Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction
Abstract
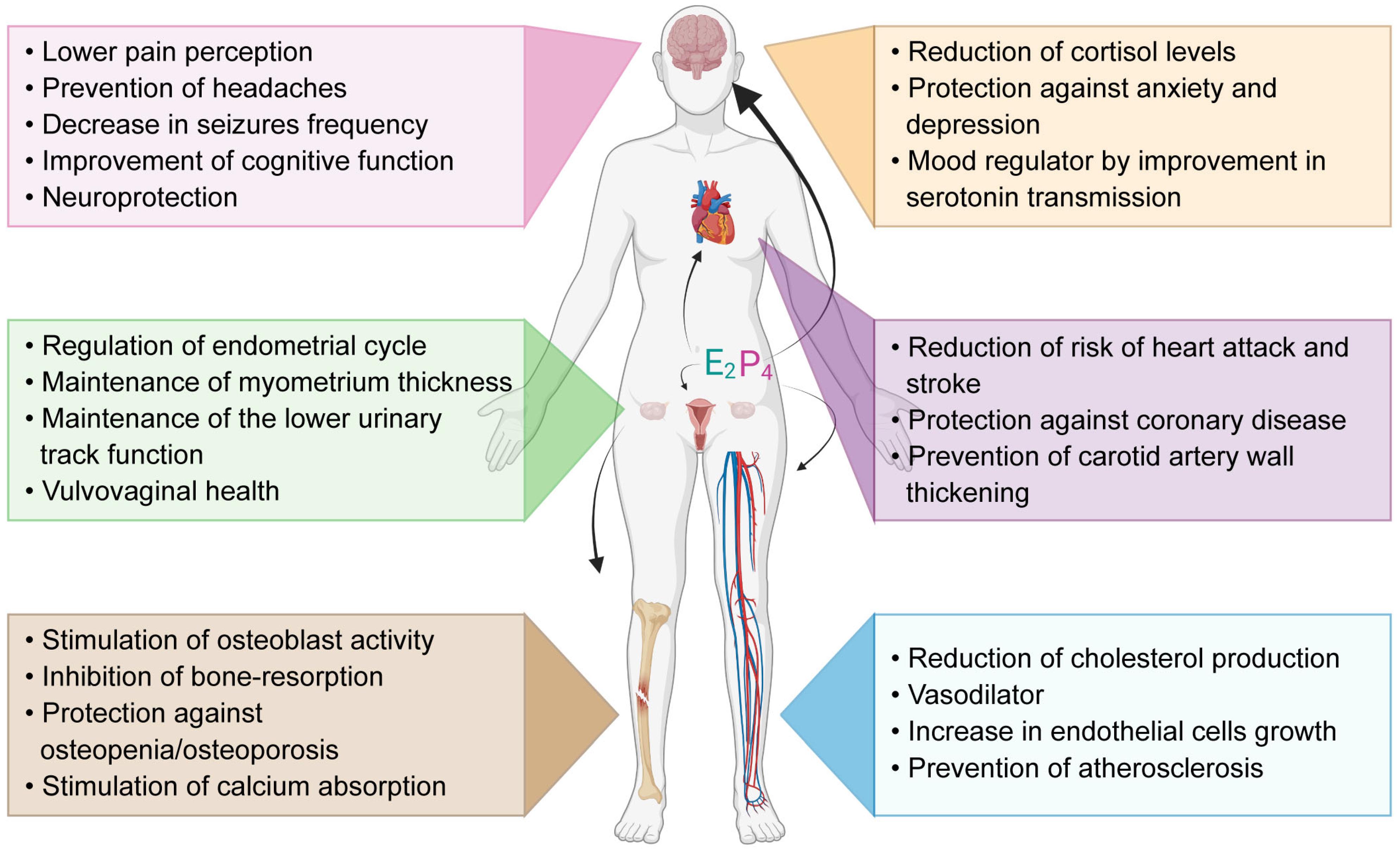
:1. Introduction
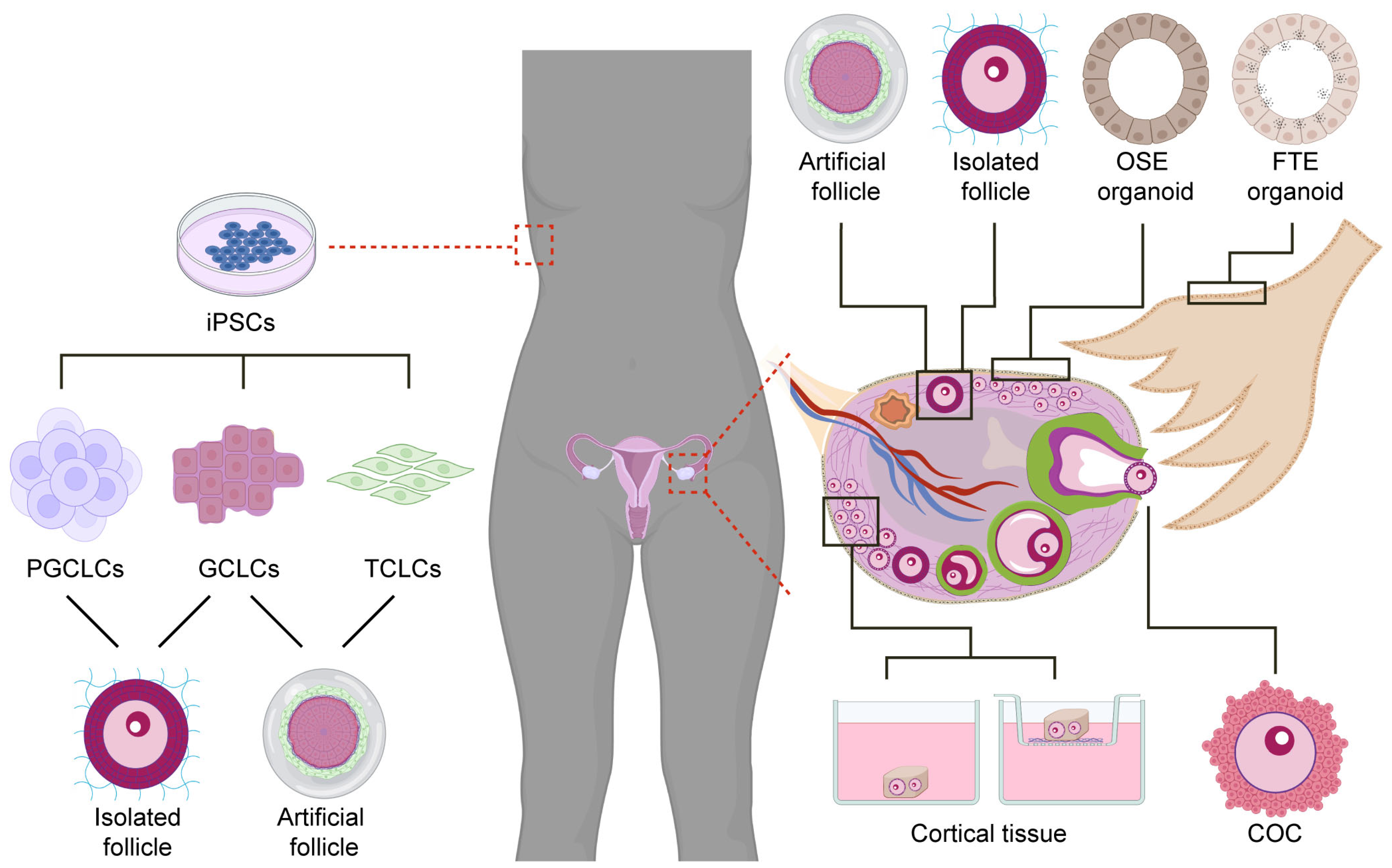
2. Ovarian Organoids to Study Oocyte Formation and Follicular Assembly
3. Ovarian Organoids to Study Follicular Growth and Oocyte Maturation
3.1. Ovarian Cortex Tissue
3.2. Isolated Antral Follicles
4. Ovarian Organoids to Study Ovarian (Somatic) Physiology and Disease
4.1. ECM Deposition and Fibrosis
4.2. Hormonal Production and Menopause
4.3. Modelling Polycystic Ovary Syndrome (PCOS)
5. Ovarian Organoids for Cancer Research
5.1. Disease Modelling for Ovarian Cancer (OC)
5.2. Drug Discovery and Drug Screening in Cancer Research
6. Clinical Applications and Limitations
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barker, R.A.; Boer, G.J.; Cattaneo, E.; Charo, R.A.; Chuva de Sousa Lopes, S.M.; Cong, Y.; Fujita, M.; Goldman, S.; Hermeren, G.; Hyun, I.; et al. The need for a standard for informed consent for collection of human fetal material. Stem Cell Rep. 2022, 17, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Van Den Broecke, R.; Van Der Elst, J.; Liu, J.; Hovatta, O.; Dhont, M. The female-to-male transsexual patient: A source of human ovarian cortical tissue for experimental use. Hum. Reprod. 2001, 16, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Casamassimi, A.; Federico, A.; Rienzo, M.; Esposito, S.; Ciccodicola, A. Transcriptome Profiling in Human Diseases: New Advances and Perspectives. Int. J. Mol. Sci. 2017, 18, 1652. [Google Scholar] [CrossRef] [Green Version]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef]
- Saitou, M.; Hayashi, K. Mammalian in vitro gametogenesis. Science 2021, 374, eaaz6830. [Google Scholar] [CrossRef]
- Richards, J.S. The Ovarian Cycle. Vitam. Horm. 2018, 107, 1–25. [Google Scholar] [CrossRef]
- Kallen, A.N.; Pal, L. Cardiovascular disease and ovarian function. Curr. Opin. Obstet. Gynecol. 2011, 23, 258–267. [Google Scholar] [CrossRef]
- Pinkerton, J.V. Hormone Therapy for Postmenopausal Women. N. Engl. J. Med. 2020, 382, 446–455. [Google Scholar] [CrossRef]
- Roeder, H.J.; Leira, E.C. Effects of the Menstrual Cycle on Neurological Disorders. Curr. Neurol. Neurosci. Rep. 2021, 21, 34. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Torbati, T.; Dutra, E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 256–262. [Google Scholar] [CrossRef]
- Gougeon, A. Human ovarian follicular development: From activation of resting follicles to preovulatory maturation. Ann. Endocrinol. 2010, 71, 132–143. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, F.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 160, R25–R39. [Google Scholar] [CrossRef]
- Fan, X.; Bialecka, M.; Moustakas, I.; Lam, E.; Torrens-Juaneda, V.; Borggreven, N.V.; Trouw, L.; Louwe, L.A.; Pilgram, G.S.K.; Mei, H.; et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Yoshihara, M.; Douagi, I.; Damdimopoulos, A.; Panula, S.; Petropoulos, S.; Lu, H.; Pettersson, K.; Palm, K.; Katayama, S.; et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 2020, 11, 1147. [Google Scholar] [CrossRef] [Green Version]
- Cox, T.R.; Erler, J.T. Molecular pathways: Connecting fibrosis and solid tumor metastasis. Clin. Cancer Res. 2014, 20, 3637–3643. [Google Scholar] [CrossRef] [Green Version]
- Salvi, A.; Hardy, L.R.; Heath, K.N.; Watry, S.; Pergande, M.R.; Cologna, S.M.; Burdette, J.E. PAX8 modulates the tumor microenvironment of high grade serous ovarian cancer through changes in the secretome. Neoplasia 2023, 36, 100866. [Google Scholar] [CrossRef]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Nery, E.D.; Ebner, D.; Montoya, M.C.; Ostling, P.; Pietiainen, V.; Price, L.S.; et al. Screening out irrelevant cell-based models of disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef] [Green Version]
- Garreta, E.; Kamm, R.D.; Chuva de Sousa Lopes, S.M.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2021, 20, 145–155. [Google Scholar] [CrossRef]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Holloway, E.M.; Wu, J.H.; Czerwinski, M.; Sweet, C.W.; Wu, A.; Tsai, Y.H.; Huang, S.; Stoddard, A.E.; Capeling, M.M.; Glass, I.; et al. Differentiation of Human Intestinal Organoids with Endogenous Vascular Endothelial Cells. Dev. Cell 2020, 54, 516–528.e517. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e1916. [Google Scholar] [CrossRef] [Green Version]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Lan, C.W.; Chen, M.J.; Jan, P.S.; Chen, H.F.; Ho, H.N. Differentiation of human embryonic stem cells into functional ovarian granulosa-like cells. J. Clin. Endocrinol. Metab. 2013, 98, 3713–3723. [Google Scholar] [CrossRef] [Green Version]
- Czukiewska, S.M.; Chuva de Sousa Lopes, S.M. Fetal germ cell development in humans, a link with infertility. Semin. Cell Dev. Biol. 2022, 131, 58–65. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Suzuki, T.; Nagamatsu, G.; Yabukami, H.; Ikegaya, M.; Kishima, M.; Kita, H.; Imamura, T.; Nakashima, K.; Nishinakamura, R.; et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science 2021, 373, eabe0237. [Google Scholar] [CrossRef]
- Chen, D.; Liu, W.; Lukianchikov, A.; Hancock, G.V.; Zimmerman, J.; Lowe, M.G.; Kim, R.; Galic, Z.; Irie, N.; Surani, M.A.; et al. Germline competency of human embryonic stem cells depends on eomesodermin. Biol. Reprod. 2017, 97, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Yokobayashi, S.; Okita, K.; Nakagawa, M.; Nakamura, T.; Yabuta, Y.; Yamamoto, T.; Saitou, M. Clonal variation of human induced pluripotent stem cells for induction into the germ cell fate. Biol. Reprod. 2017, 96, 1154–1166. [Google Scholar] [CrossRef] [Green Version]
- Overeem, A.W.; Chang, Y.W.; Moustakas, I.; Roelse, C.M.; Hillenius, S.; Van Der Helm, T.; Van Der Schrier, V.F.; Gonçalves, M.A.F.V.; Mei, H.; Freund, C.; et al. Efficient and scalable generation of primordial germ cells in 2D culture using basement membrane extract overlay. Cell Rep. Methods 2023, 3, 100488. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K.; et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Pandolfi, E.C.; Hsu, F.M.; Duhon, M.; Zheng, Y.; Goldsmith, S.; Fu, J.; Silber, S.J.; Clark, A.T. In vitro germ cell induction from fertile and infertile monozygotic twin research participants. Cell Rep. Med. 2022, 3, 100782. [Google Scholar] [CrossRef]
- Roelen, B.A.J.; Chuva de Sousa Lopes, S.M. Stay on the road: From germ cell specification to gonadal colonization in mammals. Philos. Trans. R Soc. Lond. B Biol. Sci. 2022, 377, 20210259. [Google Scholar] [CrossRef]
- Ouni, E.; Bouzin, C.; Dolmans, M.M.; Marbaix, E.; Pyr Dit Ruys, S.; Vertommen, D.; Amorim, C.A. Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum. Reprod. 2020, 35, 1391–1410. [Google Scholar] [CrossRef]
- Gosden, R.G.; Mullan, J.; Picton, H.M.; Yin, H.; Tan, S.L. Current perspective on primordial follicle cryopreservation and culture for reproductive medicine. Hum. Reprod. Update 2002, 8, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Hovatta, O.; Silye, R.; Abir, R.; Krausz, T.; Winston, R.M. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum. Reprod. 1997, 12, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.R.; Yan, J.; Lu, C.L.; Xia, X.; Yin, T.L.; Zhi, X.; Zhu, X.H.; Ding, T.; Hu, W.H.; Guo, H.Y.; et al. Human single follicle growth in vitro from cryopreserved ovarian tissue after slow freezing or vitrification. Hum. Reprod. 2016, 31, 763–773. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Lawson, M.S.; Bean, Y.; Ting, A.Y.; Pejovic, T.; De Geest, K.; Moffitt, M.; Mitalipov, S.M.; Xu, J. Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Hum. Reprod. 2021, 36, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.L.; Kryger-Baggesen, N.; Byskov, A.G.; Andersen, C.Y. Anti-Mullerian hormone initiates growth of human primordial follicles in vitro. Mol. Cell. Endocrinol. 2005, 234, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, F.; Lawson, M.S.; Tkachenko, O.Y.; Ting, A.Y.; Kahl, C.A.; Park, B.S.; Stouffer, R.R.; Bishop, C.V. Anti-Mullerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol. Reprod. 2018, 98, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.N.; Pepin, D.; Czepnik, M.; Donahoe, P.K.; Thompson, T.B. Mutational Analysis of the Putative Anti-Mullerian Hormone (AMH) Binding Interface on its Type II Receptor, AMHR2. Endocrinology 2020, 161, bqaa066. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Schoen, J. Air-liquid interface cell culture: From airway epithelium to the female reproductive tract. Reprod. Domest. Anim. 2019, 54, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Tuck, A.R.; Prakash, C.R.; Damdimopoulos, A.; Sjodin, M.O.D.; Lindberg, J.; Niklasson, B.; Pettersson, K.; Hovatta, O.; Damdimopoulou, P. Culture of human ovarian tissue in xeno-free conditions using laminin components of the human ovarian extracellular matrix. J. Assist. Reprod. Genet. 2020, 37, 2137–2150. [Google Scholar] [CrossRef]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef] [Green Version]
- Gargus, E.S.; Rogers, H.B.; McKinnon, K.E.; Edmonds, M.E.; Woodruff, T.K. Engineered reproductive tissues. Nat. Biomed. Eng. 2020, 4, 381–393. [Google Scholar] [CrossRef]
- Mancini, V.; Pensabene, V. Organs-On-Chip Models of the Female Reproductive System. Bioengineering 2019, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, J.S.; Mancini, V.; Laverde Garay, M.; Asseler, J.D.; Fan, X.; Metzemaekers, J.; Louwe, L.A.; Pilgram, G.S.K.; van der Westerlaken, L.A.J.; van Mello, N.M.; et al. Dynamic in vitro culture of cryopreserved-thawed human ovarian cortical tissue using a microfluidics platform does not improve early folliculogenesis. Front. Endocrinol. 2022, 13, 936765. [Google Scholar] [CrossRef]
- Delgado-Rosas, F.; Gaytan, M.; Morales, C.; Gomez, R.; Gaytan, F. Superficial ovarian cortex vascularization is inversely related to the follicle reserve in normal cycling ovaries and is increased in polycystic ovary syndrome. Hum. Reprod. 2009, 24, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Demeestere, I.; Delbaere, A.; Gervy, C.; Van Den Bergh, M.; Devreker, F.; Englert, Y. Effect of preantral follicle isolation technique on in-vitro follicular growth, oocyte maturation and embryo development in mice. Hum. Reprod. 2002, 17, 2152–2159. [Google Scholar] [CrossRef] [Green Version]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Vanacker, J.; Amorim, C.A. Alginate: A Versatile Biomaterial to Encapsulate Isolated Ovarian Follicles. Ann. Biomed. Eng. 2017, 45, 1633–1649. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 2015, 5, 17323. [Google Scholar] [CrossRef] [Green Version]
- Skory, R.M.; Xu, Y.; Shea, L.D.; Woodruff, T.K. Engineering the ovarian cycle using in vitro follicle culture. Hum. Reprod. 2015, 30, 1386–1395. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewski, C.E.; DiLillo, K.M.; Baker, B.M.; Arnold, K.B.; Shikanov, A. Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation. Acta Biomater. 2021, 132, 313–324. [Google Scholar] [CrossRef]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In vitro ovarian follicle growth: A comprehensive analysis of key protocol variablesdagger. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Hobeika, M.; Cernogoraz, A.; Donnez, J.; Amorim, C.A. A modified and tailored human follicle isolation procedure improves follicle recovery and survival. J. Ovarian. Res. 2017, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Pors, S.E.; Ramlose, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Andersen, C.Y.; Kristensen, S.G. Initial steps in reconstruction of the human ovary: Survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Cheng, Y.; Sun, Y.P.; Zhai, J.; Diaz-Garcia, C.; Simon, C.; Pellicer, A.; Hsueh, A.J. Ovary transplantation: To activate or not to activate. Hum. Reprod. 2015, 30, 2457–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, K.; Kawamura, N.; Hsueh, A.J. Activation of dormant follicles: A new treatment for premature ovarian failure? Curr. Opin. Obstet. Gynecol. 2016, 28, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Grosbois, J.; Bailie, E.C.; Kelsey, T.W.; Anderson, R.A.; Telfer, E.E. Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture. Hum. Reprod. 2023, 38, 444–458. [Google Scholar] [CrossRef]
- De Roo, C.; Tilleman, K.; Vercruysse, C.; Declercq, H.; T’Sjoen, G.; Weyers, S.; De Sutter, P. Texture profile analysis reveals a stiffer ovarian cortex after testosterone therapy: A pilot study. J. Assist. Reprod. Genet. 2019, 36, 1837–1843. [Google Scholar] [CrossRef] [Green Version]
- Nagamatsu, G.; Shimamoto, S.; Hamazaki, N.; Nishimura, Y.; Hayashi, K. Mechanical stress accompanied with nuclear rotation is involved in the dormant state of mouse oocytes. Sci. Adv. 2019, 5, eaav9960. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Rajareddy, S.; Reddy, P.; Du, C.; Jagarlamudi, K.; Shen, Y.; Gunnarsson, D.; Selstam, G.; Boman, K.; Liu, K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007, 134, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Angelou, K.; Grigoriadis, T.; Diakosavvas, M.; Zacharakis, D.; Athanasiou, S. The Genitourinary Syndrome of Menopause: An Overview of the Recent Data. Cureus 2020, 12, e7586. [Google Scholar] [CrossRef] [Green Version]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Lee, Y.J.; Baek, S.; Chung, Y.S.; Kim, D.H.; Lee, J.H.; Shin, Y.C.; Shin, Y.M.; Ryu, C.; Kim, H.S.; et al. Hormone autocrination by vascularized hydrogel delivery of ovary spheroids to rescue ovarian dysfunctions. Sci. Adv. 2021, 7, eabe8873. [Google Scholar] [CrossRef] [PubMed]
- Pierson Smela, M.D.; Kramme, C.C.; Fortuna, P.R.J.; Adams, J.L.; Su, A.R.; Dong, E.; Kobayashi, M.; Brixi, G.; Kavirayuni, V.S.; Tysinger, E.; et al. Directed differentiation of human iPSCs to functional ovarian granulosa-like cells via transcription factor overexpression. Elife 2023, 12, e83291. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Niu, Z.; Lin, N.; Gu, R.; Sun, Y.; Feng, Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J. Clin. Endocrinol. Metab. 2014, 99, E2269–E2276. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Ma, Y.; Tong, X.; Yang, W.; Dai, Y.; Pan, Y.; Ren, P.; Liu, L.; Fan, H.Y.; Zhang, Y.; et al. Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum. Reprod. 2020, 35, 1145–1158. [Google Scholar] [CrossRef]
- Rice, S.; Christoforidis, N.; Gadd, C.; Nikolaou, D.; Seyani, L.; Donaldson, A.; Margara, R.; Hardy, K.; Franks, S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum. Reprod. 2005, 20, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Liao, B.; Qi, X.; Yun, C.; Qiao, J.; Pang, Y. Effects of Androgen Excess-Related Metabolic Disturbances on Granulosa Cell Function and Follicular Development. Front. Endocrinol. 2022, 13, 815968. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer. 2011, 11, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Kopper, O.; de Witte, C.J.; Lohmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef]
- Maenhoudt, N.; Defraye, C.; Boretto, M.; Jan, Z.; Heremans, R.; Boeckx, B.; Hermans, F.; Arijs, I.; Cox, B.; Van Nieuwenhuysen, E.; et al. Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models. Stem Cell Rep. 2020, 14, 717–729. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- van Baal, J.; van Noorden, C.J.F.; Nieuwland, R.; Van de Vijver, K.K.; Sturk, A.; van Driel, W.J.; Kenter, G.G.; Lok, C.A.R. Development of Peritoneal Carcinomatosis in Epithelial Ovarian Cancer: A Review. J. Histochem. Cytochem. 2018, 66, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Desai, R.R.; Muthuswamy, S.K. Reinterpreting polarity and cancer: The changing landscape from tumor suppression to tumor promotion. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 103–116. [Google Scholar] [CrossRef]
- Canet-Jourdan, C.; Pages, D.L.; Nguyen-Vigouroux, C.; Cartry, J.; Zajac, O.; Desterke, C.; Lopez, J.B.; Gutierrez-Mateyron, E.; Signolle, N.; Adam, J.; et al. Patient-derived organoids identify an apico-basolateral polarity switch associated with survival in colorectal cancer. J. Cell Sci. 2022, 135, jcs259256. [Google Scholar] [CrossRef]
- Okuyama, H.; Kondo, J.; Sato, Y.; Endo, H.; Nakajima, A.; Piulats, J.M.; Tomita, Y.; Fujiwara, T.; Itoh, Y.; Mizoguchi, A.; et al. Dynamic Change of Polarity in Primary Cultured Spheroids of Human Colorectal Adenocarcinoma and Its Role in Metastasis. Am. J. Pathol. 2016, 186, 899–911. [Google Scholar] [CrossRef] [Green Version]
- Kawata, M.; Kondo, J.; Onuma, K.; Ito, Y.; Yokoi, T.; Hamanishi, J.; Mandai, M.; Kimura, T.; Inoue, M. Polarity switching of ovarian cancer cell clusters via SRC family kinase is involved in the peritoneal dissemination. Cancer Sci. 2022, 113, 3437–3448. [Google Scholar] [CrossRef]
- Lohmussaar, K.; Kopper, O.; Korving, J.; Begthel, H.; Vreuls, C.P.H.; van Es, J.H.; Clevers, H. Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids. Nat. Commun. 2020, 11, 2660. [Google Scholar] [CrossRef]
- Zhang, S.; Dolgalev, I.; Zhang, T.; Ran, H.; Levine, D.A.; Neel, B.G. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat. Commun. 2019, 10, 5367. [Google Scholar] [CrossRef] [Green Version]
- Coscia, F.; Watters, K.M.; Curtis, M.; Eckert, M.A.; Chiang, C.Y.; Tyanova, S.; Montag, A.; Lastra, R.R.; Lengyel, E.; Mann, M. Integrative proteomic profiling of ovarian cancer cell lines reveals precursor cell associated proteins and functional status. Nat. Commun. 2016, 7, 12645. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Organoids for Drug Discovery and Personalized Medicine. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Newtson, A.M.; Zhang, Y.; Devor, E.J.; Samuelson, M.I.; Thiel, K.W.; Leslie, K.K. Successful Patient-Derived Organoid Culture of Gynecologic Cancers for Disease Modeling and Drug Sensitivity Testing. Cancers 2021, 13, 2910. [Google Scholar] [CrossRef]
- Gorski, J.W.; Zhang, Z.; McCorkle, J.R.; DeJohn, J.M.; Wang, C.; Miller, R.W.; Gallion, H.H.; Dietrich, C.S.; Ueland, F.R.; Kolesar, J.M. Utilizing Patient-Derived Epithelial Ovarian Cancer Tumor Organoids to Predict Carboplatin Resistance. Biomedicines 2021, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Nanki, Y.; Chiyoda, T.; Hirasawa, A.; Ookubo, A.; Itoh, M.; Ueno, M.; Akahane, T.; Kameyama, K.; Yamagami, W.; Kataoka, F.; et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci. Rep. 2020, 10, 12581. [Google Scholar] [CrossRef]
- Tao, M.; Sun, F.; Wang, J.; Wang, Y.; Zhu, H.; Chen, M.; Liu, L.; Liu, L.; Lin, H.; Wu, X. Developing patient-derived organoids to predict PARP inhibitor response and explore resistance overcoming strategies in ovarian cancer. Pharmacol. Res. 2022, 179, 106232. [Google Scholar] [CrossRef]
- de Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lohmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef]
- Phan, N.; Hong, J.J.; Tofig, B.; Mapua, M.; Elashoff, D.; Moatamed, N.A.; Huang, J.; Memarzadeh, S.; Damoiseaux, R.; Soragni, A. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2019, 2, 78. [Google Scholar] [CrossRef]
- Lass, A. Assessment of ovarian reserve—Is there a role for ovarian biopsy? Hum. Reprod. 2001, 16, 1055–1057. [Google Scholar] [CrossRef]
- Schmidt, K.L.; Byskov, A.G.; Nyboe Andersen, A.; Muller, J.; Yding Andersen, C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum. Reprod. 2003, 18, 1158–1164. [Google Scholar] [CrossRef]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef]
- Grimm, D. EPA plan to end animal testing splits scientists. Science 2019, 365, 1231. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Valle, J.S.; Chuva de Sousa Lopes, S.M. Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction. Bioengineering 2023, 10, 832. https://doi.org/10.3390/bioengineering10070832
Del Valle JS, Chuva de Sousa Lopes SM. Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction. Bioengineering. 2023; 10(7):832. https://doi.org/10.3390/bioengineering10070832
Chicago/Turabian StyleDel Valle, Julieta S., and Susana M. Chuva de Sousa Lopes. 2023. "Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction" Bioengineering 10, no. 7: 832. https://doi.org/10.3390/bioengineering10070832
APA StyleDel Valle, J. S., & Chuva de Sousa Lopes, S. M. (2023). Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction. Bioengineering, 10(7), 832. https://doi.org/10.3390/bioengineering10070832





