Real-Time Tracking of Laryngeal Motion via the Surface Depth-Sensing Technique for Radiotherapy in Laryngeal Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Simulation, RT Planning, and Beam Delivery
2.3. Setup for the SDS Technique
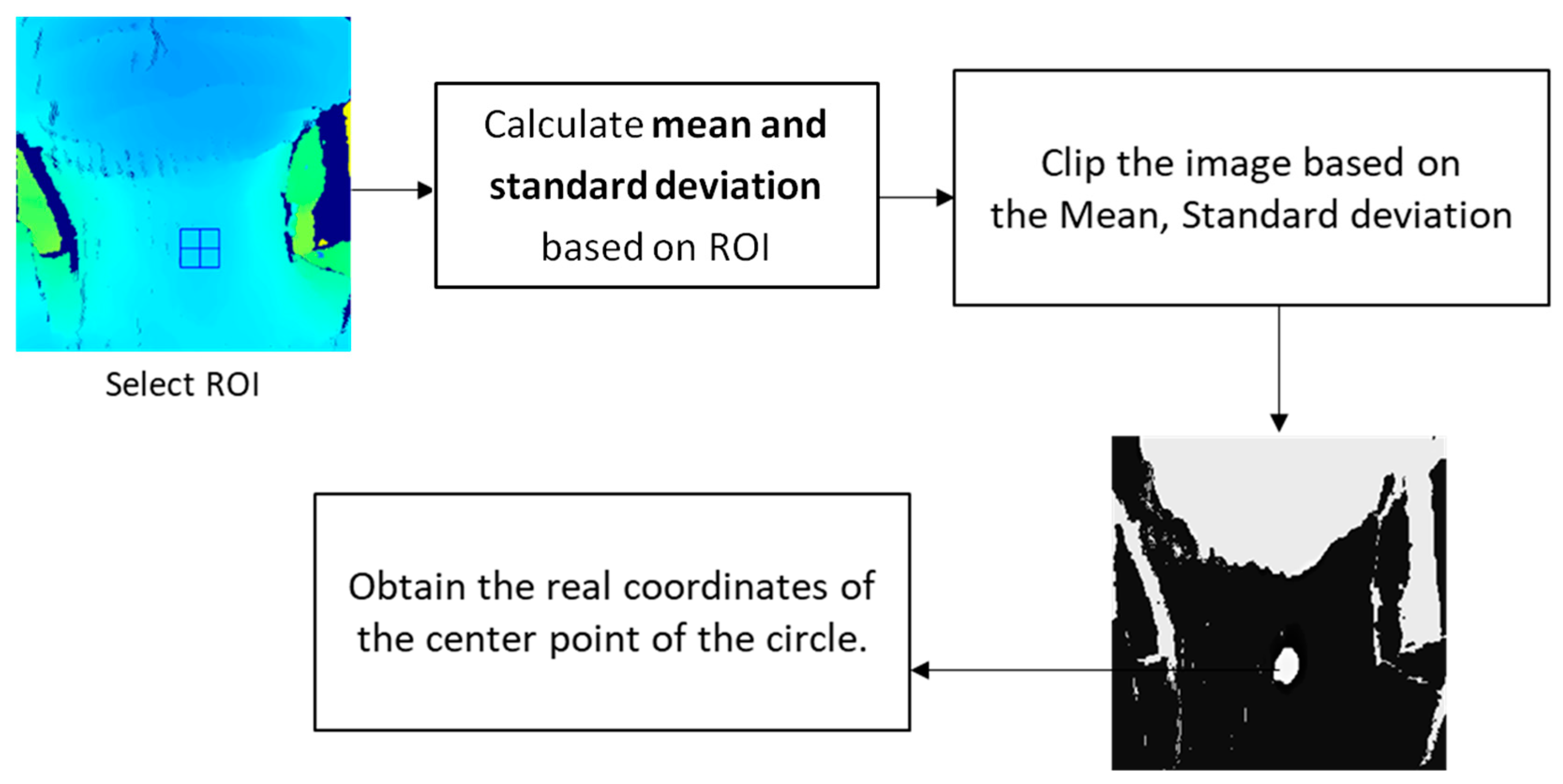
2.4. Algorithm for the Surface Depth Calculation
2.4.1. Image Optimization
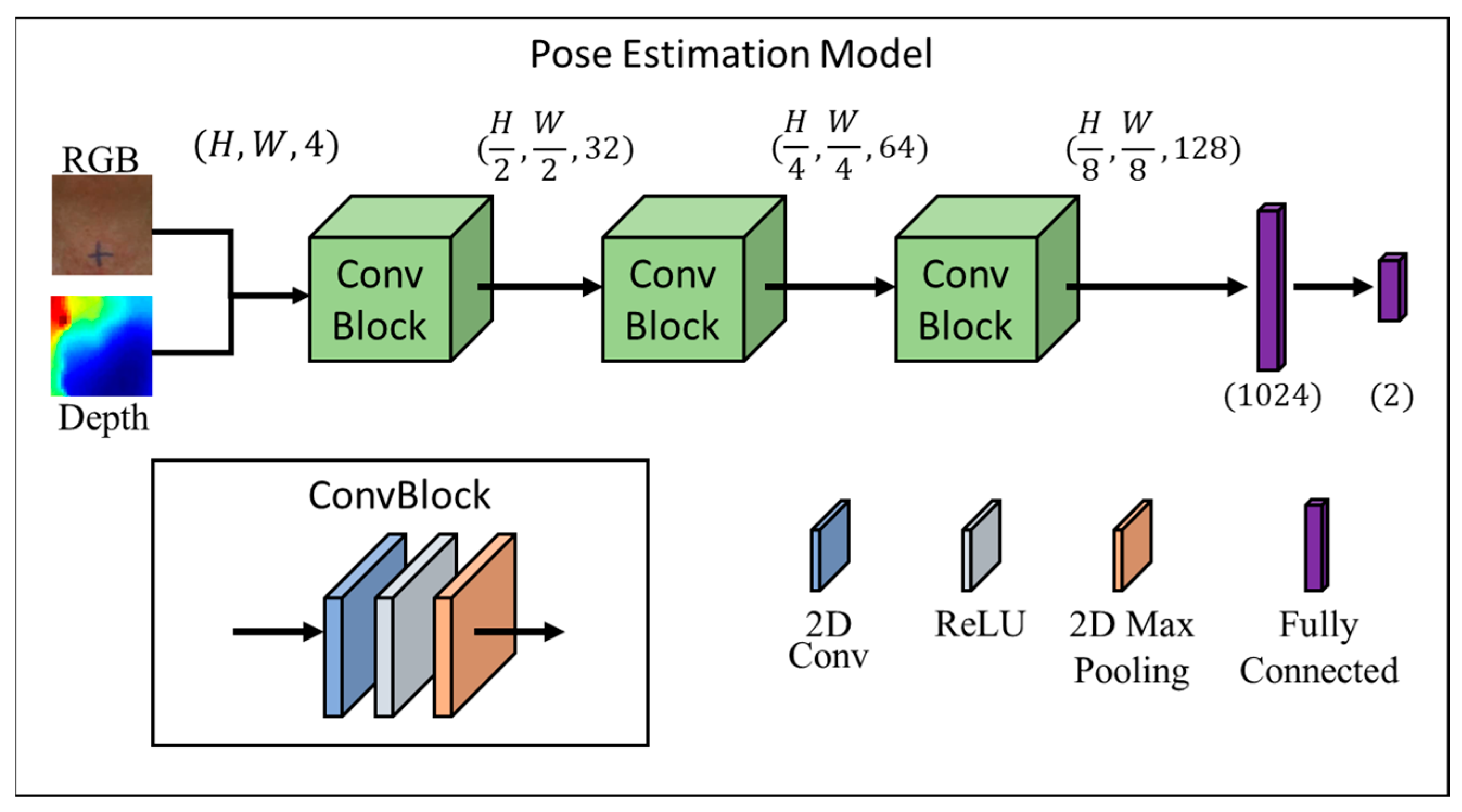
2.4.2. Algorithm for the Surface Depth Calculation
2.4.3. Preliminary Information
2.4.4. ConvBlock
2.4.5. Real-World Coordinates
2.4.6. Implementation Detail
2.5. Validation of Reproducibility
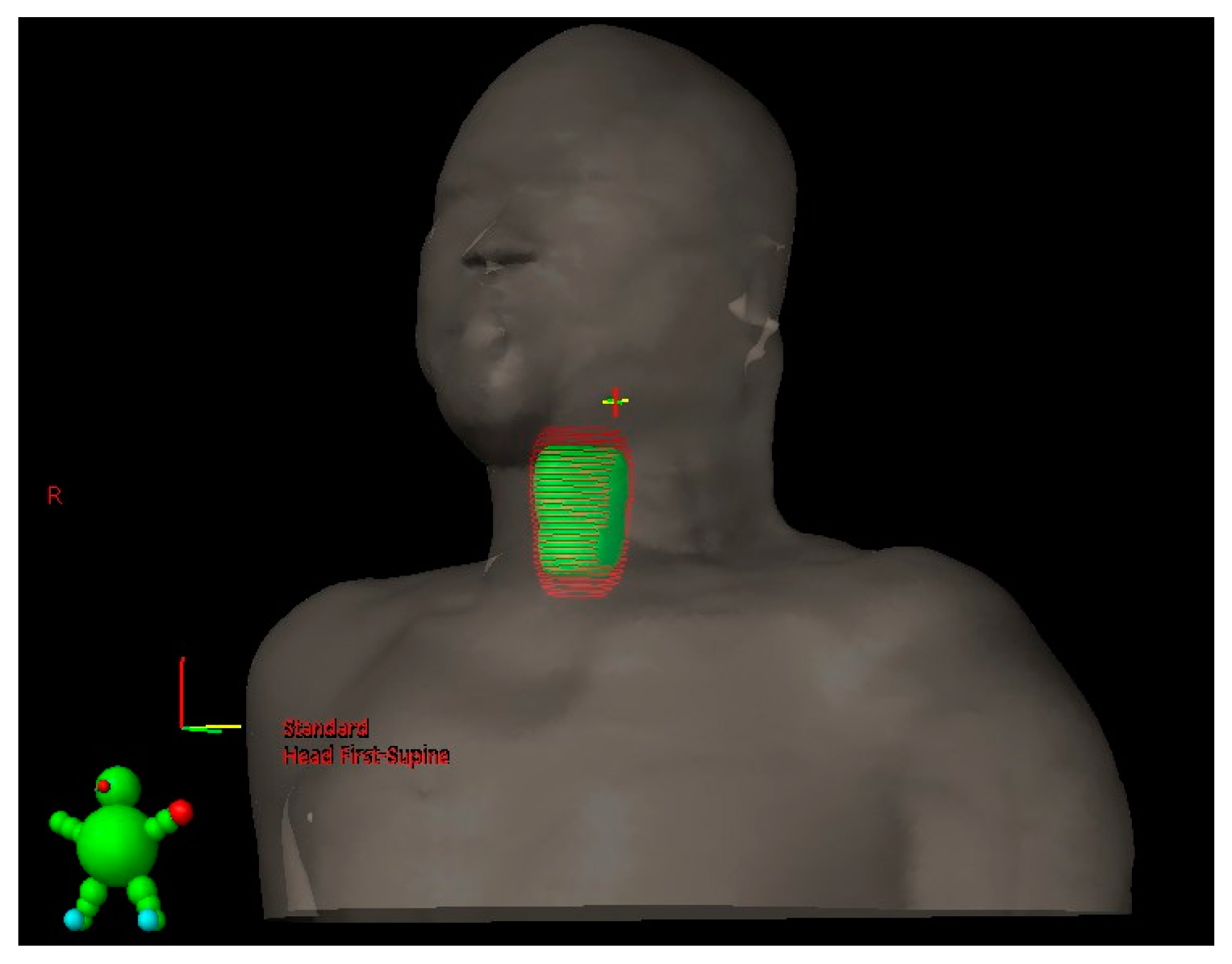
2.6. Application of SDS Data to Generate the PTV
2.7. Statistical Analysis
3. Results
3.1. Subject Enrollment
3.2. Calculation of Laryngeal Motion
3.3. PTV Margin with and without SDS
3.4. Time of Performance
3.5. Perception of Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Abdurehim, Y.; Hua, Z.; Yasin, Y.; Xukurhan, A.; Imam, I.; Yuqin, F. Transoral laser surgery versus radiotherapy: Systematic review and meta-analysis for treatment options of T1a glottic cancer. Head. Neck 2012, 34, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Warner, L.; Chudasama, J.; Kelly, C.G.; Loughran, S.; McKenzie, K.; Wight, R.; Dey, P. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst. Rev. 2014, 2014, CD002027. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.L.; Andry, G.; Chevalier, D.; Luboinski, B.; Collette, L.; Traissac, L.; de Raucourt, D.; Langendijk, J.A.; Head, E.; Neck Cancer, G. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann. Oncol. 2012, 23, 2708–2714. [Google Scholar] [CrossRef]
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; Laramore, G.E.; Endicott, J.W.; McClatchey, K.; Henderson, W.G. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar]
- Hamlet, S.; Ezzell, G.; Aref, A. Larynx motion associated with swallowing during radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 467–470. [Google Scholar] [CrossRef]
- Le, Q.T.; Fu, K.K.; Kroll, S.; Ryu, J.K.; Quivey, J.M.; Meyler, T.S.; Krieg, R.M.; Phillips, T.L. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 115–126. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nishiyama, K.; Tanaka, E.; Koizumi, M.; Chatani, M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 77–82. [Google Scholar] [CrossRef]
- Gowda, R.V.; Henk, J.M.; Mais, K.L.; Sykes, A.J.; Swindell, R.; Slevin, N.J. Three weeks radiotherapy for T1 glottic cancer: The Christie and Royal Marsden Hospital Experience. Radiother. Oncol. 2003, 68, 105–111. [Google Scholar] [CrossRef]
- Kang, B.H.; Yu, T.; Kim, J.H.; Park, J.M.; Kim, J.I.; Chung, E.J.; Kwon, S.K.; Kim, J.H.; Wu, H.G. Early Closure of a Phase 1 Clinical Trial for SABR in Early-Stage Glottic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 104–109. [Google Scholar] [CrossRef]
- Her, D.J.; Timmerman, R.D.; Nedzi, L.; Ding, C.; Pham, N.L.; Zhao, B.; Sumer, B.D. Phase 1 Fractional Dose-Escalation Study of Equipotent Stereotactic Radiation Therapy Regimens for Early-Stage Glottic Larynx Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 110–118. [Google Scholar]
- Zhao, B.; Park, Y.K.; Gu, X.; Reynolds, R.; Timmerman, R.; Sher, D.J. Surface guided motion management in glottic larynx stereotactic body radiation therapy. Radiother. Oncol. 2020, 153, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Perillo, A.; Landoni, V.; Farneti, A.; Sanguineti, G. Organ motion in linac-based SBRT for glottic cancer. Radiat. Oncol. 2021, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Paulson, E.S.; Ahunbay, E.; Schultz, C.; Li, X.A.; Wang, D. Dynamic MRI analysis of tumor and organ motion during rest and deglutition and margin assessment for radiotherapy of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e803–e812. [Google Scholar] [CrossRef]
- Gurney-Champion, O.J.; McQuaid, D.; Dunlop, A.; Wong, K.H.; Welsh, L.C.; Riddell, A.M.; Koh, D.M.; Oelfke, U.; Leach, M.O.; Nutting, C.M.; et al. MRI-based assessment of 3D intrafractional motion of head and neck cancer for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 306–316. [Google Scholar] [CrossRef]
- Bruijnen, T.; Stemkens, B.; Terhaard, C.H.J.; Lagendijk, J.J.W.; Raaijmakers, C.P.J.; Tijssen, R.H.N. Intrafraction motion quantification and planning target volume margin determination of head-and-neck tumors using cine magnetic resonance imaging. Radiother. Oncol. 2019, 130, 82–88. [Google Scholar] [CrossRef]
- Kwa, S.L.; Al-Mamgani, A.; Osman, S.O.; Gangsaas, A.; Levendag, P.C.; Heijmen, B.J. Inter- and intrafraction target motion in highly focused single vocal cord irradiation of T1a larynx cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 190–195. [Google Scholar] [CrossRef]
- Van Asselen, B.; Raaijmakers, C.P.; Lagendijk, J.J.; Terhaard, C.H. Intrafraction motions of the larynx during radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 384–390. [Google Scholar] [CrossRef]
- Bahig, H.; Nguyen-Tan, P.F.; Filion, E.; Roberge, D.; Thanomsack, P.; de Guise, J.; Blais, D.; Doucet, R.; Letourneau-Guillon, L.; Lambert, L. Larynx motion considerations in partial larynx volumetric modulated arc therapy for early glottic cancer. J. Med. Imaging Radiat. Oncol. 2017, 61, 666–673. [Google Scholar] [CrossRef]
- Kugele, M.; Mannerberg, A.; Norring Bekke, S.; Alkner, S.; Berg, L.; Mahmood, F.; Thornberg, C.; Edvardsson, A.; Back, S.A.J.; Behrens, C.F.; et al. Surface guided radiotherapy (SGRT) improves breast cancer patient setup accuracy. J. Appl. Clin. Med. Phys. 2019, 20, 61–68. [Google Scholar] [CrossRef]
- Blake, N.; Pereira, L.; Eaton, D.J.; Dobson, D. Surface-guided radiotherapy for lung cancer can reduce the number of close patient contacts without compromising initial setup accuracy. Tech. Innov. Patient Support. Radiat. Oncol. 2021, 20, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Riera, J.; Hua, K.L.; Hsiao, Y.S.; Lim, T.; Hidayati, S.C.; Cheng, W.H. A comparative study of data fusion for RGB-D based visual recognition. Pattern Recognit. Lett. 2016, 73, 1–6. [Google Scholar] [CrossRef]
- Gregoire, V.; Evans, M.; Le, Q.T.; Bourhis, J.; Budach, V.; Chen, A.; Eisbruch, A.; Feng, M.; Giralt, J.; Gupta, T.; et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: Airo, caca, Dahanca, eortc, georcc, gortec, hknpcsg, hncig, iag-kht, lprhht, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother. Oncol. 2018, 126, 3–24. [Google Scholar] [PubMed]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Zeiler, M.D.; Fergus, R. Visualizing and Understanding Convolutional Networks. In Proceedings of the Computer Vision–ECCV 2014: 13th European Conference, Zurich, Switzerland, 6–12 September 2014; Springer: Cham, Switzerland, 2014; pp. 818–833. [Google Scholar]
- Nair, V.; Hinton, G.E. Rectified linear units improve restricted boltzmann machines. In Proceedings of the 27th International Conference on Machine Learning (ICML-10), Haifa, Israel, 21–24 June 2010; Omnipress: Madison, WI, USA; pp. 807–814. [Google Scholar]
- Clarke, T.A.; Fryer, J.G. The development of camera calibration methods and models. Photogramm. Rec. 1998, 16, 51–66. [Google Scholar] [CrossRef]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. In Proceedings of the 33rd Conference on Neural Information Processing Systems (NeurIPS 2019), Vancouver, Canada, 8–14 December 2019; Neural Information Processing Systems Foundation, Inc. (NeurIPS): La Jolla, CA, USA. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. In Proceedings of the 3rd International Conference for Learning Representations, San Diego, CA, USA, 7–9 May 2015; Volume 5. [Google Scholar]
- Sekachev, B.; Manovich, N.; Zhiltsov, N.; Zhavoronkov, A.; Kalinin, D.; Hoff, B. Software for Interactive Video and Image Annotation Tool for Computer Vision; opencv/cvat: v1.1.0; Zenodo: Honolulu, HI, USA, August 2020. [Google Scholar] [CrossRef]
- Huynh, E.; Boyle, S.; Campbell, J.; Penney, J.; Mak, R.H.; Schoenfeld, J.D.; Leeman, J.E.; Williams, C.L. Technical note: Toward implementation of MR-guided radiation therapy for laryngeal cancer with healthy volunteer imaging and a custom MR-CT larynx phantom. Med. Phys. 2022, 49, 1814–1821. [Google Scholar] [CrossRef]
- Hogue, S.; Guo, X.; Morrison, R.A.; McDowell, S.; Shembel, A.C. Use of Motion Capture Technology to Study Extrinsic Laryngeal Muscle Tension and Hyperfunction. Laryngoscope 2023. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Liu, H.; Zheng, D.; Zhang, S.; Pan, Y. A Wearable Swallowing Recognition System Based on Motion and Dual Photoplethysmography Sensing of Laryngeal Movements. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; Volume 2022, pp. 13–16. [Google Scholar]
- Wang, X.; Zhong, X.; Lei, H.; Yang, N.; Gao, X.; Cheng, L. Tumor microenvironment-responsive contrast agents for specific cancer imaging: A narrative review. J. Bio-X Res. 2020, 3, 144–156. [Google Scholar] [CrossRef]

| No. | Diagnosis | Stage | Age (Years) | Sex | BMI |
|---|---|---|---|---|---|
| 1 | LC | II | 77 | Male | 23.1 |
| 2 | LC | I | 54 | Male | 24.5 |
| 3 | LC | IVA | 69 | Male | 21.2 |
| 4 | LC | II | 75 | Male | 24.4 |
| 5 | LC | I | 64 | Male | 21.6 |
| 6 | LC | I | 78 | Male | 21.9 |
| 7 | LC | I | 52 | Male | 26.0 |
| No. | LR (mm) | CC (mm) | AP (mm) | Distance (mm) |
|---|---|---|---|---|
| 1 | 2.0 | 16.0 | 3.0 | 16.4 |
| 2 | 1.7 | 29.9 | 5.6 | 30.5 |
| 3 | 2.5 | 24.6 | 3.9 | 25.0 |
| 4 | 1.6 | 16.4 | 10.5 | 19.5 |
| 5 | 1.3 | 17.5 | 5.5 | 18.4 |
| 6 | 0.1 | 23.2 | 4.6 | 23.7 |
| 7 | 2.0 | 22.5 | 11.5 | 25.3 |
| Mean ± SD | 1.6 ± 0.8 | 21.4 ± 5.1 | 6.4 ± 3.3 | 22.7 ± 4.9 |
| LR | CC | AP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Without SDS | With SDS | Difference | Without SDS | With SDS | Difference | Without SDS | With SDS | Difference | |
| Mean ± SD (mm) | 3.9 ± 1.0 | 3.2 ± 0.6 | −0.7 ± 1.0 | 4.8 ± 2.7 | 16.1 ± 6.4 | 11.3 ± 6.8 | 4.0 ± 1.0 | 5.8 ± 2.6 | 1.8 ± 2.6 |
| Percentage of change | −18% | 235% | 45% | ||||||
| p value | 0.005 | <0.001 | 0.004 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-J.; Leu, Y.-S.; Chen, J.-S.; Dai, K.-Y.; Hou, T.-C.; Chang, C.-T.; Li, C.-J.; Hua, K.-L.; Chen, Y.-J. Real-Time Tracking of Laryngeal Motion via the Surface Depth-Sensing Technique for Radiotherapy in Laryngeal Cancer Patients. Bioengineering 2023, 10, 908. https://doi.org/10.3390/bioengineering10080908
Lee W-J, Leu Y-S, Chen J-S, Dai K-Y, Hou T-C, Chang C-T, Li C-J, Hua K-L, Chen Y-J. Real-Time Tracking of Laryngeal Motion via the Surface Depth-Sensing Technique for Radiotherapy in Laryngeal Cancer Patients. Bioengineering. 2023; 10(8):908. https://doi.org/10.3390/bioengineering10080908
Chicago/Turabian StyleLee, Wan-Ju, Yi-Shing Leu, Jing-Sheng Chen, Kun-Yao Dai, Tien-Chi Hou, Chung-Ting Chang, Chi-Jung Li, Kai-Lung Hua, and Yu-Jen Chen. 2023. "Real-Time Tracking of Laryngeal Motion via the Surface Depth-Sensing Technique for Radiotherapy in Laryngeal Cancer Patients" Bioengineering 10, no. 8: 908. https://doi.org/10.3390/bioengineering10080908
APA StyleLee, W.-J., Leu, Y.-S., Chen, J.-S., Dai, K.-Y., Hou, T.-C., Chang, C.-T., Li, C.-J., Hua, K.-L., & Chen, Y.-J. (2023). Real-Time Tracking of Laryngeal Motion via the Surface Depth-Sensing Technique for Radiotherapy in Laryngeal Cancer Patients. Bioengineering, 10(8), 908. https://doi.org/10.3390/bioengineering10080908








