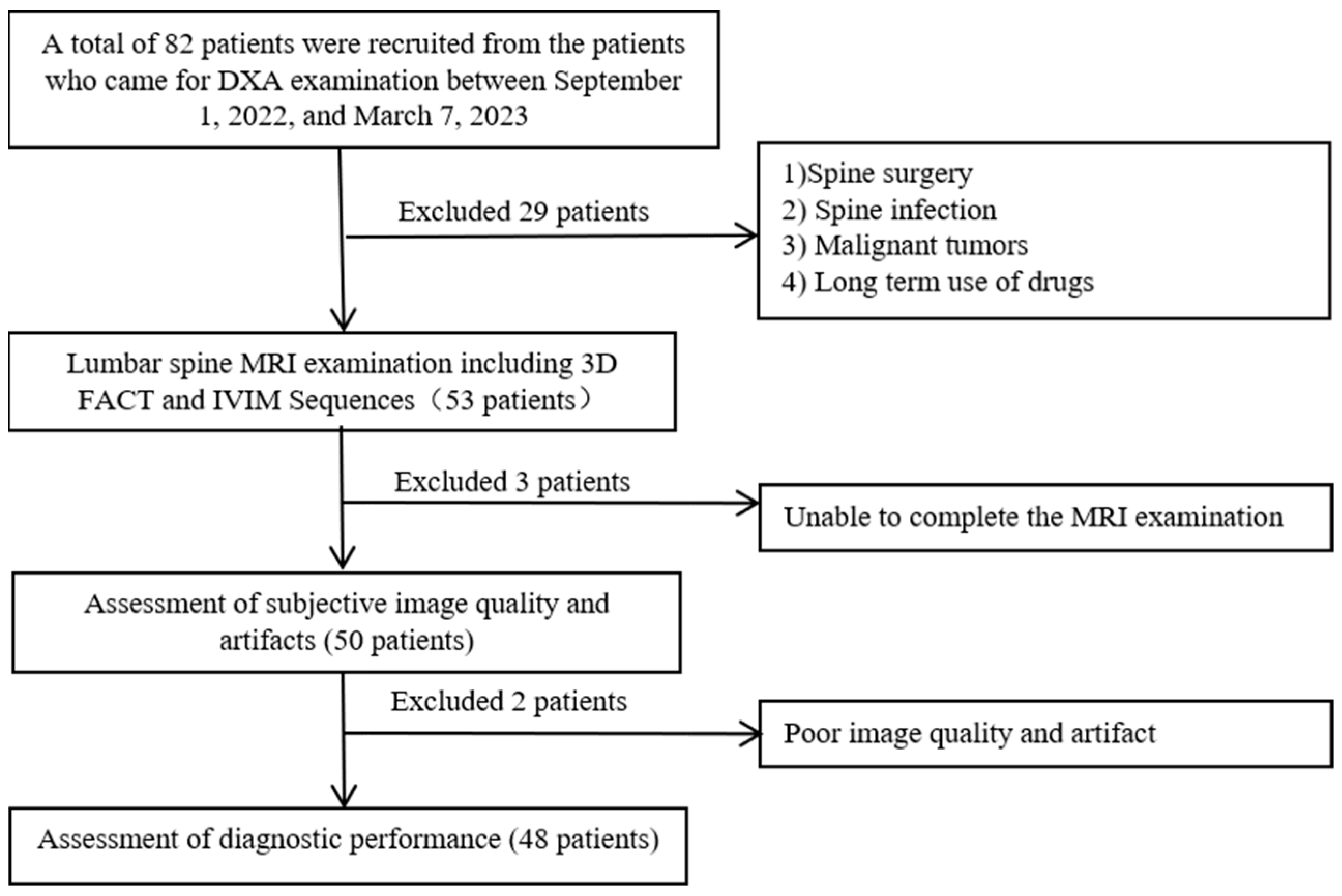
2. Materials and Methods
2.1. Study Population
2.2. BMD Examination
2.3. FRAX Evaluation
2.4. MR Examination
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
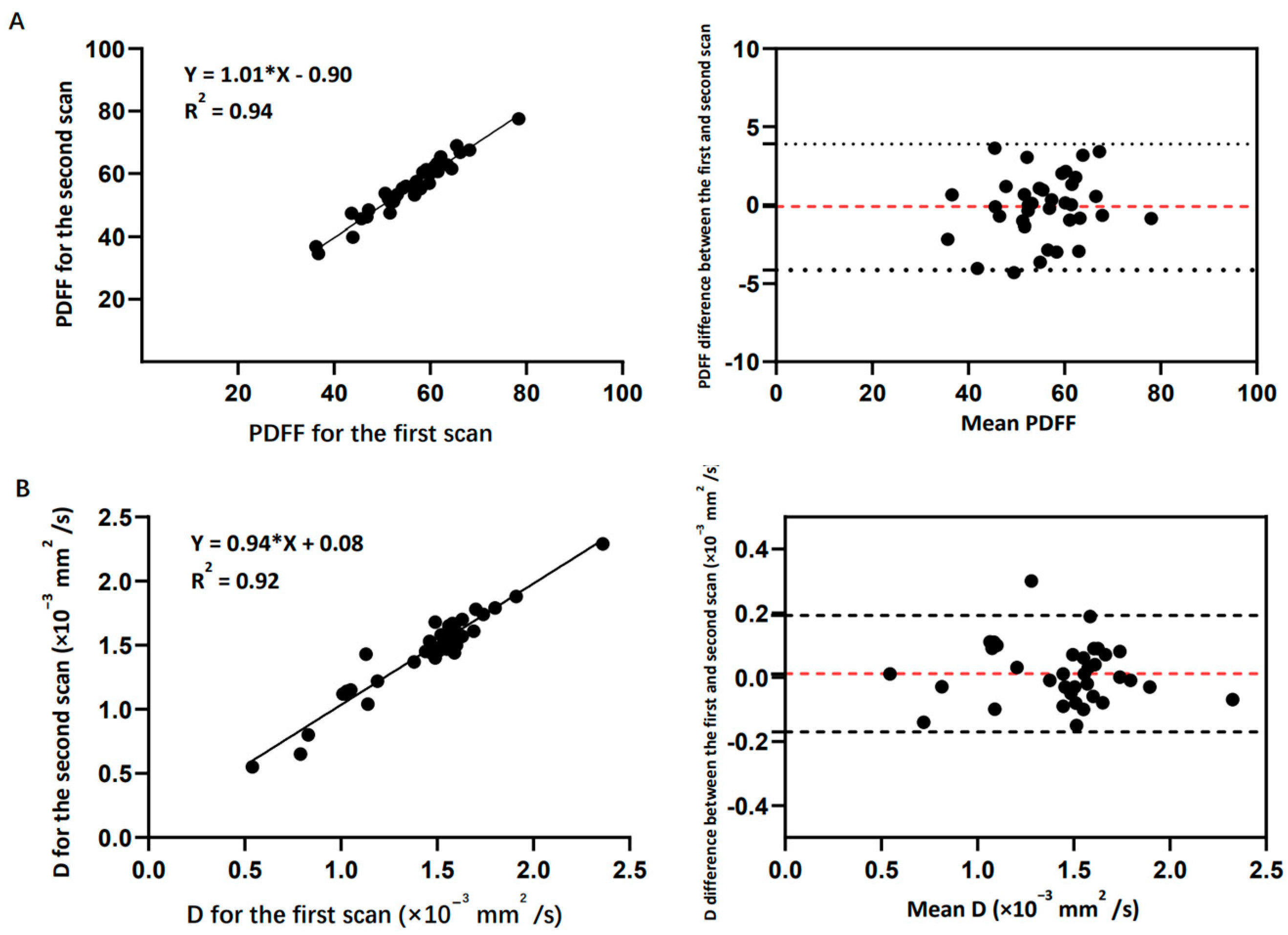
3.1. Reproducibility
3.2. Image Quality Assessment
3.3. Correlations and ROC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litwic, A.; Cooper, C.; Dennison, E. Osteoporosis therapies in 2014. Panminerva Med. 2014, 56, 273–283. [Google Scholar] [PubMed]
- Gallagher, J.C. Effect of early menopause on bone mineral density and fractures. Menopause-J. N. Am. Menopause Soc. 2007, 14, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Griffith, J.F.; Genant, H.K. New advances in imaging osteoporosis and its complications. Endocrine 2012, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Solomou, G.; Damilakis, J. Radiation Exposure in Bone Densitometry. Semin. Musculoskelet. Radiol. 2016, 20, 392–398. [Google Scholar] [CrossRef]
- Schmeel, F.C.; Luetkens, J.A.; Wagenhäuser, P.J.; Meier-Schroers, M.; Kuetting, D.L.; Feißt, A.; Gieseke, J.; Schmeel, L.C.; Träber, F.; Schild, H.H.; et al. Proton density fat fraction (PDFF) MRI for differentiation of benign and malignant vertebral lesions. Eur. Radiol. 2018, 28, 2397–2405. [Google Scholar] [CrossRef]
- Cho, G.Y.; Moy, L.; Kim, S.G.; Baete, S.H.; Moccaldi, M.; Babb, J.S.; Sodickson, D.K.; Sigmund, E.E. Sigmund, Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: Comparison with malignant status, histological subtype, and molecular prognostic factors. Eur. Radiol. 2016, 26, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Zebaze, R.; Osima, M.; Bui, M.; Lukic, M.; Wang, X.; Ghasem-Zadeh, A.; Eriksen, E.F.; Vais, A.; Shore-Lorenti, C.; Ebeling, P.R.; et al. Adding Marrow Adiposity and Cortical Porosity to Femoral Neck Areal Bone Mineral Density Improves the Discrimination of Women with Nonvertebral Fractures from Controls. J. Bone Miner. Res. 2019, 34, 1451–1460. [Google Scholar] [CrossRef]
- Margulies, J.Y.; Payzer, A.; Nyska, M.; Neuwirth, M.G.; Floman, Y.; Robin, G.C. The relationship between degenerative changes and osteoporosis in the lumbar spine. Clin. Orthop. Relat. Res. 1996, 324, 145–152. [Google Scholar] [CrossRef]
- Engelke, K.; Adams, J.E.; Armbrecht, G.; Augat, P.; Bogado, C.E.; Bouxsein, M.L.; Felsenberg, D.; Ito, M.; Prevrhal, S.; Hans, D.B.; et al. Clinical Use of Quantitative Computed Tomography and Peripheral Quantitative Computed Tomography in the Management of Osteoporosis in Adults: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 123–162. [Google Scholar] [CrossRef]
- Jung, J.Y.; Yoon, Y.C.; Kim, H.R.; Choe, B.K.; Wang, J.H.; Jung, J.Y. Knee derangements: Comparison of isotropic 3D fast spin-echo, isotropic 3D balanced fast field-echo, and conventional 2D fast spin-echo MR imaging. Radiology 2013, 268, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Lebihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Lavaljeantet, M. Separation of diffusion and perfusion in introvoexl incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Ma, X.W.; Jiang, Y.H.; Huang, D.G.; Chen, X.J.; Zhang, M.; Hao, D.J. Percentage fat fraction in magnetic resonance imaging: Upgrading the osteoporosis-detecting parameter. BMC Med. Imaging 2020, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Zhang, X.; Mei, Y.; Yi, P.; Wang, Y.; Feng, Q.; Tegola, L.L.; Guglielmi, G.; Zhang, X.; et al. Magnetic Susceptibility and Fat Content in the Lumbar Spine of Postmenopausal Women with Varying Bone Mineral Density. J. Magn. Reson. Imaging 2019, 49, 1020–1028. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Patiman Liu, B.B.; Zhang, R.; Ma, X.F.; Guo, H. Correlation between intervertebral disc degeneration and bone mineral density difference: A retrospective study of postmenopausal women using an eight-level MRI-based disc degeneration grading system. BMC Musculoskelet. Disord. 2022, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, Y.; Morio, Y.; Nagashima, H.; Hagino, H.; Teshima, R. Correlation between bone mineral density and intervertebral disk degeneration in pre- and postmenopausal women. J. Bone Miner. Metab. 2003, 21, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, K.; Zhang, Y.; Wang, L.; Xu, L.; Liu, Y.; Duanmu, Y.; Chen, D.; Tian, W.; Blake, G.M. The accurate relationship between spine bone density and bone marrow in humans. Bone 2020, 134, 115312. [Google Scholar] [CrossRef]
- Pan, J.; Lu, X.; Yang, G.; Han, Y.; Tong, X.; Wang, Y. Lumbar disc degeneration was not related to spine and hip bone mineral densities in Chinese: Facet joint osteoarthritis may confound the association. Arch. Osteoporos. 2017, 12, 118–122. [Google Scholar] [CrossRef]
- Homminga, J.; Aquarius, R.; Bulsink, V.E.; Jansen, C.T.; Verdonschot, N. Can vertebral density changes be explained by intervertebral disc degeneration? Med. Eng. Phys. 2012, 34, 453–458. [Google Scholar] [CrossRef]
- Xiao, Z.-F.; He, J.-B.; Su, G.-Y.; Chen, M.-H.; Hou, Y.; Chen, S.-D.; Lin, D.-K. Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res. Ther. 2018, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, Y.; Ketschau, J.; Ruschke, S.; Gassert, F.T.; Glanz, L.; Feuerriegel, G.C.; Gassert, F.G.; Baum, T.; Kirschke, J.S.; Braren, R.F.; et al. Associations of incidental vertebral fractures and longitudinal changes of MR-based proton density fat fraction and T2*measurements of vertebral bone marrow. Front. Endocrinol. 2022, 13, 1046547. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.-P.; Hernando, D.; Meffert, P.J.; Reeder, S.; Hosten, N.; Laqua, R.; Steveling, A.; Ender, S.; Schröder, H.; Pillich, D.-T. Proton-density fat fraction and simultaneous R2*estimation as an MRI tool for assessment of osteoporosis. Eur. Radiol. 2013, 23, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Greve, T.; Rayudu, N.M.; Dieckmeyer, M.; Boehm, C.; Ruschke, S.; Burian, E.; Kloth, C.; Kirschke, J.S.; Karampinos, D.C.; Baum, T.; et al. Finite Element Analysis of Osteoporotic and Osteoblastic Vertebrae and Its Association With the Proton Density Fat Fraction From Chemical Shift Encoding-Based Water-Fat MRI—A Preliminary Study. Front. Endocrinol. 2022, 13, 900356. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Sigurdsson, S.; Hue, T.F.; Lang, T.F.; Harris, T.B.; Rosen, C.J.; Vittinghoff, E.; Siggeirsdottir, K.; Sigurdsson, G.; Oskarsdottir, D.; et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J. Clin. Endocrinol. Metab. 2013, 98, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Li, X.; Baum, T.; Yap, S.P.; Karampinos, D.C.; Schwartz, A.V.; Link, T.M. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J. Bone Min. Res. 2013, 28, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Fazeli, P.K.; Miller, K.K.; Misra, M.; Torriani, M.; Thomas, B.J.; Ghomi, R.H.; Rosen, C.J.; Klibanski, A. Increased bone marrow fat in anorexia nervosa. J. Clin. Endocrinol. Metab. 2009, 94, 2129–2136. [Google Scholar] [CrossRef]
- Yang, I.Y.; Cui, Y.F.; Wiens, C.N.; Wade, T.P.; Friesen-Waldner, L.J.; McKenzie, C.A. Fat Fraction Bias Correction Using T-1 Estimates and Flip Angle Mapping. J. Magn. Reson. Imaging 2014, 39, 217–223. [Google Scholar] [CrossRef]
- Karampinos, D.C.; Ruschke, S.; Dieckmeyer, M.; Eggers, H.; Kooijman, H.; Rummeny, E.J.; Bauer, J.S.; Baum, T. Modeling of T-2* decay in vertebral bone marrow fat quantification. Nmr. Biomed. 2015, 28, 1535–1542. [Google Scholar] [CrossRef]
- Peng, H.; Zou, C.; Cheng, C.; Tie, C.; Qiao, Y.; Wan, Q.; Lv, J.; He, Q.; Liang, D.; Liu, X. Fat-water separation based on Transition REgion Extraction (TREE). Magn. Reson. Med. 2019, 82, 436–448. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, C.; Liang, C.; Liu, X.; Zheng, H. Fat-Water Separation Using a Region-Growing Algorithm with Self-Feeding Phasor Estimation. Magn. Reson. Med. 2017, 77, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Cho, J.H.; Lee, H.K.; Lee, S.J.; Park, C.S.; Dong, K.R.; Park, Y.S.; Chung, W.K.; Lee, J.W.; Kim, H.S.; et al. Correlations between the MR Diffusion-weighted Image (DWI) and the bone mineral density (BMD) as a function of the soft tissue thickness-focus on phantom and patient. J. Korean Phys. Soc. 2013, 62, 684–694. [Google Scholar] [CrossRef]
- Koyama, H.; Yoshihara, H.; Kotera, M.; Tamura, T.; Sugimura, K. The quantitative diagnostic capability of routine MR imaging and diffusion-weighted imaging in osteoporosis patients. Clin. Imaging 2013, 37, 925–929. [Google Scholar] [CrossRef]
- Turner, R.; Lebihan, D.; Maier, J.; Vavrek, R.; Hedges, L.K.; Pekar, J. Echo-planer imaging of intravoxel incoherent motion. Radiology 1990, 177, 407–414. [Google Scholar] [CrossRef]
- Yamada, I.; Aung, W.; Himeno, Y.; Nakagawa, T.; Shibuya, H. Diffusion coefficients in abdominal organs and hepatic lesions: Evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 1999, 210, 617–623. [Google Scholar] [CrossRef] [PubMed]




| All Subjects (n = 48) | Normal (n = 14) | Osteopenia (n = 15) | Osteoporosis (n = 19) | p | |
|---|---|---|---|---|---|
| Ages (years) | 55.9 ± 15.2 | 46.4 ± 12.7 | 58 ± 16.2 | 66.5 ± 8.3 | <0.05 * |
| BMI (kg/m2) | 23.7 ± 3.5 | 23.8 ± 3 | 23.3 ± 3.4 | 24 ± 4.5 | 0.860 |
| BMD (mg/cm2) | 918 ± 148 | 1062 ± 69.1 | 877 ± 62.5 | 766.8 ± 98.1 | <0.05 * |
| PDFF (%) | 50.4 ± 12.5 | 41.7 ± 11.1 | 55.3 ± 12.6 | 56.8 ± 6.7 | <0.05 * |
| D (×10−3 mm2/s) | 1.4 ± 0.3 | 1.6 ± 0.2 | 1.5 ± 0.58 | 1.2 ± 0.2 | <0.05 * |
| FRAX score (%) | 3.6 ± 2.8 | 2.6 ± 2.2 | 3.8 ± 2.6 | 5.4 ± 2.8 | <0.05 * |
| Subjective Image Quality | 3D FACT (Reader1/2) | IVIM (Reader1/2) |
|---|---|---|
| Grade 1 | 0/0 | 1/1 |
| Grade 2 | 2/2 | 0/0 |
| Grade 3 | 45/39 | 90/105 |
| Grade 4 | 128/136 | 68/48 |
| Artifacts | ||
| Grade 1 | 0/0 | 1/1 |
| Grade 2 | 2/2 | 0/0 |
| Grade 3 | 93/66 | 108/120 |
| Grade 4 | 64/100 | 44/28 |
| Parameters | BMD | FRAX |
|---|---|---|
| PDFF | ||
| R | −0.393 ** | 0.706 ** |
| p | 0.005 | <0.001 |
| D | ||
| R | 0.321 * | −0.334 * |
| p | 0.024 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Guo, Q.; Yang, Y.; Feng, H.; Zhao, Y.; Guo, P.; Li, D.; Du, X.; Song, Q. Feasibility Study of 3D FACT and IVIM Sequences in the Evaluation of Female Osteoporosis. Bioengineering 2023, 10, 710. https://doi.org/10.3390/bioengineering10060710
Zhang S, Guo Q, Yang Y, Feng H, Zhao Y, Guo P, Li D, Du X, Song Q. Feasibility Study of 3D FACT and IVIM Sequences in the Evaluation of Female Osteoporosis. Bioengineering. 2023; 10(6):710. https://doi.org/10.3390/bioengineering10060710
Chicago/Turabian StyleZhang, Shuo, Qianrui Guo, Yang Yang, Hongbo Feng, Yan Zhao, Peng Guo, Di Li, Xuemei Du, and Qingwei Song. 2023. "Feasibility Study of 3D FACT and IVIM Sequences in the Evaluation of Female Osteoporosis" Bioengineering 10, no. 6: 710. https://doi.org/10.3390/bioengineering10060710
APA StyleZhang, S., Guo, Q., Yang, Y., Feng, H., Zhao, Y., Guo, P., Li, D., Du, X., & Song, Q. (2023). Feasibility Study of 3D FACT and IVIM Sequences in the Evaluation of Female Osteoporosis. Bioengineering, 10(6), 710. https://doi.org/10.3390/bioengineering10060710









