Gait Analysis in Neurorehabilitation: From Research to Clinical Practice
Abstract
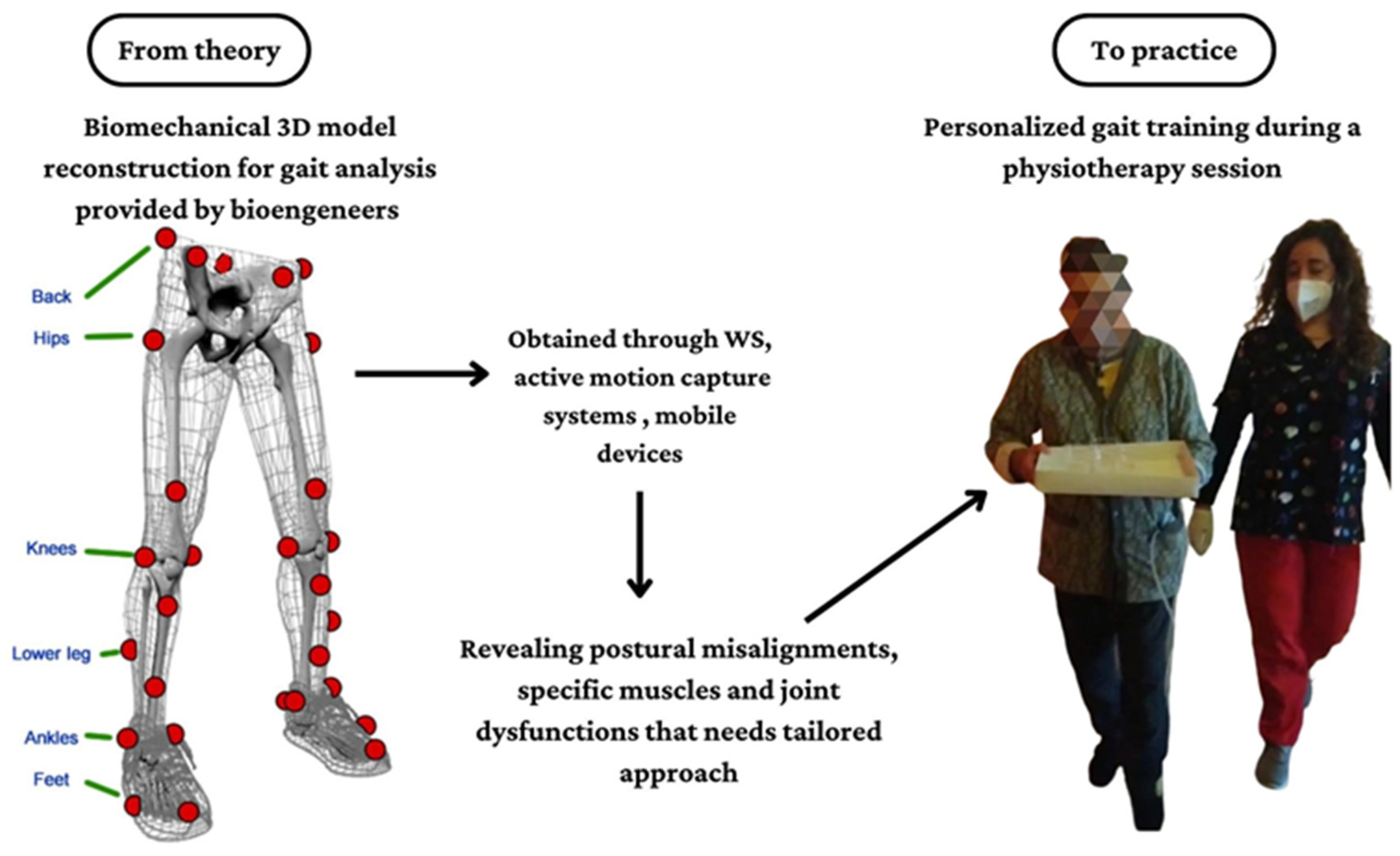
:1. Introduction
2. Search Strategy
3. Neurodegenerative Disorders
3.1. Parkinson’s Disease
3.2. Multiple Sclerosis
3.3. Cerebellar Ataxia
4. Acquired Brain Injury
4.1. Stroke
4.2. Traumatic Brain Injury
5. Discussion
5.1. Clinical Considerations about Gait and Postural Dysfunctions
5.2. Clinical Implications of NWS
5.3. Clinical Implications of WS
5.4. Future Directions of Gait Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Neurological Disorders AND | Gait Analysis Systems |
| “Parkinson’s disease” OR “multiple sclerosis” OR “cerebellar ataxia” OR “ataxia” OR “neurodegenerative disorders” OR “acquired brain injury” OR “stroke” OR “traumatic brain injury” | “wearable sensors” OR “gait platforms” OR “non-wearable sensors” OR “instrumented gait analysis” OR “objective gait evaluation” OR “inertial measurement units” OR “motion capture systems” OR “mobile application” OR “artificial intelligence” |
References
- Salchow-Hömmen, C.; Skrobot, M.; Jochner, M.C.E.; Schauer, T.; Kühn, A.A.; Wenger, N. Review—Emerging Portable Technologies for Gait Analysis in Neurological Disorders. Front. Hum. Neurosci. 2022, 16, 768575. [Google Scholar] [CrossRef]
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef] [Green Version]
- Loyd, B.J.; Dibble, L.E.; Weightman, M.M.; Pelo, R.; Hoppes, C.W.; Lester, M.; King, L.A.; Fino, P.C. Volitional Head Movement Deficits and Alterations in Gait Speed Following Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 2022, 38, E223–E232. [Google Scholar] [CrossRef]
- di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef] [PubMed]
- Coca-Tapia, M.; Cuesta-Gómez, A.; Molina-Rueda, F.; Carratalá-Tejada, M. Gait Pattern in People with Multiple Sclerosis: A Systematic Review. Diagnostics 2021, 11, 584. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. J. 1999, 5, 363–368. [Google Scholar] [CrossRef]
- Tunca, C.; Pehlivan, N.; Ak, N.; Arnrich, B.; Salur, G.; Ersoy, C. Inertial Sensor-Based Robust Gait Analysis in Non-Hospital Settings for Neurological Disorders. Sensors 2017, 17, 825. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Paul, S.; Mourya, G.K.; Kumar, N.; Hussain, M. Recent Trends and Practices toward Assessment and Rehabilitation of Neurodegenerative Disorders: Insights from Human Gait. Front. Neurosci. 2022, 16, 859298. [Google Scholar] [CrossRef] [PubMed]
- Hulleck, A.A.; Mohan, D.M.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, M.; De Luca, R.; Torregrossa, W.; Tonin, P.; Calabrò, R.S. Moving toward Appropriate Motor Assessment Tools in People Affected by Severe Acquired Brain Injury: A Scoping Review with Clinical Advices. Healthcare 2022, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Bhidayasiri, R.; Martinez-Martin, P. Clinical Assessments in Parkinson’s Disease: Scales and Monitoring. Int. Rev. Neurobiol. 2017, 132, 129–182. [Google Scholar] [CrossRef]
- Wang, C.; Ruiz, A.; Mao-Draayer, Y. Assessment and Treatment Strategies for a Multiple Sclerosis Relapse. J. Immunol. Clin. Res. 2016, 5, 1032. [Google Scholar] [PubMed]
- Power, L.; Pathirana, P.; Horne, M.; Milne, S.; Marriott, A.; Szmulewicz, D.J. Instrumented Objective Clinical Examination of Cerebellar Ataxia: The Upper and Lower Limb—A Review. Cerebellum 2022, 21, 145–158. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [Green Version]
- Schniepp, R.; Huppert, A.; Decker, J.; Schenkel, F.; Schlick, C.; Rasoul, A.; Dieterich, M.; Brandt, T.; Jahn, K.; Wuehr, M. Fall prediction in neurological gait disorders: Differential contributions from clinical assessment, gait analysis, and daily-life mobility monitoring. J. Neurol. 2021, 268, 3421–3434. [Google Scholar] [CrossRef] [PubMed]
- Pieruccini-Faria, F.; Black, S.E.; Masellis, M.; Smith, E.E.; Almeida, Q.J.; Li, K.Z.H.; Bherer, L.; Camicioli, R.; Montero-Odasso, M. Gait variability across neurodegenerative and cognitive disorders: Results from the Canadian Consortium of Neurodegeneration in Aging (CCNA) and the Gait and Brain Study. Alzheimer’s Dement. 2021, 17, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Cicirelli, G.; Impedovo, D.; Dentamaro, V.; Marani, R.; Pirlo, G.; D’Orazio, T.R. Human Gait Analysis in Neurodegenerative Diseases: A Review. IEEE J. Biomed. Health Inform. 2022, 26, 229–242. [Google Scholar] [CrossRef]
- Marković, V.; Stanković, I.; Radovanović, S.; Petrović, I.; Lukić, M.J.; Mišković, N.D.; Svetel, M.; Kostić, V. Gait alterations in Parkinson’s disease at the stage of hemiparkinsonism—A longitudinal study. PLoS ONE 2022, 17, e0269886. [Google Scholar] [CrossRef]
- Pistacchi, M.; Gioulis, M.; Sanson, F.; De Giovannini, E.; Filippi, G.; Rossetto, F.; Zambito Marsala, S. Gait analysis and clinical correlations in early Parkinson’s disease. Funct. Neurol. 2017, 32, 28–34. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use. IEEE J. Biomed. Health Inform. 2015, 20, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Potter, M.V.; Cain, S.M.; Ojeda, L.V.; Gurchiek, R.D.; McGinnis, R.S.; Perkins, N.C. Evaluation of Error-State Kalman Filter Method for Estimating Human Lower-Limb Kinematics during Various Walking Gaits. Sensors 2022, 22, 8398. [Google Scholar] [CrossRef]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb. Clin. Neurol. 2018, 159, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.C.; Doorenbosch, C.A.; Knol, D.L.; de Groot, V.; Beckerman, H. Newly Identified Gait Patterns in Patients with Multiple Sclerosis May Be Related to Push-off Quality. Phys. Ther. 2016, 96, 1744–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Straudi, S.; Morelli, M.; Basaglia, N. Evaluation of Clinical Gait Analysis parameters in patients affected by Multiple Sclerosis: Analysis of kinematics. Clin. Biomech. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Sehle, A.; Mündermann, A.; Starrost, K.; Sailer, S.; Becher, I.; Dettmers, C.; Vieten, M. Objective assessment of motor fatigue in multiple sclerosis using kinematic gait analysis: A pilot study. J. Neuroeng. Rehabil. 2011, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Combden, O.; Jiang, X.; Buragadda, S.; Newell, C.J.; Williams, M.C.; Critch, A.L.; Ploughman, M. Machine learning corroborates subjective ratings of walking and balance difficulty in multiple sclerosis. Front. Artif. Intell. 2022, 5, 952312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, D.H.; Yang, Y.; Ha, S.W.; Han, J.H. Gait Patterns in Parkinson’s Disease with or without Cognitive Impairment. Dement. Neurocogn. Disord. 2018, 17, 57–65. [Google Scholar] [CrossRef] [PubMed]
- di Biase, L.; Raiano, L.; Caminiti, M.L.; Pecoraro, P.M.; Di Lazzaro, V. Parkinson’s Disease Wearable Gait Analysis: Kinematic and Dynamic Markers for Diagnosis. Sensors 2022, 22, 8773. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.Z.U.; Buckley, C.; Mico-Amigo, M.E.; Kirk, C.; Dunne-Willows, M.; Mazza, C.; Shi, J.Q.; Alcock, L.; Rochester, L.; Del Din, S. Accelerometry-Based Digital Gait Characteristics for Classification of Parkinson’s Disease: What Counts? IEEE Open J. Eng. Med. Biol. 2020, 1, 65–73. [Google Scholar] [CrossRef]
- Jakob, V.; Küderle, A.; Kluge, F.; Klucken, J.; Eskofier, B.M.; Winkler, J.; Winterholler, M.; Gassner, H. Validation of a Sensor-Based Gait Analysis System with a Gold-Standard Motion Capture System in Patients with Parkinson’s Disease. Sensors 2021, 21, 7680. [Google Scholar] [CrossRef]
- Alberto, S.; Cabral, S.; Proença, J.; Pona-Ferreira, F.; Leitão, M.; Bouça-Machado, R.; Kauppila, L.A.; Veloso, A.P.; Costa, R.M.; Ferreira, J.J.; et al. Validation of quantitative gait analysis systems for Parkinson’s disease for use in supervised and unsupervised environments. BMC Neurol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Z.; Qiu, S.; Zhao, H.Y.; Wang, C.; Shi, X.; Lin, F. A Wearable Gait Analysis and Recognition Method for Parkinson’s Disease Based on Error State Kalman Filter. IEEE J. Biomed. Health Inform. 2022, 26, 4165–4175. [Google Scholar] [CrossRef]
- Lizama, L.E.C.; Khan, F.; Lee, P.V.S.; Galea, M.P. The use of laboratory gait analysis for understanding gait deterioration in people with multiple sclerosis. Mult. Scler. J. 2016, 22, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Buckley, E.; Mazzà, C.; McNeill, A. A systematic review of the gait characteristics associated with Cerebellar Ataxia. Gait Posture 2018, 60, 154–163. [Google Scholar] [CrossRef]
- Serrao, M.; Pierelli, F.; Ranavolo, A.; Draicchio, F.; Conte, C.; Don, R.; Di Fabio, R.; LeRose, M.; Padua, L.; Sandrini, G.; et al. Gait Pattern in Inherited Cerebellar Ataxias. Cerebellum 2011, 11, 194–211. [Google Scholar] [CrossRef]
- Matsushima, A.; Yoshida, K.; Genno, H.; Murata, A.; Matsuzawa, S.; Nakamura, K.; Nakamura, A.; Ikeda, S.-I. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias 2015, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Nguyen, H.; Enriquez, A.; Morsy, L.; Curtis, M.; Piser, T.; Kenney, C.; Stephen, C.D.; Gupta, A.S.; Schmahmann, J.D.; et al. Assessment of gait and balance impairment in people with spinocerebellar ataxia using wearable sensors. Neurol. Sci. 2021, 43, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Ilg, W.; Seemann, J.; Giese, M.; Traschütz, A.; Schöls, L.; Timmann, D.; Synofzik, M. Real-life gait assessment in degenerative cerebellar ataxia: Toward ecologically valid biomarkers. Neurology 2020, 95, e1199–e1210. [Google Scholar] [CrossRef]
- Ferrarello, F.; Bianchi, V.A.M.; Baccini, M.; Rubbieri, G.; Mossello, E.; Cavallini, M.C.; Marchionni, N.; Di Bari, M. Tools for Observational Gait Analysis in Patients With Stroke: A Systematic Review. Phys. Ther. 2013, 93, 1673–1685. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Taly, A.B. Post-stroke Gait Analysis in Rehabilitation Set-up: Observational or Instrumental! Neurol. India 2019, 67, 1041–1042. [Google Scholar] [CrossRef]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Alali, S.I.I.I.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.; Colomer, C.; Alcañiz, M.; Llorens, R. Gait analysis with the Kinect v2: Normative study with healthy individuals and comprehensive study of its sensitivity, validity, and reliability in individuals with stroke. J. Neuroeng. Rehabil. 2019, 16, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tang, R.; Wang, H.; Yu, X.; Li, Y.; Wang, C.; Wang, L.; Qie, S. The Validity and Reliability of a New Intelligent Three-Dimensional Gait Analysis System in Healthy Subjects and Patients with Post-Stroke. Sensors 2022, 22, 9425. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Tok, F. Gait Disturbances in Patients with Stroke. PM&R 2014, 6, 635–642. [Google Scholar] [CrossRef]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Laudanski, A.; Brouwer, B.; Li, Q. Measurement of Lower Limb Joint Kinematics using Inertial Sensors During Stair Ascent and Descent in Healthy Older Adults and Stroke Survivors. J. Health Eng. 2013, 4, 555–576. [Google Scholar] [CrossRef] [Green Version]
- Dever, A.; Powell, D.; Graham, L.; Mason, R.; Das, J.; Marshall, S.J.; Vitorio, R.; Godfrey, A.; Stuart, S. Gait Impairment in Traumatic Brain Injury: A Systematic Review. Sensors 2022, 22, 1480. [Google Scholar] [CrossRef]
- Reidy, J.; Mobbs, R.; Kim, J.; Brown, E.; Mobbs, R. Clinical gait characteristics in the early post-concussion phase: A systematic review. J. Clin. Neurosci. 2023, 107, 184–191. [Google Scholar] [CrossRef]
- Martini, D.N.; Parrington, L.; Stuart, S.; Fino, P.C.; King, L.A. Gait Performance in People with Symptomatic, Chronic Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 218–224. [Google Scholar] [CrossRef]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Bustos, A.O.; Allevi, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Gait Quality Assessment in Survivors from Severe Traumatic Brain Injury: An Instrumented Approach Based on Inertial Sensors. Sensors 2019, 19, 5315. [Google Scholar] [CrossRef] [Green Version]
- Pitt, W.; Chen, S.-H.; Chou, L.-S. Using IMU-based kinematic markers to monitor dual-task gait balance control recovery in acutely concussed individuals. Clin. Biomech. 2020, 80, 105145. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Stuart, S.; Woo, W.; Godfrey, A. Gait analysis in neurological populations: Progression in the use of wearables. Med. Eng. Phys. 2020, 87, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Klebe, S.; Baecker, C.; Zechlin, C.; Friege, L.; Pohle, S.; Deuschl, G. Prevalence of gait disorders in hospitalized neurological patients. Mov. Disord. 2004, 20, 89–94. [Google Scholar] [CrossRef]
- Ge, H.-L.; Chen, X.-Y.; Lin, Y.-X.; Ge, T.-J.; Yu, L.-H.; Lin, Z.-Y.; Wu, X.-Y.; Kang, D.-Z.; Ding, C.-Y. The prevalence of freezing of gait in Parkinson’s disease and in patients with different disease durations and severities. Chin. Neurosurg. J. 2020, 6, 17. [Google Scholar] [CrossRef]
- Tasseel-Ponche, S.; Delafontaine, A.; Godefroy, O.; Yelnik, A.P.; Doutrellot, P.-L.; Duchossoy, C.; Hyra, M.; Sader, T.; Diouf, M. Walking speed at the acute and subacute stroke stage: A descriptive meta-analysis. Front. Neurol. 2022, 13, 989622. [Google Scholar] [CrossRef]
- Moon, Y.; Sung, J.; An, R.; Hernandez, M.E.; Sosnoff, J.J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 2016, 47, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Enzastiga, D.; Casamento-Moran, A.; Christou, E.A.; Lodha, N. Increased temporal stride variability contributes to impaired gait coordination after stroke. Sci. Rep. 2022, 12, 12679. [Google Scholar] [CrossRef]
- Niechwiej-Szwedo, E.; Inness, E.; Howe, J.; Jaglal, S.; McIlroy, W.; Verrier, M. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture 2007, 25, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.W., 3rd; Merkas, J.; Pilutti, L.A. The Effect of Exercise Training on Gait, Balance, and Physical Fitness Asymmetries in Persons with Chronic Neurological Conditions: A Systematic Review of Randomized Controlled Trials. Front. Physiol. 2020, 11, 585765. [Google Scholar] [CrossRef]
- Bishnoi, A.; Shankar, M.; Lee, R.; Hu, Y.; Hernandez, M.E. Effects of Therapeutic Intervention on Spatiotemporal Gait Parameters in Adults With Neurologic Disorder: Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2023, 104, 451–474. [Google Scholar] [CrossRef]
- Keshner, E.A.; Lamontagne, A. The Untapped Potential of Virtual Reality in Rehabilitation of Balance and Gait in Neurological Disorders. Front. Virtual Real. 2021, 2, 641650. [Google Scholar] [CrossRef] [PubMed]
- Peppe, A.; Chiavalon, C.; Pasqualetti, P.; Crovato, D.; Caltagirone, C. Does gait analysis quantify motor rehabilitation efficacy in Parkinson’s disease patients? Gait Posture 2007, 26, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Guner, S.; Inanici, F. Yoga therapy and ambulatory multiple sclerosis Assessment of gait analysis parameters, fatigue and balance. J. Bodyw. Mov. Ther. 2015, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.-D.; Markham, A.; Service, K.; Reini, S.; Wolf, E.; Sessoms, P. A systematic literature review of the use and effectiveness of the Computer Assisted Rehabilitation Environment for research and rehabilitation as it relates to the wounded warrior. Work 2015, 50, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Lemaire, E.D. Temporal-spatial gait parameter models of very slow walking. Gait Posture 2018, 61, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Imoto, D.; Hirano, S.; Mukaino, M.; Saitoh, E.; Otaka, Y. A novel gait analysis system for detecting abnormal hemiparetic gait patterns during robot-assisted gait training: A criterion validity study among healthy adults. Front. Neurorobot. 2022, 16, 1047376. [Google Scholar] [CrossRef] [PubMed]
- Khera, P.; Kumar, N. Role of machine learning in gait analysis: A review. J. Med. Eng. Technol. 2020, 44, 441–467. [Google Scholar] [CrossRef]
- Alfayeed, S.M.; Saini, B.S. Human Gait Analysis Using Machine Learning: A Review. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; pp. 550–554. [Google Scholar]
- Slemenšek, J.; Fister, I.; Geršak, J.; Bratina, B.; van Midden, V.M.; Pirtošek, Z.; Šafarič, R. Human Gait Activity Recognition Machine Learning Methods. Sensors 2023, 23, 745. [Google Scholar] [CrossRef]
- Fricke, C.; Alizadeh, J.; Zakhary, N.; Woost, T.B.; Bogdan, M.; Classen, J. Evaluation of Three Machine Learning Algorithms for the Automatic Classification of EMG Patterns in Gait Disorders. Front. Neurol. 2021, 12, 666458. [Google Scholar] [CrossRef]
- Liuzzi, P.; Carpinella, I.; Anastasi, D.; Gervasoni, E.; Lencioni, T.; Bertoni, R.; Carrozza, M.C.; Cattaneo, D.; Ferrarin, M.; Mannini, A. Machine learning based estimation of dynamic balance and gait adaptability in persons with neurological diseases using inertial sensors. Sci. Rep. 2023, 13, 8640. [Google Scholar] [CrossRef]
- Rastegari, E.; Azizian, S.; Ali, H. Machine learning and similarity network approaches to support automatic classification of Parkinson’s diseases using accelerometer-based gait analysis. In Proceedings of the 52nd Annual Hawaii International Conference on System Sciences, Maui, HI, USA, 8–11 January 2019; pp. 4231–4242. [Google Scholar]
- Harris, E.J.; Khoo, I.-H.; Demircan, E. A Survey of Human Gait-Based Artificial Intelligence Applications. Front. Robot. AI 2022, 8, 749274. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Androwis, G.; Adamovich, S.; Su, H.; Nunez, E.; Zhou, X. Reinforcement Learning and Control of a Lower Extremity Exoskeleton for Squat Assistance. Front. Robot. AI 2021, 8, 702845. [Google Scholar] [CrossRef] [PubMed]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Schmidt, G.; Maetzler, W. A Deep Learning Approach for Gait Event Detection from a Single Shank-Worn IMU: Validation in Healthy and Neurological Cohorts. Sensors 2022, 22, 3859. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Motl, R.W.; Sowers, R.; Hernandez, M.E. A Vision-Based Framework for Predicting Multiple Sclerosis and Parkinson’s Disease Gait Dysfunctions—A Deep Learning Approach. IEEE J. Biomed. Health Inform. 2023, 27, 190–201. [Google Scholar] [CrossRef]




| Reference n° | Gait Analysis System | Technology Description | Neurological Disorder | Clinical Implication | |
|---|---|---|---|---|---|
| Non- Wearable Sensors | Wearable Sensors | ||||
| [15] | X | X | Three-dimensional gait analysis in laboratory, including optometric system, a dynamometric platform, and ad hoc software. | PD with 1.5–2 H&Y stage | Reduced gait speed and step length, showing bilateral extra rotation of knee, ankle, and foot. |
| [16] | X | Triaxial accelerometer-based device placed on the fifth lumbar vertebrae and a double-sided tape. | PD with 1–3 H&Y stage | NA | |
| [17] | X | Instrumented force-sensitive insole placed in patients’ shoes, with eight pressure-sensitive sensors. | PD with 2–3 H&Y stage | Stride-to-stride variability due to bradykinesia, loss of muscle synergies in the lower limb, and lack of rhythmicity. | |
| [18] | X | X | Motion-capture based gait analysis compared to mobile sensor (inertial sensors) gait analysis, which were integrated in the mid-sole of the athletic shoes. | PD with 1–4 H&Y stage | Reduced gait speed, stride time, and length; increased duration stance phase time accompanied by a synchronic decreasing duration of swing phase time. |
| [19] | X | X | Gait assessment through an optoelectronic (48 retroflected markers), inertial, and a smartphone-based capture system. | PD with <3 H&Y stage | NA |
| [20] | X | Wearable device compared to Opti Track system, using an error state Kalman filter algorithm. | PD | NA | |
| [21] | X | Stereophotogrammetric system (Vicon Motion Systems Ltd., Oxford, UK) and reflective markers to estimate joints’ angles. | MS with a score of ≤5–6 | MS patients showed reduced gait speed, which correlated with a decrease in cadence, step length, and swing time, and an increase in stance time. Additionally, authors found an increased pelvic tilt, which negatively correlates with the 6MWT. | |
| [22] | X | X | Wireless AS200 system, comprising three line-scanning camera system and 11 active infrared markers attached on body’s patient, with a 2-mm accuracy. | MS with a mean score of 3.6 in EDSS | MS patients manifested changes in variability of movement gait patterns due to fatigue, altered motor coordination linked to additional activity of the antagonists, or insufficient strength produced by the agonists. |
| [23] | X | Walkway sensor and machine learning (XGB) process to distinguish MS patients’ degree of severity based on their gait features. | MS with a mean score of 2.11 in EDSS | Step time and step width were considered as the most important variables to distinguish level of severity of MS subjects. | |
| [9] | X | X | SMART-E stereophotogrammetric system (BTS, Milan, Italy) with eight infrared cameras (for acquiring kinematic data). Sensorized pathway with 2 piezoelectric force platforms (for acquiring kinetic data), 22 retro-reflective spherical markers for lower-body segments, and 15 markers for the upper body, placed on specific anatomic sites. | Spino-CA autosomal dominant (type 1 and 2) and Friedreich’s ataxia as recessive ataxia | Loss of lower limbs control during gait and of ability to stabilize a walking strategy over time. CA patients definitively lack a stable gait control behavior since the cerebellum functions of motor behavior and developing new motor patterns are altered. |
| [24] | X | Triaxial accelerometer. | Spino-CA with a mean score of 3.9 for stance and gait in SARA | Gait velocity, cadence, step length, step regularity, and step repeatability are strongly correlated with disease duration. | |
| [25] | X | Seven inertial sensors while performing two independent trials of gait and balance assessments. | CA | NA | |
| [26] | X | Three Opal inertial sensors were attached on both feet and the posterior trunk at the level of L5 with elastic Velcro bands. | Spino-CA with a mean score of 3.6 for stance and gait in SARA | Minimal changes in gait spatial–temporal parameters can be considered as accurate markers for CA progression. | |
| Reference n° | Gait Analysis System | Technology Description | Neurological Disorder | Clinical Implication | |
|---|---|---|---|---|---|
| NWS | WS | ||||
| [38] | X | A 10 m walkway with a pressure sensitive mat. Spatial–temporal parameters were registered using GaitRite mat, which contains a total of 13,824 sensors. | Post-stroke patients (both ischemic and hemorrhagic) | Most useful gait parameters are step length, swing time, and stance time. In addition, authors stated that asymmetry time values are not reliable parameters to assess gait in post-stroke patients. | |
| [39] | X | Inertial Measurment Unit (IMU) system (Xsens Technology B.V., Enschede, The Netherlands, Hengelo) composed of seven inertial sensors. | Post-stroke patients | NA | |
| [40] | X | Kinect v2, which included an 8-core Intel® in addition to an ad hoc application designed to register the 3D position and orientation of the 25 human joints provided by the Kinect v2. | Post-stroke patients (both ischemic and hemorrhagic) | Results indicated that patients with a higher fall risk manifested lower gait velocity and cadence, a shorter stride and step length, and higher double support time. Additionally, the risk of falling was related to increased trunk and pelvic obliquity and tilt, and to decreased hip flexion–extension and ankle height variation. | |
| [41] | X | Odonate 3D motion capture system in a mobile terminal and a workstation. This innovative a binocular depth camera combined with an artificial intelligence system to capture, analyze, and calculate gait parameters automatically. | Post-stroke patients | Alterations were found in spatial–temporal and kinematic parameters; thus, this new system can perform an objective gait assessment in five minutes, also in a home-based setting. | |
| [42] | X | Five synchronized IMUs. | Severe TBI patients | Severe TBI patients present serious difficulties in maintaining balance during gait, especially movements of the head, which are the most impaired, probably related to vestibular dysfunctions due to traumatic events. Additionally, authors suggested to assess gait through dynamic balance skills during curved trajectories as in Figure-of-8 Walk Test. | |
| [43] | X | Three IMUs were attached with elastic straps over both lateral ankles to detect gait phases and over the fifth lumbar vertebrae. | TBI | TBI patients manifest great imbalances in dynamic balance, especially in antero-medial weight shifting, when compared with healthy control subjects. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, M.; De Nunzio, A.M.; Quartarone, A.; Militi, A.; Petralito, F.; Calabrò, R.S. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering 2023, 10, 785. https://doi.org/10.3390/bioengineering10070785
Bonanno M, De Nunzio AM, Quartarone A, Militi A, Petralito F, Calabrò RS. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering. 2023; 10(7):785. https://doi.org/10.3390/bioengineering10070785
Chicago/Turabian StyleBonanno, Mirjam, Alessandro Marco De Nunzio, Angelo Quartarone, Annalisa Militi, Francesco Petralito, and Rocco Salvatore Calabrò. 2023. "Gait Analysis in Neurorehabilitation: From Research to Clinical Practice" Bioengineering 10, no. 7: 785. https://doi.org/10.3390/bioengineering10070785







