Intervertebral Disc Progenitors: Lessons Learned from Single-Cell RNA Sequencing and the Role in Intervertebral Disc Regeneration
Abstract
1. Introduction
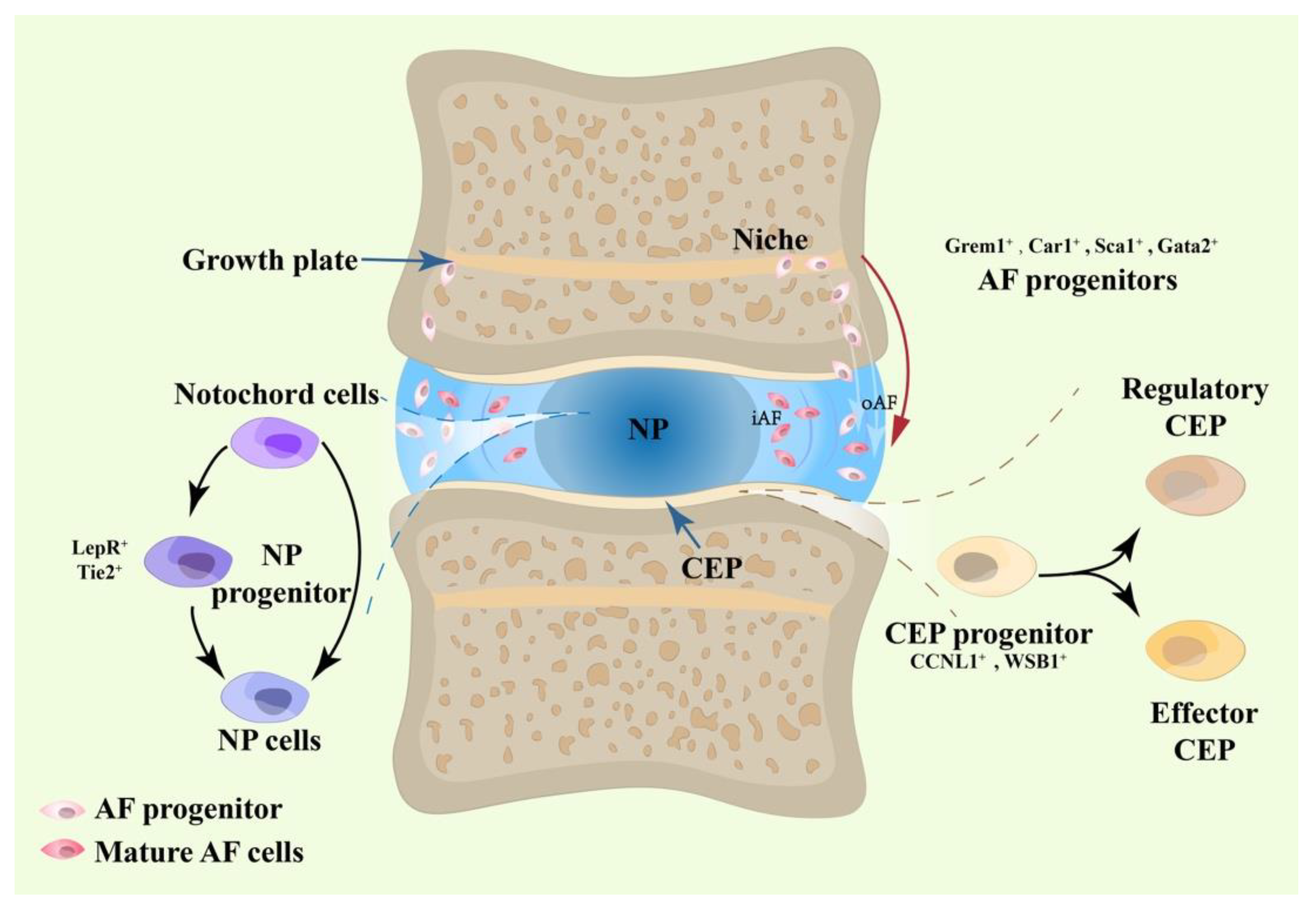
2. The Heterogeneity of IVD Cells: Evidence from Single-Cell RNA Sequencing
2.1. NP Progenitors
2.2. AF Progenitors
2.3. CEP Progenitors
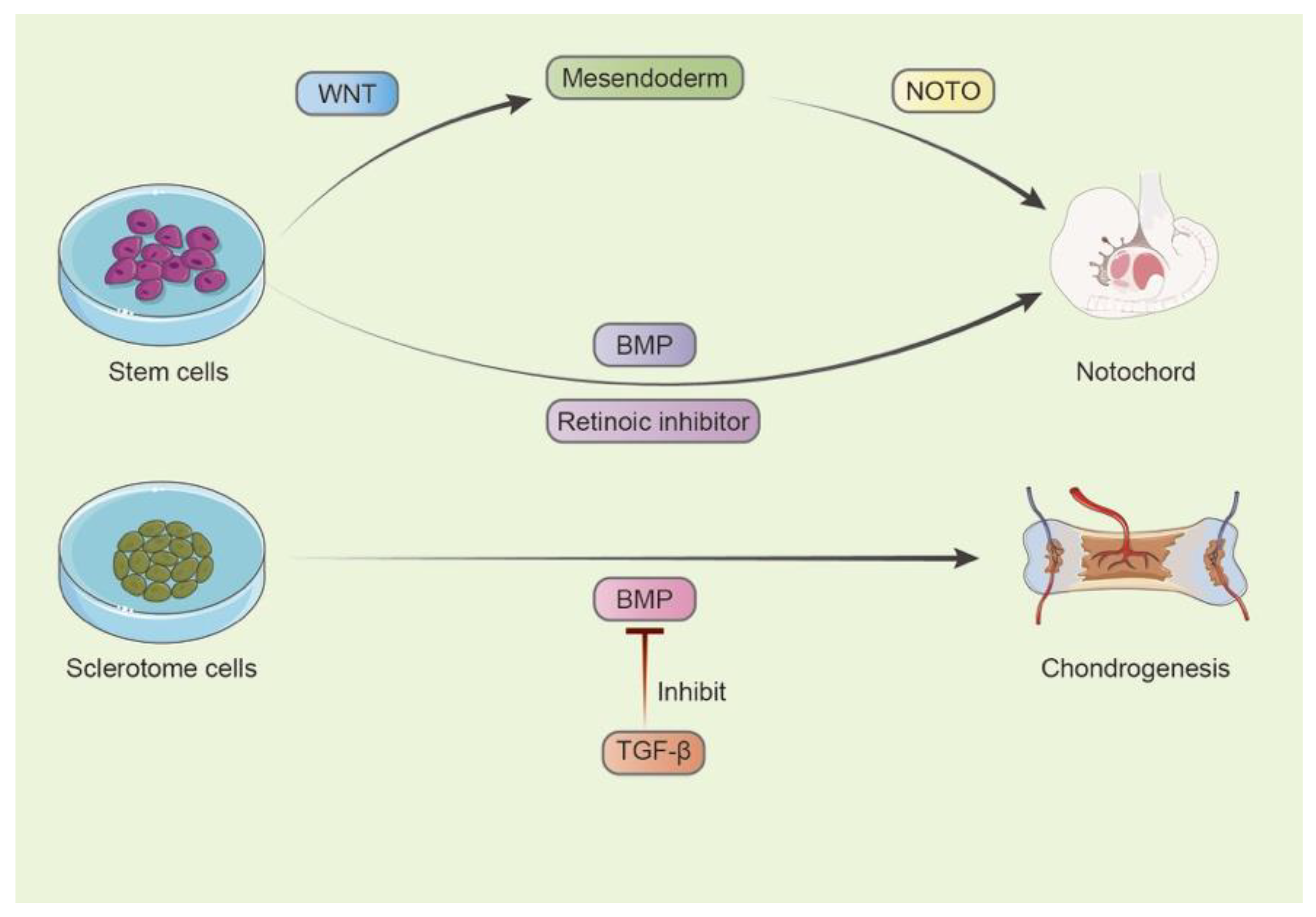
3. Key Pathways in the Early Development of IVD Progenitors
4. Characteristic of Disc Progenitor Cells Compared to Traditional MSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Jt. Surg. 2006, 88 (Suppl. S2), 21–24. [Google Scholar] [CrossRef]
- Freemont, A.J.; Peacock, T.E.; Goupille, P.; Hoyland, J.A.; O’Brien, J.; Jayson, M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Kepler, C.K.; Markova, D.Z.; Dibra, F.; Yadla, S.; Vaccaro, A.R.; Risbud, M.V.; Albert, T.J.; Anderson, D.G. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1β in painful human intervertebral discs. Spine 2013, 38, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Williams, N.H.; Sutton, A.J.; Burton, K.; Din, N.U.; Matar, H.E.; Hendry, M.; Phillips, C.J.; Nafees, S.; Fitzsimmons, D.; et al. Comparative clinical effectiveness of management strategies for sciatica: Systematic review and network meta-analyses. Spine J. 2015, 15, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.C.; Cho, S.K.; Giannarelli, C.; Iatridis, J.C.; Purmessur, D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthr. Cartil. 2015, 23, 487–496. [Google Scholar] [CrossRef]
- Brand-Saberi, B.; Christ, B. Evolution and development of distinct cell lineages derived from somites. Curr. Top. Dev. Biol. 2000, 48, 1–42. [Google Scholar]
- Liu, S.; Liang, H.; Lee, S.M.; Li, Z.; Zhang, J.; Fei, Q. Isolation and identification of stem cells from degenerated human inter-vertebral discs and their migration characteristics. Acta Biochim. Biophys. Sin. 2017, 49, 101–109. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Zhao, Y.; Wang, D.; Shi, Q.; Ding, Z.; Wang, Y.; Gao, B.; Yan, M. Revealing the Key MSCs Niches and Pathogenic Genes in Influencing CEP Homeostasis: A Conjoint Analysis of Single-Cell and WGCNA. Front. Immunol. 2022, 13, 933721. [Google Scholar] [CrossRef]
- Sakai, D.; Andersson, G.B. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Lyu, F.J.; Cheung, K.M.; Zheng, Z.; Wang, H.; Sakai, D.; Leung, V.Y. IVD progenitor cells: A new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 2019, 15, 102–112. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J.; Bach, F.C.; Tekari, A.; Sagawa, N.; Nakamura, Y.; Chan, S.C.W.; Nakai, T.; Creemers, L.B.; Frauchiger, D.A.; et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR Spine 2018, 1, e1018. [Google Scholar] [CrossRef]
- Bach, F.C.; Zhang, Y.; Miranda-Bedate, A.; Verdonschot, L.C.; Bergknut, N.; Creemers, L.B.; Ito, K.; Sakai, D.; Chan, D.; Meij, B.P.; et al. Increased caveolin-1 in intervertebral disc degeneration facilitates repair. Arthritis Res. Ther. 2016, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Ma, K.; He, R.; Xiong, L.; Hu, Y.; Deng, X.; Yang, A.; Ma, X.; Shao, Z. Comparison of different methods for the isolation and purification of rat nucleus pulposus-derived mesenchymal stem cells. Connect. Tissue Res. 2020, 61, 426–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Li, Y.; Xu, H. Mechanism of the Mitogen-Activated Protein Kinases/Mammalian Target of Rapamycin Pathway in the Process of Cartilage Endplate Stem Cell Degeneration Induced by Tension Load. Glob. Spine J. 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Sun, C.; Zou, F.; Wang, H.; Lu, F.; Song, J.; Liu, S.; Xia, X.; Jiang, J.; Ma, X. Carbohydrate sulfotransferase 3 (CHST3) overexpression promotes cartilage endplate-derived stem cells (CESCs) to regulate molecular mechanisms related to repair of intervertebral disc degeneration by rat nucleus pulposus. J. Cell. Mol. Med. 2021, 25, 6006–6017. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhang, W.; Zhou, P.; Yuan, Z.; Zhu, C.; Wang, H.; Li, J.; Zhou, F.; Yang, Q.; Yang, H.; et al. Substrate Topography Regulates Differentiation of Annulus Fibrosus-Derived Stem Cells via CAV1-YAP-Mediated Mecha-notransduction. ACS Biomater. Sci. Eng. 2021, 7, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, D.; Luo, B.; Wang, D.; Jia, H.; Peng, P.; Shang, Q.; Mao, J.; Gao, C.; Peng, Y.; et al. Decoding the annulus fibrosus cell atlas by scRNA-seq to develop an inducible composite hydrogel: A novel strategy for disc reconstruction. Bioact. Mater. 2022, 14, 350–363. [Google Scholar] [CrossRef]
- Jiang, W.; Glaeser, J.D.; Salehi, K.; Kaneda, G.; Mathkar, P.; Wagner, A.; Ho, R.; Sheyn, D. Single-cell atlas unveils cellular heterogeneity and novel markers in human neonatal and adult intervertebral discs. iScience 2022, 25, 104504. [Google Scholar] [CrossRef]
- Panebianco, C.J.; Dave, A.; Charytonowicz, D.; Sebra, R.; Iatridis, J.C. Single-cell RNA-sequencing atlas of bovine caudal in-tervertebral discs: Discovery of heterogeneous cell populations with distinct roles in homeostasis. FASEB J. 2021, 35, e21919. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, Y.; Wang, Z.; Zhang, Z.; Chen, B.; Yang, J.; Zeng, B.; Gao, Y.; Jiang, C.; Huang, Y.; et al. Single-Cell RNA-Seq Analysis Reveals Macrophage Involved in the Progression of Human Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2021, 9, 833420. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, B.; Xing, W.; Xie, Z.; Luo, Z.; Zou, W. Discovery and Application of Postnatal Nucleus Pulposus Progenitors Essential for Intervertebral Disc Homeostasis and Degeneration. Adv. Sci. 2022, 9, e2104888. [Google Scholar] [CrossRef]
- Gan, Y.; He, J.; Zhu, J.; Xu, Z.; Wang, Z.; Yan, J.; Hu, O.; Bai, Z.; Chen, L.; Xie, Y.; et al. Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus pro-genitors in human intervertebral discs. Bone Res. 2021, 9, 37. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Huang, L.; Shi, K.; Wang, J.; Zhu, C.; Li, L.; Zhang, L.; Feng, G.; Liu, L.; et al. Novel biomarkers of intervertebral disc cells and evidence of stem cells in the intervertebral disc. Osteoarthr. Cartil. 2021, 29, 389–401. [Google Scholar] [CrossRef]
- Calió, M.; Gantenbein, B.; Egli, M.; Poveda, L.; Ille, F. The Cellular Composition of Bovine Coccygeal Intervertebral Discs: A Comprehensive Single-Cell RNAseq Analysis. Int. J. Mol. Sci. 2021, 22, 4917. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yin, J.; Xu, X.; Fan, J.; Wang, D.; Zheng, C.; Lu, W.; Cheng, P.; Sun, J.; Wang, D.; et al. Leptin receptor-expressing cells represent a distinct subpopulation of notochord-derived cells and are essential for disc ho-moeostasis. J. Orthop. Transl. 2020, 21, 91–99. [Google Scholar]
- Li, X.; Yang, S.; Qin, L.; Yang, S. Type II collagen-positive embryonic progenitors are the major contributors to spine and in-tervertebral disc development and repair. Stem Cells Transl. Med. 2021, 10, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, H.; Zhang, W.; Mao, H.; Li, B. Single-cell RNA sequencing reveals resident progenitor and vasculariza-tion-associated cell subpopulations in rat annulus fibrosus. J. Orthop. Transl. 2023, 38, 256–267. [Google Scholar]
- Luo, L.; Jian, X.; Sun, H.; Qin, J.; Wang, Y.; Zhang, J.; Shen, Z.; Yang, D.; Li, C.; Zhao, P.; et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells 2021, 39, 467–481. [Google Scholar] [CrossRef]
- He, Z.; Jia, M.; Yu, Y.; Yuan, C.; Wang, J. Roles of SDF-1/CXCR4 axis in cartilage endplate stem cells mediated promotion of nucleus pulposus cells proliferation. Biochem. Biophys. Res. Commun. 2018, 506, 94–101. [Google Scholar] [CrossRef]
- Luo, L.; Gong, J.; Wang, Z.; Liu, Y.; Cao, J.; Qin, J.; Zuo, R.; Zhang, H.; Wang, S.; Zhao, P.; et al. Injectable cartilage matrix hydrogel loaded with cartilage endplate stem cells engineered to release exosomes for non-invasive treatment of intervertebral disc degeneration. Bioact. Mater. 2022, 15, 29–43. [Google Scholar] [CrossRef]
- Colombier, P.; Halgand, B.; Chédeville, C.; Chariau, C.; François-Campion, V.; Kilens, S.; Vedrenne, N.; Clouet, J.; David, L.; Guicheux, J.; et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells 2020, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Urban, J.P.; Luk, K.D. Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol. 2014, 10, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hernandez, M.E.; Khan, N.M.; Trochez, C.M.; Yoon, T.; Maye, P.; Presciutti, S.M.; Gibson, G.; Drissi, H. Derivation of notochordal cells from human embryonic stem cells reveals unique regulatory networks by single cell-transcriptomics. J. Cell. Physiol. 2020, 235, 5241–5255. [Google Scholar] [CrossRef]
- Ban, G.I.; Williams, S.; Serra, R. Antagonism of BMP signaling is insufficient to induce fibrous differentiation in primary scle-rotome. Exp. Cell Res. 2019, 378, 11–20. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the Osteogenic Potentials and Bone Regeneration Ca-pacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef]
- Frauchiger, D.A.; Heeb, S.R.; May, R.D.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Differentiation of MSC and annulus fi-brosus cells on genetically engineered silk fleece-membrane-composites enriched for GDF-6 or TGF-β3. J. Orthop. Res. 2018, 36, 1324–1333. [Google Scholar] [CrossRef]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus re-construction. Bioact. Mater. 2021, 6, 179–190. [Google Scholar] [CrossRef]
- Steffen, F.; Bertolo, A.; Affentranger, R.; Ferguson, S.J.; Stoyanov, J. Treatment of Naturally Degenerated Canine Lumbosacral Intervertebral Discs with Autologous Mesenchymal Stromal Cells and Collagen Microcarriers: A Prospective Clinical Study. Cell Transplant. 2019, 28, 201–211. [Google Scholar] [CrossRef]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, Z.; Dang, M.; Rambhia, K.J.; Ma, P.X. Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification. Biomaterials 2020, 256, 120213. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, X.; Cao, F.; Zhang, X.; Wu, J. Bone Mesenchymal Stem Cells Promote Extracellular Matrix Remodeling of Degenerated Nucleus Pulposus Cells via the miR-101-3p/EIF4G2 Axis. Front. Bioeng. Biotechnol. 2021, 9, 642502. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.P.; Jakub, G.; Harasymczuk, J.; Jagodziński, P.P. Transforming growth factor β mediates communication of co-cultured human nucleus pulposus cells and mesenchymal stem cells. J. Orthop. Res. 2018, 36, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.R.; Ma, H.L.; Wang, J.P.; Chang, M.C.; Liu, C.L.; Chen, T.H.; Hung, S.C. Use of Allogeneic Hypoxic Mesenchymal Stem Cells for Treating Disc Degeneration in Rabbits. J. Orthop. Res. 2019, 37, 1440–1450. [Google Scholar] [CrossRef]
- Omlor, G.W.; Lorenz, S.; Nerlich, A.G.; Guehring, T.; Richter, W. Disc cell therapy with bone-marrow-derived autologous mesenchymal stromal cells in a large porcine disc degeneration model. Eur. Spine J. 2018, 27, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Ukeba, D.; Yamada, K.; Suyama, T.; Lebl, D.R.; Tsujimoto, T.; Nonoyama, T.; Sugino, H.; Iwasaki, N.; Watanabe, M.; Matsuzaki, Y.; et al. Combination of ultra-purified stem cells with an in situ-forming bioresorbable gel enhances intervertebral disc regeneration. EBioMedicine 2022, 76, 103845. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Deng, G.; Ma, J.; Huang, X.; Yu, J.; Xi, Y.; Ye, X. Transplantation of Hypoxic-Preconditioned Bone Mesenchymal Stem Cells Retards Intervertebral Disc Degeneration via En-hancing Implanted Cell Survival and Migration in Rats. Stem Cells Int. 2018, 2018, 7564159. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Tang, X.; Feng, R.; Yao, G.; Chen, W.; Li, W.; Liang, J.; Feng, X.; Sun, L. Mesenchymal Stem Cells Promote the Osteogenesis in Collagen-Induced Arthritic Mice through the Inhibition of TNF-α. Stem Cells Int. 2018, 2018, 4069032. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, X.; Yu, J.; Shang, Y.; Tu, M.; Cheang, L.H.; Zhang, J. Comparison of nucleus pulposus stem/progenitor cells isolated from degenerated intervertebral discs with umbilical cord de-rived mesenchymal stem cells. Exp. Cell Res. 2017, 361, 324–332. [Google Scholar] [CrossRef]
- Matta, A.; Karim, M.Z.; Gerami, H.; Benigno, B.; Erwin, W.M. A comparative study of mesenchymal stem cell transplantation and NTG-101 molecular therapy to treat degenerative disc disease. Sci. Rep. 2021, 11, 14804. [Google Scholar] [CrossRef]
- Khalid, S.; Ekram, S.; Salim, A.; Chaudhry, G.R.; Khan, I. Transcription regulators differentiate mesenchymal stem cells into chondroprogenitors, and their in vivo implantation regenerated the intervertebral disc degeneration. World J. Stem Cells 2022, 14, 163–182. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Gao, L.; Jiang, S.; Ruan, D. Human Wharton’s Jelly Cells Activate Degenerative Nucleus Pulposus Cells In Vitro. Tissue Eng. Part A 2018, 24, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cruet, M.; Beeravolu, N.; McKee, C.; Brougham, J.; Khan, I.; Bakshi, S. Potential of Human Nucleus Pulposus-Like Cells Derived From Umbilical Cord to Treat Degenerative Disc Disease. Neurosurgery 2019, 84, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Ekram, S.; Khalid, S.; Bashir, I.; Salim, A.; Khan, I. Human umbilical cord-derived mesenchymal stem cells and their chon-droprogenitor derivatives reduced pain and inflammation signaling and promote regeneration in a rat intervertebral disc de-generation model. Mol. Cell. Biochem. 2021, 476, 3191–3205. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Z.; Li, R.; Tian, J.; Sun, G.; Li, L.; Wu, D.; Ding, S.; Zhou, C. A novel biomimetic scaffold with hUCMSCs for lumbar fusion. J. Mater. Chem. B 2017, 5, 5996–6007. [Google Scholar] [CrossRef]
- Dai, X.; Guan, Y.; Zhang, Z.; Xiong, Y.; Liu, C.; Li, H.; Liu, B. Comparison of the differentiation abilities of bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells toward nucleus pulposus-like cells in three-dimensional culture. Exp. Ther. Med. 2021, 22, 1018. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, S.J.; Liu, C.; Wang, J.; Hu, B.; Xu, H.G. Sod2 and catalase improve pathological conditions of intervertebral disc degeneration by modifying human adipose-derived mesenchymal stem cells. Life Sci. 2021, 267, 118929. [Google Scholar] [CrossRef] [PubMed]
- Borem, R.; Madeline, A.; Bowman, M.; Gill, S.; Tokish, J.; Mercuri, J. Differential Effector Response of Amnion- and Adi-pose-Derived Mesenchymal Stem Cells to Inflammation; Implications for Intradiscal Therapy. J. Orthop. Res. 2019, 37, 2445–2456. [Google Scholar] [CrossRef]
- Frapin, L.; Clouet, J.; Chédeville, C.; Moraru, C.; Samarut, E.; Henry, N.; André, M.; Bord, E.; Halgand, B.; Lesoeur, J.; et al. Controlled release of biological factors for endogenous progenitor cell migration and intervertebral disc extracellular matrix remodelling. Biomaterials 2020, 253, 120107. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, Y.; Park, S.; Muttigi, M.S.; Kim, J.; Park, H.; Lee, S. Matrilin3/TGFβ3 gelatin microparticles promote chondrogenesis, prevent hypertrophy, and induce paracrine release in MSC spheroid for disc regeneration. NPJ Regen. Med. 2021, 6, 50. [Google Scholar] [CrossRef]
- González-Cubero, E.; González-Fernández, M.L.; Olivera, E.R.; Villar-Suárez, V. Extracellular vesicle and soluble fractions of adipose tissue-derived mesenchymal stem cells secretome induce inflammatory cytokines modulation in an in vitro model of discogenic pain. Spine J. 2022, 22, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, D.; Wang, C.; Xia, K.; Wang, J.; Zhou, X.; Ying, L.; Shu, J.; Huang, X.; Xu, H.; et al. Injectable kartogenin and apocynin loaded micelle enhances the alleviation of intervertebral disc degeneration by adi-pose-derived stem cell. Bioact. Mater. 2021, 6, 3568–3579. [Google Scholar] [CrossRef]
- Friedmann, A.; Baertel, A.; Schmitt, C.; Ludtka, C.; Milosevic, J.; Meisel, H.J.; Goehre, F.; Schwan, S. Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model. Int. J. Mol. Sci. 2021, 22, 4248. [Google Scholar] [CrossRef]
- Muttigi, M.S.; Kim, B.J.; Kumar, H.; Park, S.; Choi, U.Y.; Han, I.; Park, H.; Lee, S.H. Efficacy of matrilin-3-primed adipose-derived mesenchymal stem cell spheroids in a rabbit model of disc degeneration. Stem Cell Res. Ther. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guerrero, J.; Croft, A.S.; Albers, C.E.; Häckel, S.; Gantenbein, B. Spheroid-Like Cultures for Expanding Angiopoietin Receptor-1 (aka. Tie2) Positive Cells from the Human Intervertebral Disc. Int. J. Mol. Sci. 2020, 21, 9423. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Wang, R.; Shi, Q.; Yuan, M.; Jin, M.; Li, D. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J. Bone Miner. Metab. 2019, 37, 455–466. [Google Scholar] [CrossRef]
- Wu, H.; Shang, Y.; Yu, J.; Zeng, X.; Lin, J.; Tu, M.; Cheang, L.H.; Zhang, J. Regenerative potential of human nucleus pulposus resident stem/progenitor cells declines with ageing and intervertebral disc degeneration. Int. J. Mol. Med. 2018, 42, 2193–2202. [Google Scholar] [CrossRef]
- Brown, S.J.; Turner, S.A.; Balain, B.S.; Davidson, N.T.; Roberts, S. Is Osteogenic Differentiation of Human Nucleus Pulposus Cells a Possibility for Biological Spinal Fusion? Cartilage 2020, 11, 181–191. [Google Scholar] [CrossRef]
- Zeng, X.; Lin, J.; Wu, H.; Yu, J.; Tu, M.; Cheang, L.H.; Zhang, J. Effect of Conditioned Medium from Human Umbilical Cord-Derived Mesenchymal Stromal Cells on Rejuvenation of Nucleus Pulposus Derived Stem/Progenitor Cells from Degenerated Intervertebral Disc. Int. J. Stem Cells 2020, 13, 257–267. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Qi, J.; Wang, J.; Zhou, Q.; Hu, F.; Liang, J.; Li, C.; Zhang, W.; Zhang, X. CD24 identifies nucleus pulposus progenitors/notochordal cells for disc regeneration. J. Biol. Eng. 2018, 12, 35. [Google Scholar] [CrossRef]
- Ying, J.W.; Wen, T.Y.; Pei, S.S.; Su, L.H.; Ruan, D.K. Stromal cell-derived factor-1α promotes recruitment and differentiation of nucleus pulposus-derived stem cells. World J. Stem Cells 2019, 11, 196–211. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, Z.; Cui, M.; Liu, S.; Wu, W.; Chen, M.; Wu, Y.; Qu, Y.; Lin, H.; Chen, S.; et al. HIF1A Alleviates compression-induced apoptosis of nucleus pulposus derived stem cells via upregulating autophagy. Autophagy 2021, 17, 3338–3360. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.S.; Li, D.D.; Wang, C.G.; Ying, L.W.; Wang, J.K.; Yang, B.; Shu, J.W.; Huang, X.P.; Zhang, Y.A.; Yu, C.; et al. An esterase-responsive ibuprofen nano-micelle pre-modified embryo derived nucleus pulposus progenitor cells promote the regeneration of intervertebral disc degeneration. Bioact. Mater. 2023, 21, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nan, L.P.; Zhou, S.F.; Liu, Y.; Wang, Z.Y.; Wang, J.C.; Feng, X.M.; Zhang, L. Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells Int. 2019, 2019, 8496025. [Google Scholar] [CrossRef]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.; et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef]
- Li, X.C.; Wang, M.S.; Liu, W.; Zhong, C.F.; Deng, G.B.; Luo, S.J.; Huang, C.M. Co-culturing nucleus pulposus mesenchymal stem cells with notochordal cell-rich nucleus pulposus explants attenuates tumor necrosis factor-α-induced senescence. Stem Cell Res. Ther. 2018, 9, 171. [Google Scholar] [CrossRef]
- Li, Z.; Ye, D.; Dai, L.; Xu, Y.; Wu, H.; Luo, W.; Liu, Y.; Yao, X.; Wang, P.; Miao, H.; et al. Single-Cell RNA Sequencing Reveals the Difference in Human Normal and Degenerative Nucleus Pulposus Tissue Profiles and Cellular Interactions. Front. Cell Dev. Biol. 2022, 10, 910626. [Google Scholar] [CrossRef]
- Blanco, J.F.; Graciani, I.F.; Sanchez-Guijo, F.M.; Muntión, S.; Hernandez-Campo, P.; Santamaria, C.; Carrancio, S.; Barbado, M.V.; Cruz, G.; Gutierrez-Cosío, S.; et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: Comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine 2010, 35, 2259–2265. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, F.; Deng, C.; He, F.; Zhang, Y.; Shen, H.; Chen, Z.; Qian, L. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging 2019, 11, 10252–10265. [Google Scholar] [CrossRef]
- Gao, C.; Ning, B.; Sang, C.; Zhang, Y. Rapamycin prevents the intervertebral disc degeneration via inhibiting differentiation and senescence of annulus fibrosus cells. Aging 2018, 10, 131–143. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, P.; Li, B. Identification and Characterizations of Annulus Fibrosus-Derived Stem Cells. Methods Mol Biol. 2018, 1842, 207–216. [Google Scholar]
- Kaji, D.A.; Montero, A.M.; Patel, R.; Huang, A.H. Transcriptional profiling of mESC-derived tendon and fibrocartilage cell fate switch. Nat. Commun. 2021, 12, 4208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chu, G.; Yuan, Z.; Wang, H.; Zhang, W.; Mao, Y.; Zhu, X.; Chen, W.; Yang, H.; Li, B. Regulation of differentiation of annulus fibrosus-derived stem cells using heterogeneous electrospun fibrous scaffolds. J. Orthop. Transl. 2021, 26, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gong, J.; Zhang, H.; Qin, J.; Li, C.; Zhang, J.; Tang, Y.; Zhang, Y.; Chen, J.; Zhou, Y.; et al. Cartilage Endplate Stem Cells Transdifferentiate into Nucleus Pulposus Cells via Autocrine Exosomes. Front. Cell Dev. Biol. 2021, 9, 648201. [Google Scholar]
- Bhujel, B.; Yang, S.S.; Kim, H.R.; Kim, S.B.; Min, B.H.; Choi, B.H.; Han, I. An Injectable Engineered Cartilage Gel Improves Intervertebral Disc Repair in a Rat Nucleotomy Model. Int. J. Mol. Sci. 2023, 24, 3146. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P.; et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506–7524. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Chen, P.; Ma, C.Y.; Li, C.; Au, T.Y.K.; Tam, V.; Peng, Y.; Wu, R.; Cheung, K.M.C.; et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep. 2020, 30, 2791–2806.e5. [Google Scholar] [CrossRef]
- Kamatani, T.; Hagizawa, H.; Yarimitsu, S.; Morioka, M.; Koyamatsu, S.; Sugimoto, M.; Kodama, J.; Yamane, J.; Ishiguro, H.; Shichino, S.; et al. Human iPS cell-derived cartilaginous tissue spatially and functionally replaces nucleus pulposus. Biomaterials 2022, 284, 121491. [Google Scholar] [CrossRef] [PubMed]
| Source | Species | Digestion | Expansion | Function | Phenotype | Ref |
|---|---|---|---|---|---|---|
| NP | Human | Collagenase and pronase | αMEM with 10% FBS | CFU-S adolescent > older (25%) (limited) | Tie2+, GD2+ | [11] |
| NP | Rat | 0.25% type II collagenase | MSC complete medium | Colony-forming ability and multipotency | CD44+, CD73+, CD90+, CD105+, Sox2+, Nanog+, Oct4+ | [13] |
| CEP | Rabbit | 0.25% EDTA-trypsin and 0.2% type II collagenase | DMEM/F12 medium with 20% FBS | Multipotency | CD90+, CD105+, ACAN, Sox9+, Col2A+ | [14] |
| CEP (degenerated) | Human | 0.25% type II collagenase | DMEM/F12 medium with 10% FCS | Osteogenic Chondroblastic | CD73+, CD90+, CD105+ | [15] |
| AF | Rabbit | Type I and II collagenase (150 U/mL) | αMEM with 15% FBS | Proliferation | iAF: Col2a1, Acan oAF: Col1a1 | [16] |
| AF | Mouse | Collagenase P | DMEM/F12 medium with 10% FBS | Fibrogenic Chondrogenic | CD44+, Col1a1+, Col2a1+ | [17] |
| Phenotype | Differentiation | Expansion | Therapeutic Potential | Animal Models | |
|---|---|---|---|---|---|
| BMSC | CD29+, CD90+, CD105+, CD146+ | Osteocytes Adipocytes Chondrocytes NP cells AF cells | BMSC = NP progenitor | (1) IVD matrix promotion (2) Local cell regeneration (3) Differentiation (4) Immunomodulation | Rabbit Canine Porcine Rat Sheep |
| UCSC | CD29+, CD44+, CD73+, CD90+, CD105+, CD166+ | Osteocytes Adipocytes Chondrocytes NP cells | UCSC < NP progenitor | (1) IVD matrix promotion (2) Local cell regeneration (3) Differentiation (4) Immunomodulation | Rabbit Rat |
| ADSC | CD73+, CD105+, CD44+, Sca-1+ | Osteocytes Adipocytes Chondrocytes NP cells | / | (1) IVD matrix promotion (2) Local cell regeneration (3) Differentiation (4) Immunomodulation | Rabbit Mice Rat Sheep |
| NP progenitor | CD24+, CD44+, CD55+ CD70+ CD73+, CD82+, CD90+, CD105+, Tie2+, UTS2R+, PDGFRA+, KRT15+, Col2a1+, Col1a1+, Sox4+, LepR+ | Osteocytes Adipocytes Chondrocytes NP cells CEP cells | NP progenitor = BMSC NP progenitor > UCSC (NP progenitor = AF progenitor = CEP progenitor) | (1) IVD matrix promotion (2) Local cell regeneration (3) Differentiation (4) Immunomodulation | Rat |
| AF progenitor | Scx+, Oct4+, SSEA4+, neucleostemin+, Col2+ CD29+, CD44+, CD69+, Grem1+, CD105+, Gata2+, Tnfaip3+, Car1+, LepR+ | Osteocytes Adipocytes Chondrocytes AF cells | (NP progenitor = AF progenitor = CEP progenitor) | (1) IVD matrix promotion (2) Differentiation | |
| CEP progenitor | CD29+, CD44+, CD73+, CD90+, CD105+, CCNL1+, WSB1+ | Osteocytes Adipocytes Chondrocytes NP cells CEP cells | (NP progenitor = AF progenitor = CEP progenitor) | (1) IVD matrix promotion (2) Local cell regeneration (3) Differentiation (4) Immunomodulation | Rat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.-D.; Huang, Y.-C.; Lin, J.-L.; Li, W.-S. Intervertebral Disc Progenitors: Lessons Learned from Single-Cell RNA Sequencing and the Role in Intervertebral Disc Regeneration. Bioengineering 2023, 10, 713. https://doi.org/10.3390/bioengineering10060713
Zhao Y-D, Huang Y-C, Lin J-L, Li W-S. Intervertebral Disc Progenitors: Lessons Learned from Single-Cell RNA Sequencing and the Role in Intervertebral Disc Regeneration. Bioengineering. 2023; 10(6):713. https://doi.org/10.3390/bioengineering10060713
Chicago/Turabian StyleZhao, Yu-Dong, Yong-Can Huang, Jia-Liang Lin, and Wei-Shi Li. 2023. "Intervertebral Disc Progenitors: Lessons Learned from Single-Cell RNA Sequencing and the Role in Intervertebral Disc Regeneration" Bioengineering 10, no. 6: 713. https://doi.org/10.3390/bioengineering10060713
APA StyleZhao, Y.-D., Huang, Y.-C., Lin, J.-L., & Li, W.-S. (2023). Intervertebral Disc Progenitors: Lessons Learned from Single-Cell RNA Sequencing and the Role in Intervertebral Disc Regeneration. Bioengineering, 10(6), 713. https://doi.org/10.3390/bioengineering10060713







