Multifunctional Sodium Hyaluronate/Chitosan Foam Used as an Absorbable Hemostatic Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sodium Hyaluronate/Carboxymethyl Chitosan Absorbable Hemostatic Foam (SHCF)
2.3. Characterization of SHCF
2.4. Water Absorption Performance Test
2.5. Gel-Forming Performance Test
2.6. In Vitro Dynamic Coagulation
2.7. In Vitro Red Blood Cell (RBC) Adsorption
2.8. In Vivo Hemostasis and Anti-Adhesion Evaluation
2.9. Evaluation of Cytotoxicity and Hemolysis Rate
2.10. Inflammatory Factors Level Test
2.11. In Vitro Degradation Test
2.12. Metabolic Studies
2.13. Subcutaneous Implantation Test
3. Results and Discussion
3.1. Synthesis and Characterization of SHCF
3.2. Liquid Absorption and Gel-Forming Properties of SHCF
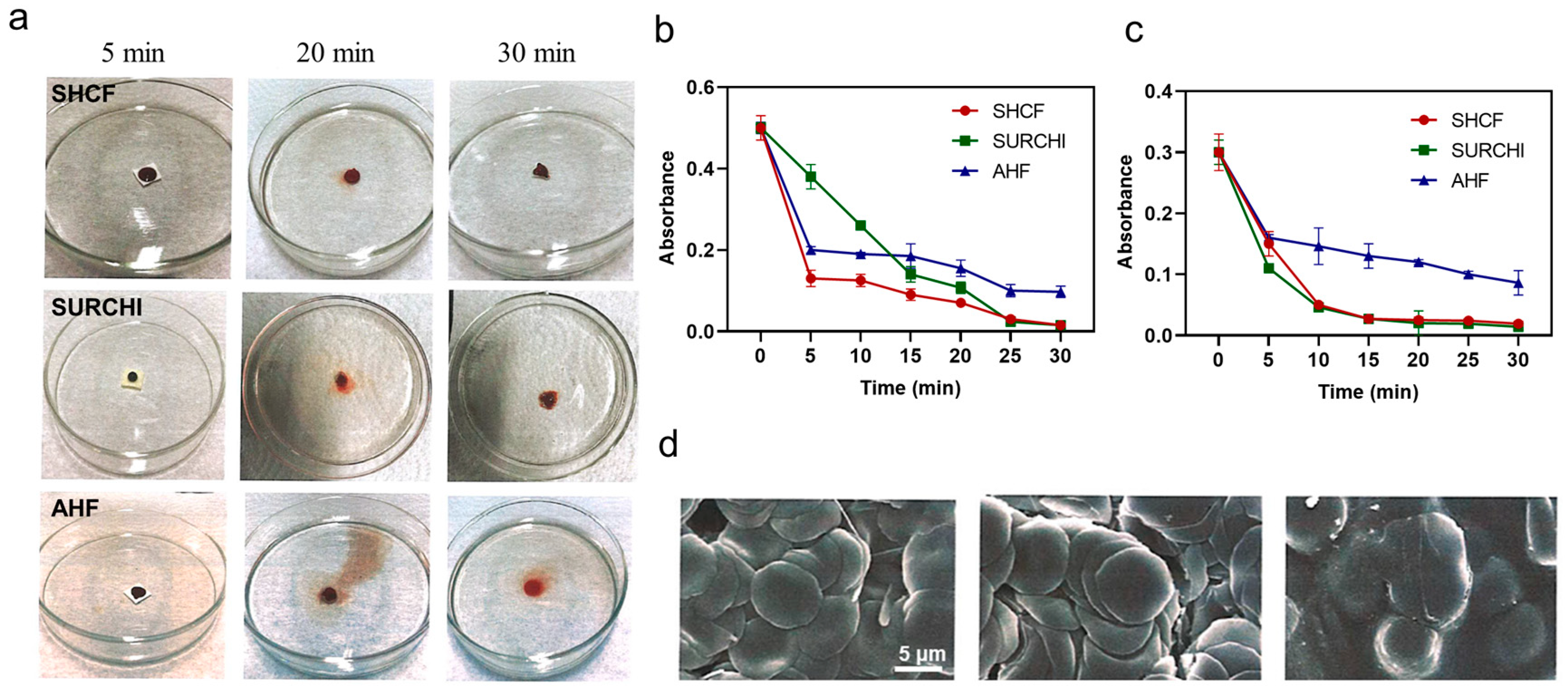
3.3. In Vitro Coagulation and RBC Enrichment Capacity of SHCF
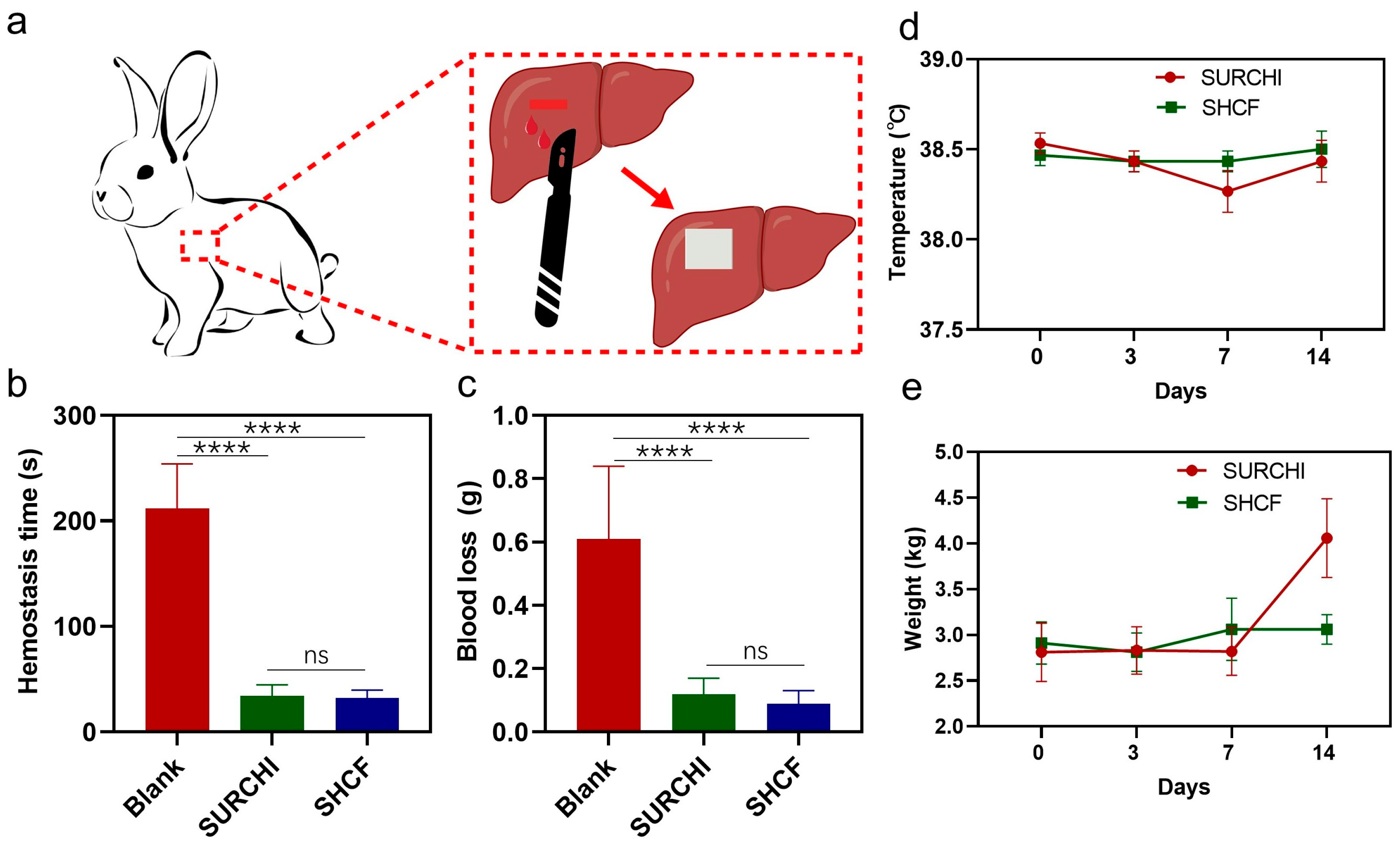
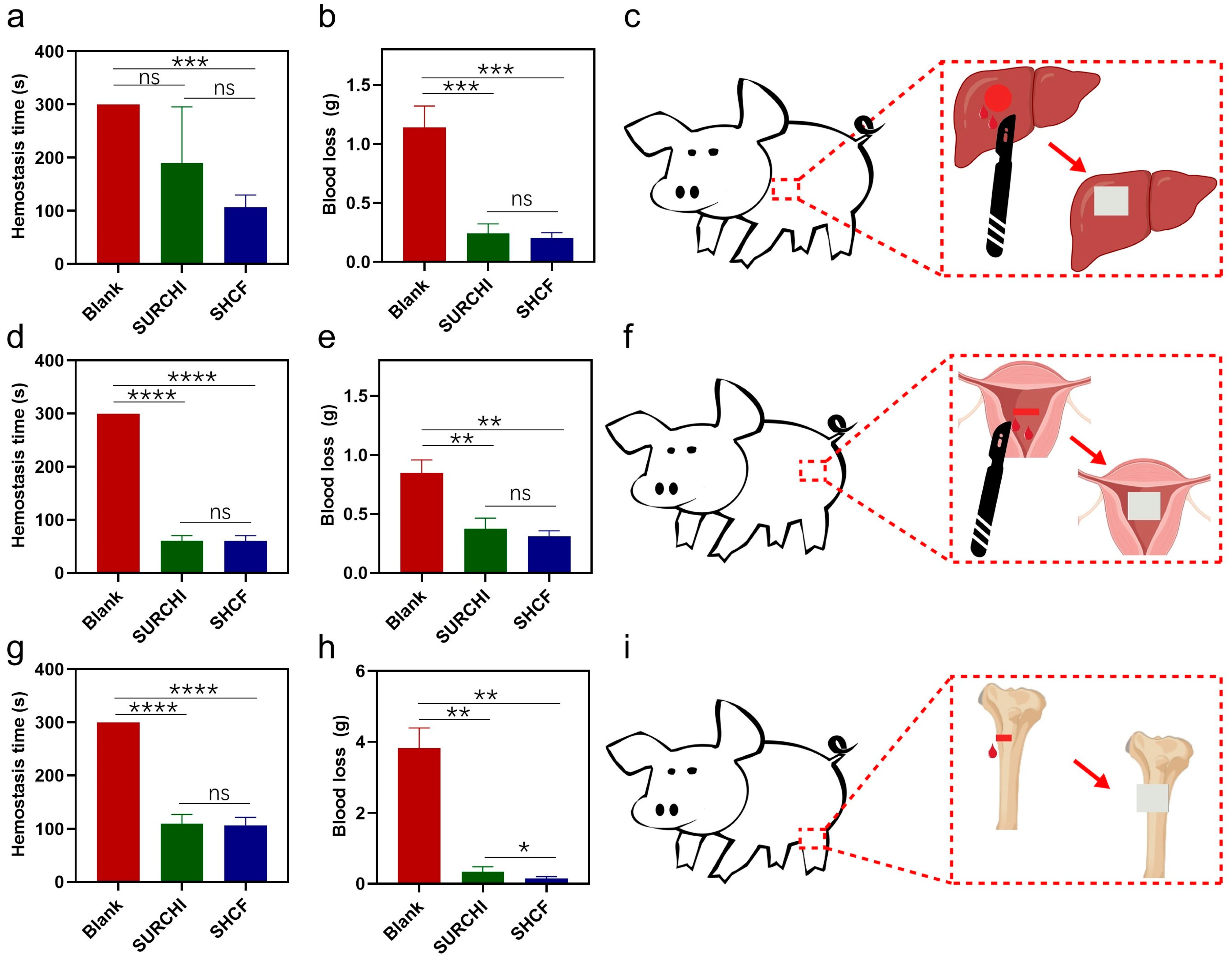
3.4. In Vivo Hemostatic Properties of SHCF
3.5. Anti-Adhesive Properties of SHCF
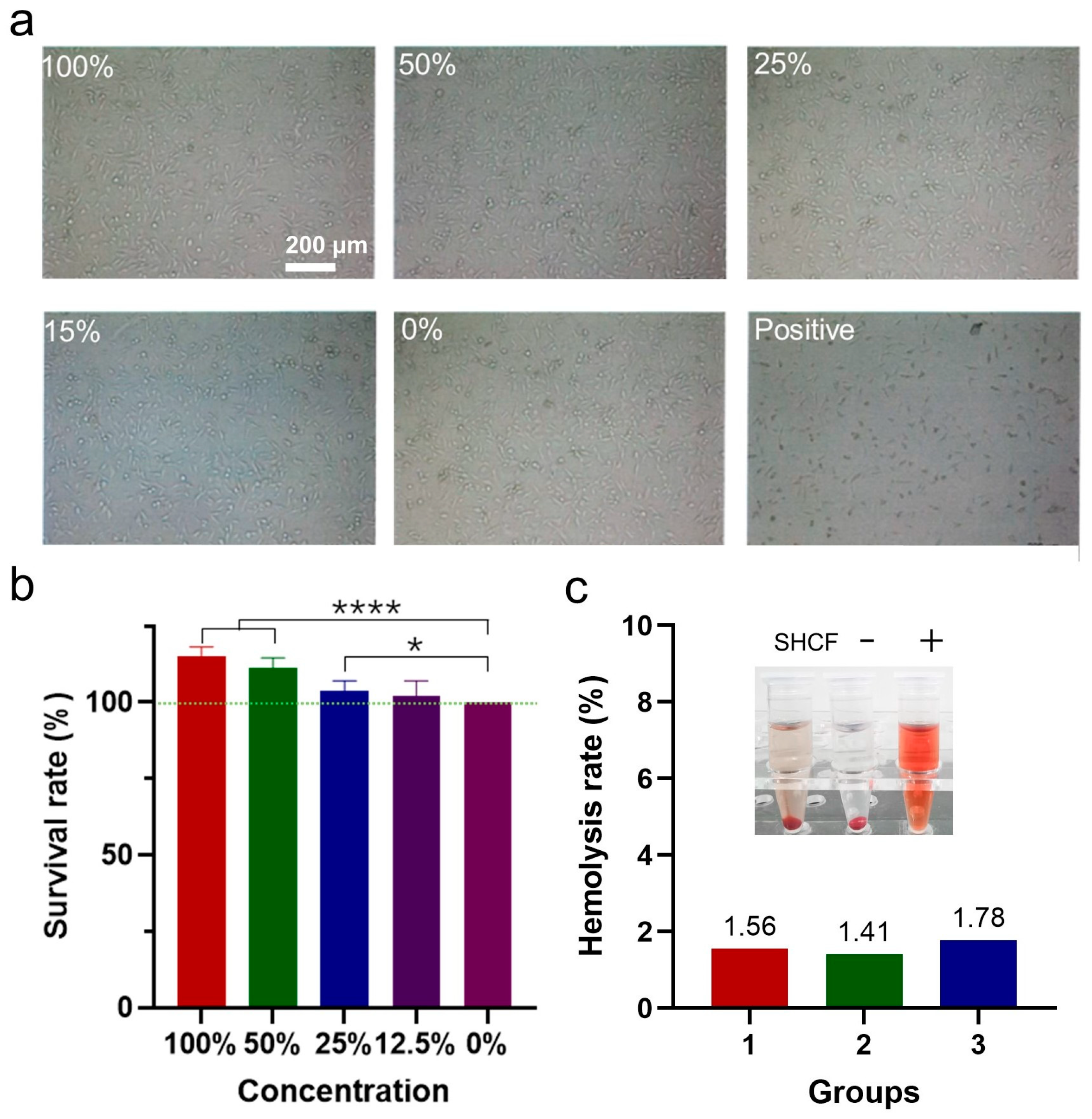
3.6. Biosafety of SHCF
3.7. Degradation and Metabolic Properties of SHCF
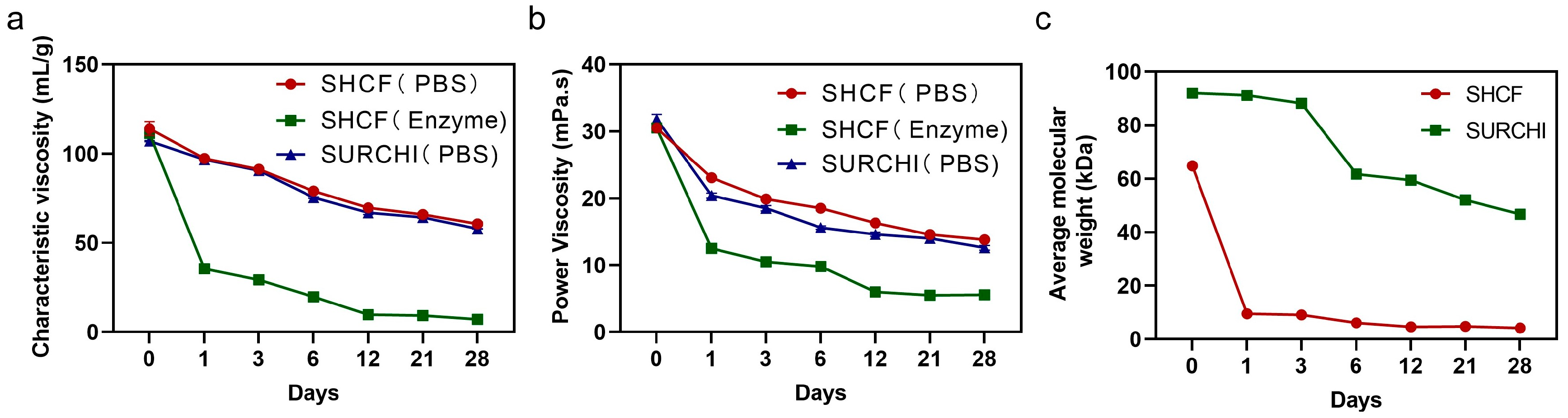
3.8. Absorbable Properties of SHCF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Carcao, M. Inherited abnormalities of coagulation: Hemophilia, von Willebrand disease, and beyond. Pediatr. Clin. 2013, 60, 1419–1441. [Google Scholar]
- Hong, C.; Alser, O.; Gebran, A.; Joo, W.; Kokoroskos, N.; Velmahos, G.; Olsen, B.D.; Hammond, P.T. Modulating Nanoparticle Size to Understand Factors Affecting Hemostatic Efficacy and Maximize Survival in a Lethal Inferior Vena Cava Injury Model. ACS Nano 2022, 16, 2494–2510. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef]
- Li, X.-F.; Lu, P.; Jia, H.-R.; Li, G.; Zhu, B.; Wang, X.; Wu, F.-G. Emerging materials for hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Yu, L.; Shang, X.; Chen, H.; Xiao, L.; Zhu, Y.; Fan, J. A tightly-bonded and flexible mesoporous zeolite-cotton hybrid hemostat. Nat. Commun. 2019, 10, 1932. [Google Scholar] [CrossRef]
- Harkins, A.L.; Duri, S.; Kloth, L.C.; Tran, C.D. Chitosan–cellulose composite for wound dressing material. Part 2. Antimicrobial activity, blood absorption ability, and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1199–1206. [Google Scholar] [CrossRef]
- Zhai, Z.; Xu, K.; Mei, L.; Wu, C.; Liu, J.; Liu, Z.; Wan, L.; Zhong, W. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing. Soft Matter 2019, 15, 8603–8610. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Colloids Surf. B Biointerfaces 2015, 136, 1026–1034. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef]
- Wu, B.; Du, F.; Wenjing, A.; Li, G.; Wang, X. Graphene-based hemostatic sponge. Chin. Chem. Lett. 2022, 33, 703–713. [Google Scholar] [CrossRef]
- Maesen, T. The zeolite scene–anoverview. Introd. Zeolite Mol. Sieves 2007, 38, 1. [Google Scholar]
- Wang, L.; You, X.; Dai, C.; Tong, T.; Wu, J. Hemostatic nanotechnologies for external and internal hemorrhage management. Biomater. Sci. 2020, 8, 4396–4412. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. International journal of biological macromolecules novel electrospun chitosan/polyvinyl alcohol/zinc oxide nano fi brous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Fathi, P.; Sikorski, M.; Christodoulides, K.; Langan, K.; Choi, Y.S.; Titcomb, M.; Ghodasara, A.; Wonodi, O.; Thaker, H.; Vural, M. Zeolite-loaded alginate-chitosan hydrogel beads as a topical hemostat. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1662–1671. [Google Scholar] [CrossRef]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef]
- Sena, M.J.; Douglas, G.; Gerlach, T.; Grayson, J.K.; Pichakron, K.O.; Zierold, D. A pilot study of the use of kaolin-impregnated gauze (Combat Gauze) for packing high-grade hepatic injuries in a hypothermic coagulopathic swine model. J. Surg. Res. 2013, 183, 704–709. [Google Scholar] [CrossRef]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.; Yang, Q.; Mao, L.; Yang, H. Emerging nanoclay composite for effective hemostasis. Adv. Funct. Mater. 2018, 28, 1704452. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, X.; Zhang, L.; Wang, H.; Zhang, T.; Bai, R.; Sun, Q.; Wang, X.; Yu, T.; Wu, D. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Sarrafian, T.L.; Mao, X.; Varela, C.E.; Roche, E.T.; Griffiths, L.G.; Nabzdyk, C.S.; Zhao, X. Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue. Nat. Biomed. Eng. 2021, 5, 1131–1142. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Y.; Nie, J.; Lu, W.; Yang, L.; Zhang, Z.; Yin, H.; Wang, Z.; Hu, Q. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int. J. Biol. Macromol. 2015, 75, 322–329. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, S.; Wang, X.; Zhang, Y.; Chen, L.; Zhang, L. Noncompressible hemostasis and bone regeneration induced by an absorbable bioadhesive self-healing hydrogel. Adv. Funct. Mater. 2021, 31, 2009189. [Google Scholar] [CrossRef]
- Gabay, M. Absorbable hemostatic agents. Am. J. Health-Syst. Pharm. 2006, 63, 1244–1253. [Google Scholar] [CrossRef]
- Chen, S.; Carlson, M.A.; Zhang, Y.S.; Hu, Y.; Xie, J. Fabrication of injectable and superelastic nanofiber rectangle matrices (“peanuts”) and their potential applications in hemostasis. Biomaterials 2018, 179, 46–59. [Google Scholar] [CrossRef]
- Yang, X.; Chen, M.; Li, P.; Ji, Z.; Wang, M.; Feng, Y.; Shi, C. Fabricating poly (vinyl alcohol)/gelatin composite sponges with high absorbency and water-triggered expansion for noncompressible hemorrhage and wound healing. J. Mater. Chem. B 2021, 9, 1568–1582. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef]
- Sawada, D.; Ogawa, Y.; Kimura, S.; Nishiyama, Y.; Langan, P.; Wada, M. Solid–solvent molecular interactions observed in crystal structures of β-chitin complexes. Cellulose 2014, 21, 1007–1014. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Ouaïssi, M.; Gaujoux, S.; Veyrie, N.; Denève, E.; Brigand, C.; Castel, B.; Duron, J.; Rault, A.; Slim, K.; Nocca, D. Post-operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J. Visc. Surg. 2012, 149, e104–e114. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A.; Zarzycki, A.N.; Jeon, K.; Deniset, J.F.; Fedak, P.W. Post-operative adhesions: A comprehensive review of mechanisms. Biomedicines 2021, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, H.; Chen, L.; Bao, X.; Li, Y.; Chen, L.; Xu, G.; Ye, X.; Ding, J. Comparative studies of thermogels in preventing post-operative adhesions and corresponding mechanisms. Biomater. Sci. 2014, 2, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Hassanabad, A.; Zarzycki, A.N.; Jeon, K.; Dundas, J.A.; Vasanthan, V.; Deniset, J.F.; Fedak, P.W. Prevention of post-operative adhesions: A comprehensive review of present and emerging strategies. Biomolecules 2021, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Lim, C.; Israelachvili, J.N.; Hwang, D.S. Strong adhesion and cohesion of chitosan in aqueous solutions. Langmuir 2013, 29, 14222–14229. [Google Scholar] [CrossRef] [PubMed]
- Mati-Baouche, N.; Elchinger, P.-H.; de Baynast, H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdara, N.; Ashutosh Tiwari, N. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, S.; Zhang, H.; Dong, L.; Cong, Y.; Sun, S.; Sun, X. Polysaccharides composite materials for rapid hemostasis. J. Drug Deliv. Sci. Technol. 2021, 66, 102890. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; You, R.; Tariq, Z.; Huang, J.; Li, M.; Yan, S. Silk fibroin/hyaluronic acid porous scaffold for dermal wound healing. Fibers Polym. 2017, 18, 1056–1063. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Li, Y.; Hu, Y.; Cheng, G.; Ye, E.; Shen, C.; Xu, F.-J. Hemostatic porous sponges of cross-linked hyaluronic acid/cationized dextran by one self-foaming process. Mater. Sci. Eng. C 2018, 83, 160–168. [Google Scholar] [CrossRef]
- An, S.; Jeon, E.J.; Jeon, J.; Cho, S.-W. A serotonin-modified hyaluronic acid hydrogel for multifunctional hemostatic adhesives inspired by a platelet coagulation mediator. Mater. Horiz. 2019, 6, 1169–1178. [Google Scholar] [CrossRef]
- Wang, C.; Liang, Y.; Huang, Y.; Li, M.; Guo, B. Porous photothermal antibacterial antioxidant dual–crosslinked cryogel based on hyaluronic acid/polydopamine for non-compressible hemostasis and infectious wound repair. J. Mater. Sci. Technol. 2022, 121, 207–219. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, L.; Zhang, Q.; Sun, Y.; Chen, C.; Xu, H.; Chai, Y.; Lang, M. In situ thiolated alginate hydrogel: Instant formation and its application in hemostasis. J. Biomater. Appl. 2016, 31, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ji, Z.; Xu, M.; Liu, C.; Ye, X.; Zhang, W.; Li, S.; Wang, D.; Zhang, W.; Chen, J. Microspheres of carboxymethyl chitosan, sodium alginate, and collagen as a hemostatic agent in vivo. ACS Biomater. Sci. Eng. 2018, 4, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhou, X.; Wang, P.; Li, J.; Wu, Z.; Jiao, Z.; Guo, M.; Wang, Z.; Wang, Y.; Wang, L. Biodegradable alginate-based sponge with antibacterial and shape memory properties for penetrating wound hemostasis. Compos. Part B Eng. 2022, 247, 110263. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Shi, Y.; Feng, Y.; Ren, X.; Shi, C. Fabricating antimicrobial peptide-immobilized starch sponges for hemorrhage control and antibacterial treatment. Carbohydr. Polym. 2019, 222, 115012. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- Lan, G.; Li, Q.; Lu, F.; Yu, K.; Lu, B.; Bao, R.; Dai, F. Improvement of platelet aggregation and rapid induction of hemostasis in chitosan dressing using silver nanoparticles. Cellulose 2020, 27, 385–400. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Liu, Z.-H.; Kuo, C.-Y.; Chen, J.-P. Photo-crosslinked hyaluronic acid/carboxymethyl cellulose composite hydrogel as a dural substitute to prevent post-surgical adhesion. Int. J. Mol. Sci. 2022, 23, 6177. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Mao, S.-H.; Tsai, M.-J.; Chou, P.-Y.; Liao, C.-H.; Chen, J.-P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef]
- Lih, E.; Oh, S.H.; Joung, Y.K.; Lee, J.H.; Han, D.K. Polymers for cell/tissue anti-adhesion. Prog. Polym. Sci. 2015, 44, 28–61. [Google Scholar] [CrossRef]
- Akrami, M.; Samimi, S.; Alipour, M.; Bardania, H.; Ramezanpour, S.; Najafi, N.; Hosseinkhani, S.; Kamankesh, M.; Haririan, I.; Hassanshahi, F. Potential anticancer activity of a new pro-apoptotic peptide–thioctic acid gold nanoparticle platform. Nanotechnology 2021, 32, 145101. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Silva, J.C.; Colaco, B.; Gama, A.; Duarte-Araújo, M.; Fernandes, M.H.; Bettencourt, A.; Gomes, P. In vivo tissue response and antibacterial efficacy of minocycline delivery system based on polymethylmethacrylate bone cement. J. Biomater. Appl. 2018, 33, 380–391. [Google Scholar] [CrossRef] [PubMed]





| H&E Morphological Characteristics of Tissue | ||||
|---|---|---|---|---|
| Inflammatory Response | Angiogenesis | Material Degradation | New Tissue Formation | |
| Week 4 | +++ | 0 | + | +/++ |
| Week 12 | +/++ | ++ | +++ | +/++ |
| Week 16 | +/++ | +++ | +++ | +/++ |
| Week 20 | + | 0 | +++ | ++ |
| Week 24 | + | 0 | +++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Du, F.; Yuan, Q. Multifunctional Sodium Hyaluronate/Chitosan Foam Used as an Absorbable Hemostatic Material. Bioengineering 2023, 10, 868. https://doi.org/10.3390/bioengineering10070868
Chen R, Du F, Yuan Q. Multifunctional Sodium Hyaluronate/Chitosan Foam Used as an Absorbable Hemostatic Material. Bioengineering. 2023; 10(7):868. https://doi.org/10.3390/bioengineering10070868
Chicago/Turabian StyleChen, Ran, Fanglin Du, and Qipeng Yuan. 2023. "Multifunctional Sodium Hyaluronate/Chitosan Foam Used as an Absorbable Hemostatic Material" Bioengineering 10, no. 7: 868. https://doi.org/10.3390/bioengineering10070868
APA StyleChen, R., Du, F., & Yuan, Q. (2023). Multifunctional Sodium Hyaluronate/Chitosan Foam Used as an Absorbable Hemostatic Material. Bioengineering, 10(7), 868. https://doi.org/10.3390/bioengineering10070868







