Efficient Decellularization of the Full-Thickness Rat-Derived Abdominal Wall to Produce Acellular Biologic Scaffolds for Tissue Reconstruction: Promising Evidence Acquired from In Vitro Results
Abstract
:1. Introduction
2. Methods
2.1. Abdominal Wall Excision
2.2. Decellularization of Whole Rat Abdominal Wall Samples
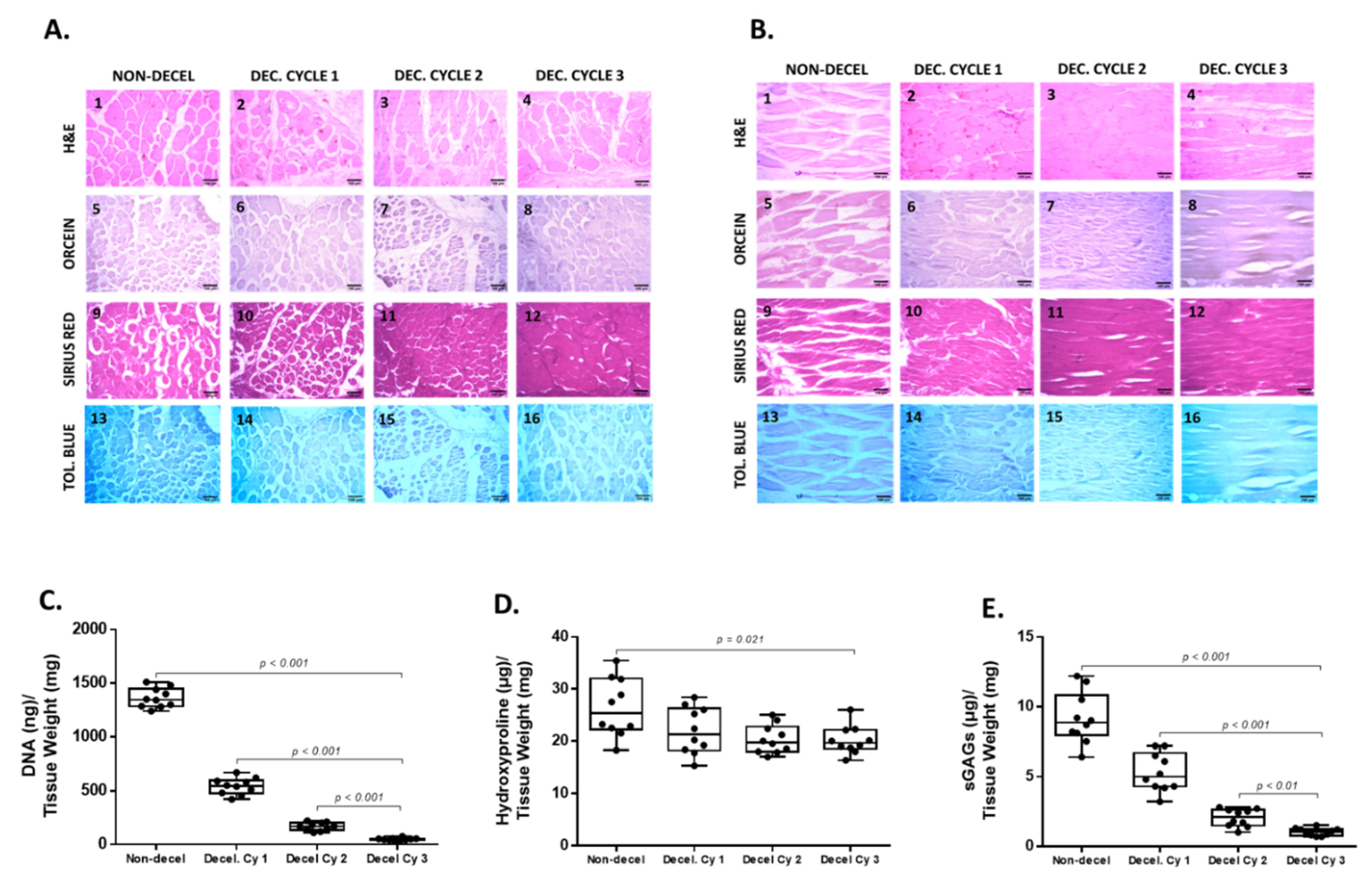
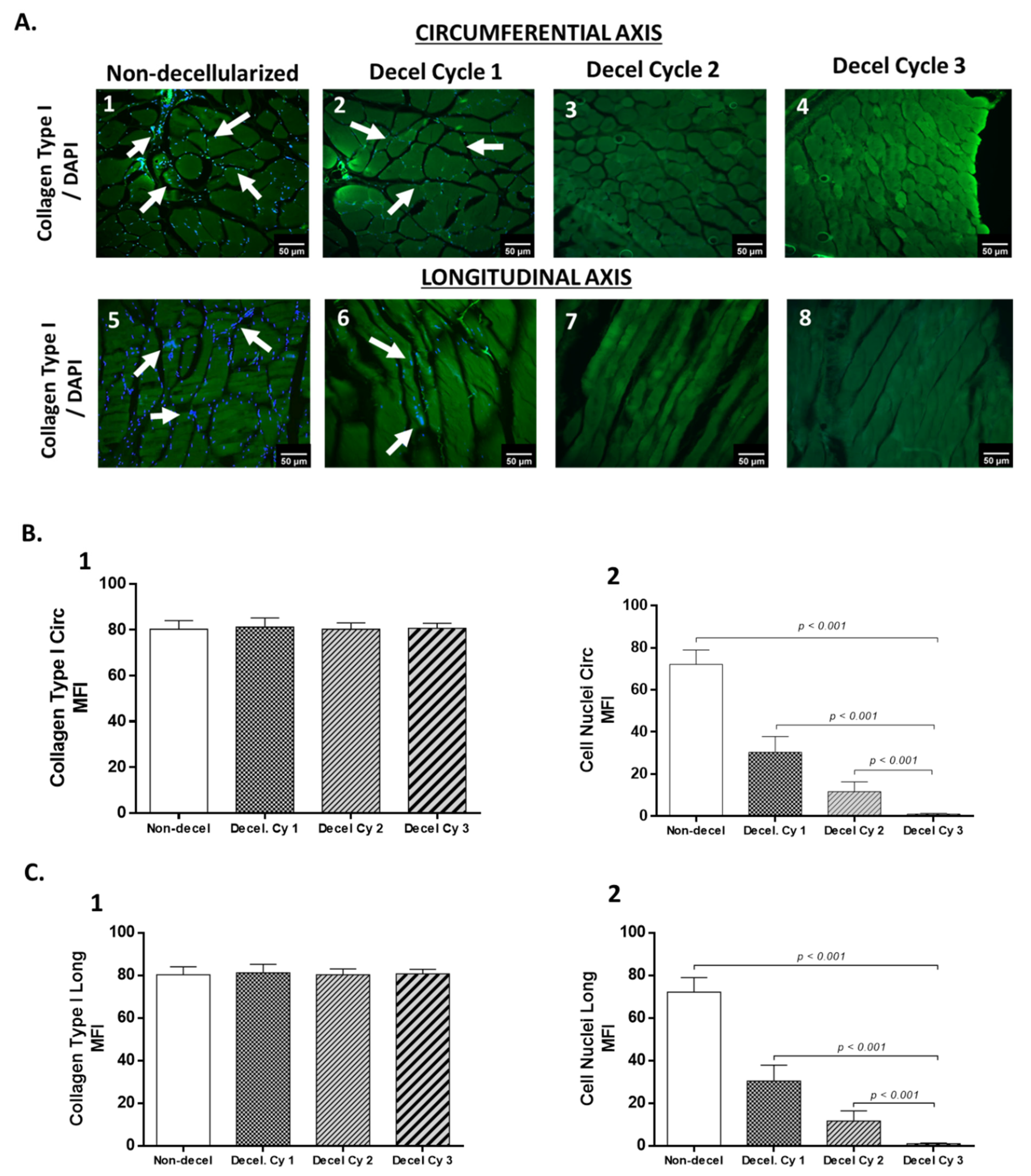
2.3. Histological Analysis
2.4. Biochemical Analysis
2.5. DNA Quantification
2.6. Biomechanical Analysis
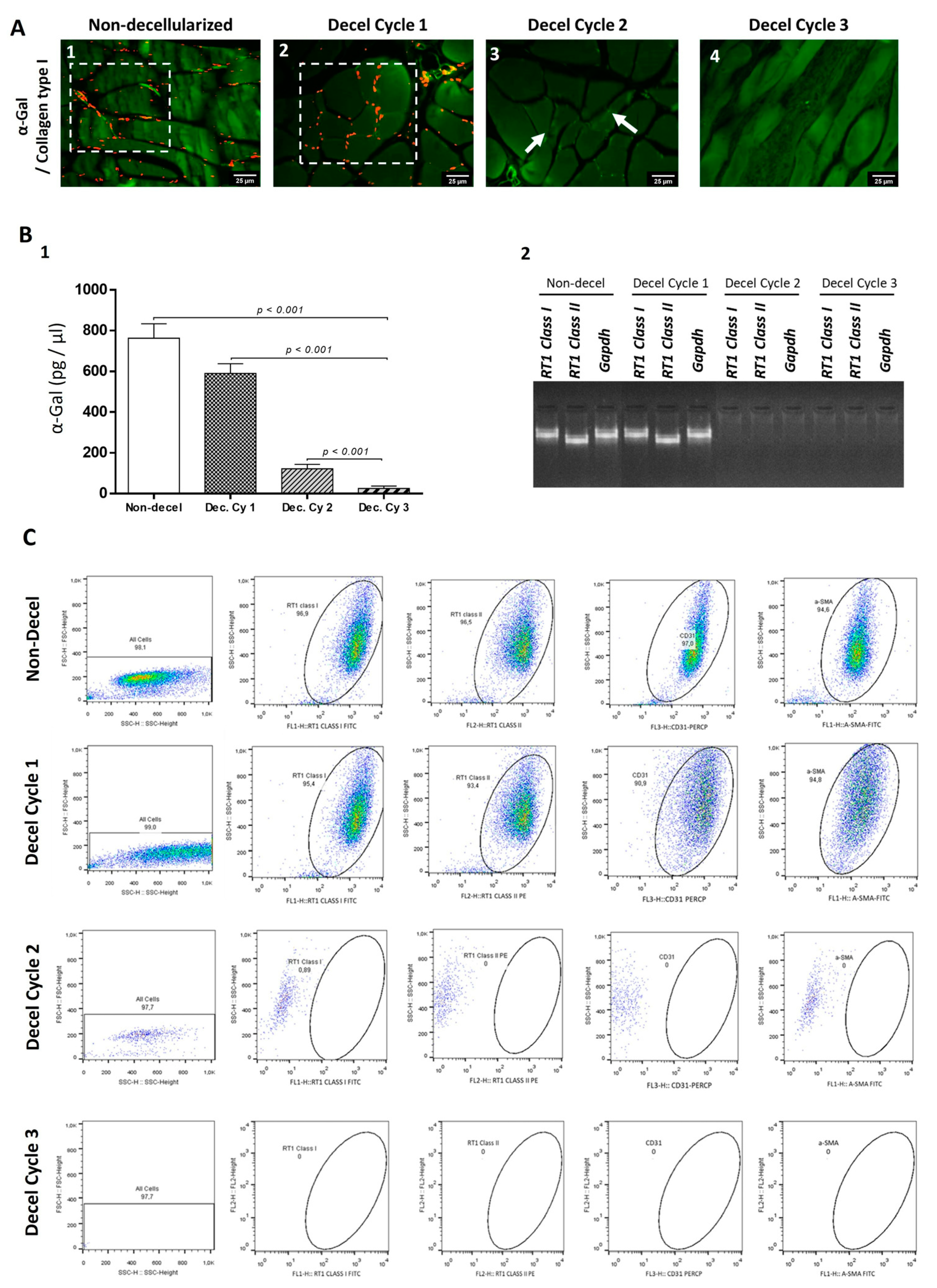
2.7. Determination of Acellular Whole Abdominal Wall Immunogenicity
2.8. Statistical Analysis
3. Results
3.1. Histological and Biochemical Evaluation of Decellularized Full-Thickness Rat-Derived Abdominal Wall
3.2. Evaluation of Biomechanical Properties
3.3. Evaluation of Immunogenicity of Decellularized Whole Abdominal Wall Scaffolds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meintjes, J.; Yan, S.; Zhou, L.; Zheng, S.; Zheng, M. Synthetic, biological and composite scaffolds for abdominal wall reconstruction. Expert Rev. Med. Devices 2011, 8, 275–288. [Google Scholar] [CrossRef]
- Hope, W.W.; Abdul, W.; Winters, R. Abdominal Wall Reconstruction. [Updated 25 July 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431108/ (accessed on 23 June 2023).
- Kingsnorth, A.; LeBlanc, K. Hernias: Inguinal and incisional. Lancet 2003, 362, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, R.W.; Hop, W.C.; van den Tol, M.P.; de Lange, D.C.; Braaksma, M.M.; IJzermans, J.N.; Boelhouwer, R.U.; de Vries, B.C.; Salu, M.K.; Wereldsma, J.C.; et al. A comparison of suture repair with mesh repair for incisional hernia. N. Engl. J. Med. 2000, 343, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Klinge, U.; Si, Z.Y.; Zheng, H.; Schumpelick, V.; Bhardwaj, R.S.; Klosterhalfen, B. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur. Surg. Res. 2000, 32, 43–48. [Google Scholar] [CrossRef]
- Klinge, U.; Zheng, H.; Si, Z.; Schumpelick, V.; Bhardwaj, R.S.; Muys, L.; Klosterhalfen, B. Expression of the extracellular matrix proteins collagen I, collagen III and fibronectin and matrix metalloproteinase-1 and -13 in the skin of patients with inguinal hernia. Eur. Surg. Res. 1999, 31, 480–490. [Google Scholar] [CrossRef]
- Zheng, H.; Si, Z.; Kasperk, R.; Bhardwaj, R.S.; Schumpelick, V.; Klinge, U.; Klosterhalfen, B. Recurrent inguinal hernia: Disease of the collagen matrix? World J. Surg. 2002, 26, 401–408. [Google Scholar]
- Bringman, S.; Conze, J.; Cuccurullo, D.; Deprest, J.; Junge, K.; Klosterhalfen, B.; Parra-Davila, E.; Ramshaw, B.; Schumpelick, V. Hernia repair: The search for ideal meshes. Hernia 2010, 14, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Cobb, W.S. A Current Review of Synthetic Meshes in Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2018, 142, 64S–71S. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Pavan, P.G.; Natali, A.N. Synthetic surgical meshes used in abdominal wall surgery: Part I-materials and structural conformation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 689–699. [Google Scholar] [CrossRef]
- Todros, S.; Pavan, P.G.; Pachera, P.; Natali, A.N. Synthetic surgical meshes used in abdominal wall surgery: Part II-Biomechanical aspects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 892–903. [Google Scholar] [CrossRef]
- Finch, D.; Mehmood, S.; Varghese, J. Abdominal wall reconstruction using biosynthetic absorbable mesh in high-risk complex ventral hernia. Swiss Med. Wkly. 2021, 151, w20449. [Google Scholar] [CrossRef]
- Fatkhudinov, T.; Tsedik, L.; Arutyunyan, I.; Lokhonina, A.; Makarov, A.; Korshunov, A.; Elchaninov, A.; Kananykhina, E.; Vasyukova, O.; Usman, N.; et al. Evaluation of resorbable polydioxanone and polyglycolic acid meshes in a rat model of ventral hernia repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Baumann, D.P.; Butler, C.E. Bioprosthetic mesh in abdominal wall reconstruction. Semin. Plast. Surg. 2012, 26, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Rahimnejad, M.; Derakhshanfar, S.; Zhong, W. Biomaterials and tissue engineering for scar management in wound care. Burn. Trauma 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wissing, T.B.; Bonito, V.; van Haaften, E.E.; van Doeselaar, M.; Brugmans, M.M.C.P.; Janssen, H.M.; Bouten, C.V.C.; Smits, A.I.P.M. Macrophage-Driven Biomaterial Degradation Depends on Scaffold Microarchitecture. Front. Bioeng. Biotechnol. 2019, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.S.; Bryant, S.J. The Host Response in Tissue Engineering: Crosstalk Between Immune cells and Cell-laden Scaffolds. Curr. Opin. Biomed. Eng. 2018, 6, 58–65. [Google Scholar] [CrossRef]
- Deeken, C.R.; Lake, S.P. Mechanical properties of the abdominal wall and biomaterials utilized for hernia repair. J. Mech. Behav. Biomed. Mater. 2017, 74, 411–427. [Google Scholar] [CrossRef]
- Nisiewicz, M.; Hughes, T.; Plymale, M.A.; Davenport, D.L.; Roth, J.S. Abdominal wall reconstruction with large polypropylene mesh: Is bigger better? Hernia 2019, 23, 1003–1008. [Google Scholar] [CrossRef]
- Gui, D.; Spada, P.L.; Di Mugno, M.; Sermoneta, D.; Runfola, M.; Rossi, S. Abdominal wall closure with ePTFE—Goretex Dual Mesh after detensive laparotomy for abdominal compartment syndrome. Acta Biomed. 2003, 74 (Suppl. S2), 51–54. [Google Scholar]
- Ünek, T.; Sökmen, S.; Egeli, T.; Avkan Oğuz, V.; Ellidokuz, H.; Obuz, F. The results of expanded-polytetrafluoroethylene mesh repair in difficult abdominal wall defects. Asian J. Surg. 2019, 42, 131–143. [Google Scholar] [CrossRef]
- Kanitra, J.J.; Hess, A.L.; Haan, P.S.; Anderson, C.I.; Kavuturu, S. Hernia recurrence and infection rate in elective complex abdominal wall repair using biologic mesh. BMC Surg. 2019, 19, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darehzereshki, A.; Goldfarb, M.; Zehetner, J.; Moazzez, A.; Lipham, J.C.; Mason, R.J.; Katkhouda, N. Biologic versus nonbiologic mesh in ventral hernia repair: A systematic review and meta-analysis. World J. Surg. 2014, 38, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bellows, C.F.; Alder, A.; Helton, W.S. Abdominal wall reconstruction using biological tissue grafts: Present status and future opportunities. Expert Rev. Med. Devices 2006, 3, 657–675. [Google Scholar] [CrossRef]
- Costa, A.; Adamo, S.; Gossetti, F.; D’Amore, L.; Ceci, F.; Negro, P.; Bruzzone, P. Biological Scaffolds for Abdominal Wall Repair: Future in Clinical Application? Materials 2019, 12, 2375. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Baganizi, D.R.; Sahu, R.; Dosunmu, E.; Chaudhari, A.; Vig, K.; Pillai, S.R.; Singh, S.R.; Dennis, V.A. Immunological challenges associated with artificial skin grafts: Available solutions and stem cells in future design of synthetic skin. J. Biol. Eng. 2017, 13, 11–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velnar, T.; Bunc, G.; Klobucar, R.; Gradisnik, L. Biomaterials and host versus graft response: A short review. Bosn. J. Basic Med. Sci. 2016, 16, 82–90. [Google Scholar] [CrossRef] [Green Version]
- de Castro Brás, L.E.; Shurey, S.; Sibbons, P.D. Evaluation of crosslinked and non-crosslinked biologic prostheses for abdominal hernia repair. Hernia 2012, 16, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future Perspectives in Small-Diameter Vascular Graft Engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 675–683. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2021, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Hasem Arefi, A.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Ranjbar, R.; Seifalian, A.; Rahbar, M. Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. J. Mater. Res. Technol. 2021, 15, 1217–1251. [Google Scholar] [CrossRef]
- Yazdanian, M.; Alam, M.; Abbasi, K.; Rahbar, M.; Farjood, A.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Hesam Arefi, A. Synthetic materials in craniofacial regenerative medicine: A comprehensive overview. Front. Bioeng. Biotechnol. 2022, 9, 987195. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Díaz, D.M.; Sánchez, L.V.; Valencia-Vásquez, A.; Quintero, J.F.; Muñoz, L.V.; Bernal, A.F.; Osorio, G.; Guerra, Á.; Buitrago, J. Decellularization and In Vivo Recellularization of Abdominal Porcine Fascial Tissue. Tissue Eng. Regen. Med. 2021, 18, 369–376. [Google Scholar] [CrossRef]
- Buell, J.F.; Helm, J.; Mckillop, I.H.; Iglesias, B.; Pashos, N.; Hooper, P. Decellularized biologic muscle-fascia abdominal wall scaffold graft. Surgery 2021, 169, 595–602. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Lin, Y.N.; Chen, Y.J.; Periasamy, S.; Yen, K.C.; Hsieh, D.J. Efficacy of Supercritical Fluid Decellularized Porcine Acellular Dermal Matrix in the Post-Repair of Full-Thickness Abdominal Wall Defects in the Rabbit Hernia Model. Processes 2022, 10, 2588. [Google Scholar] [CrossRef]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef]
- Mallis, P.; Oikonomidis, C.; Dimou, Z.; Stavropoulos-Giokas, C.; Michalopoulos, E.; Katsimpoulas, M. Optimizing Decellularization Strategies for the Efficient Production of Whole Rat Kidney Scaffolds. Tissue Eng. Regen. Med. 2021, 18, 623–640. [Google Scholar] [CrossRef]
- Schmitt, A.; Csiki, R.; Tron, A.; Saldamli, B.; Tübel, J.; Florian, K.; Siebenlist, S.; Balmayor, E.; Burgkart, R. Optimized protocol for whole organ decellularization. Eur. J. Med. Res. 2017, 22, 31. [Google Scholar] [CrossRef] [Green Version]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubenberger, A.V.; Woodruff, M.A.; Bai, H.; Muller, D.J.; Hutmacher, D.W. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation. Biomaterials 2010, 31, 2827–2835. [Google Scholar] [CrossRef]
- Gullberg, D.E.; Lundgren-Akerlund, E. Collagen-binding I domain integrins--what do they do? Prog. Histochem. Cytochem. 2002, 37, 3–54. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Duffy, G.P.; Schindeler, A.; O’brien, F.J. Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types. J. Biomed. Mater. Res. A 2016, 104, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.I.; Wang, J.; Mort, J.S.; Komatova, S.V. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Front. Phys. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Shishido, S.; Bönig, H.; Kim, Y.M. Role of integrin alpha4 in drug resistance of leukemia. Front. Oncol. 2014, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Wang, J.; Li, W.; Hao, X.; Hang, Q. Roles of Integrins in Gastrointestinal Cancer Metastasis. Front. Mol. Biosci. 2021, 8, 708779. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Montes, G.S. Biology of collagen-proteoglycan interaction. Arch. Histol. Jpn. 1983, 46, 589–629. [Google Scholar] [CrossRef] [Green Version]
- Mattson, J.M.; Turcotte, R.; Zhang, Y. Glycosaminoglycans contribute to extracellular matrix fiber recruitment and arterial wall mechanics. Biomech. Model. Mechanobiol. 2017, 16, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Korossis, S.A.; Wilshaw, S.P.; Jennings, L.M.; Fisher, J.; Ingham, E. Development and characterization of acellular porcine pulmonary valve scaffolds for tissue engineering. Tissue Eng. Part A 2014, 20, 2963–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Mallis, P.; Katsimpoulas, M.; Kostakis, A.; Dipresa, D.; Korossis, S.; Papapanagiotou, A.; Kassi, E.; Stavropoulos-Giokas, C.; Michalopoulos, E. Vitrified Human Umbilical Arteries as Potential Grafts for Vascular Tissue Engineering. Tissue Eng. Regen. Med. 2020, 17, 285–299. [Google Scholar] [CrossRef]
- Arai, S.; Lacerda, C.; Orton, E.C. Tissue-gel electrophoresis enhances antigen removal from porcine aortic valve and bovine pericardium. J. Heart Valve Dis. 2010, 19, 753–758. [Google Scholar]
- Korhonen, R.K.; Julkunen, P.; Wilson, W.; Herzog, W. Importance of collagen orientation and depth-dependent fixed charge densities of cartilage on mechanical behavior of chondrocytes. J. Biomech. Eng. 2008, 130, 021003. [Google Scholar] [CrossRef]
- Sexton, A.J.; Turmaine, M.; Cai, W.Q.; Burnstock, G. A study of the ultrastructure of developing human umbilical vessels. J. Anat. 1996, 188, 75–85. [Google Scholar]
- Stehbens, W.E.; Wakefield, J.S.; Gilbert-Barness, E.; Zuccollo, J.M. Histopathology and ultrastructure of human umbilical blood vessels. Fetal Pediatr. Pathol. 2005, 24, 297–315. [Google Scholar] [CrossRef]
- Sassani, S.G.; Tsangaris, S.; Sokolis, D.P. Layer- and region-specific material characterization of ascending thoracic aortic aneurysms by microstructure-based models. J. Biomech. 2015, 48, 3757–3765. [Google Scholar] [CrossRef]
- Roy, S.; Silacci, P.; Stergiopulos, N. Biomechanical properties of decellularized porcine common carotid arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1567–H1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galili, U. Biosynthesis of α-Gal Epitopes (Galα1-3Galβ1-4GlcNAc-R) and Their Unique Potential in Future α-Gal Therapies. Front. Mol. Biosci. 2021, 8, 746883. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef]
- Ekser, B.; Cooper, D.K. Overcoming the barriers to xenotransplantation: Prospects for the future. Expert Rev. Clin. Immunol. 2010, 6, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, R. Gal knockout and beyond. Am. J. Transpl. 2007, 7, 5–11. [Google Scholar] [CrossRef]
- Milland, J.; Christiansen, D.; Sandrin, M.S. Alpha1,3-galactosyltransferase knockout pigs are available for xenotransplantation: Are glycosyltransferases still relevant? Immunol. Cell Biol. 2005, 83, 687–693. [Google Scholar] [CrossRef]
- Wu, L.C.; Kuo, Y.J.; Sun, F.W.; Chen, C.H.; Chiang, C.J.; Weng, P.W.; Tsuang, Y.H.; Huang, Y.Y. Optimized decellularization protocol including α-Gal epitope reduction for fabrication of an acellular porcine annulus fibrosus scaffold. Cell Tissue Bank. 2017, 18, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef]
- Joziasse, D.H.; Oriol, R. Xenotransplantation: The importance of the Galalpha1,3Gal epitope in hyperacute vascular rejection. Biochim. Biophys. Acta 1999, 1455, 403–418. [Google Scholar] [CrossRef] [Green Version]
- Günther, E.; Walter, L. The major histocompatibility complex of the rat (Rattus norvegicus). Immunogenetics 2001, 53, 520–542. [Google Scholar] [CrossRef]
- Walter, L.; Hurt, P.; Himmelbauer, H.; Sudbrak, R.; Günther, E. Physical mapping of the major histocompatibility complex class II and class III regions of the rat. Immunogenetics 2002, 54, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Sokolis, D.P.; Makridakis, M.; Zoidakis, J.; Velentzas, A.D.; Katsimpoulas, M.; Vlahou, A.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Insights into Biomechanical and Proteomic Characteristics of Small Diameter Vascular Grafts Utilizing the Human Umbilical Artery. Biomedicines 2020, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Chachlaki, P.; Katsimpoulas, M.; Stavropoulos-Giokas, C.; Michalopoulos, E. Optimization of Decellularization Procedure in Rat Esophagus for Possible Development of a Tissue Engineered Construct. Bioengineering 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallis, P.; Boulari, D.; Chachlaki, P.; Stavropoulos Giokas, C.; Michalopoulos, E. Vitrified Wharton’s jelly tissue as a biomaterial for multiple tissue engineering applications. Gynecol. Endocrinol. 2020, 36, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Boulari, D.; Michalopoulos, E.; Dinou, A.; Spyropoulou-Vlachou, M.; Stavropoulos-Giokas, C. Evaluation of HLA-G Expression in Multipotent Mesenchymal Stromal Cells Derived from Vitrified Wharton’s Jelly Tissue. Bioengineering 2018, 5, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Gene ID | Forward Primer | Reverse Primer | Amplicon Size |
|---|---|---|---|---|
| RT1 Class I | 309627 | CAGATCCCCCAAAGGCACAT | CAGATCCCCCAAAGGCACAT | 253 |
| RT1 Class II | 309622 | CTTCCTTACCCGGGTGGAAC | TCTGATCACGAGCCGTATGC | 316 |
| Gapdh | 24383 | GGCCCGGAGTCTAAAGTAGC | GGCGGCCAGTTACCATAAGT | 234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skepastianos, G.; Mallis, P.; Kostopoulos, E.; Michalopoulos, E.; Skepastianos, V.; Palazi, C.; Pannuto, L.; Tsourouflis, G. Efficient Decellularization of the Full-Thickness Rat-Derived Abdominal Wall to Produce Acellular Biologic Scaffolds for Tissue Reconstruction: Promising Evidence Acquired from In Vitro Results. Bioengineering 2023, 10, 913. https://doi.org/10.3390/bioengineering10080913
Skepastianos G, Mallis P, Kostopoulos E, Michalopoulos E, Skepastianos V, Palazi C, Pannuto L, Tsourouflis G. Efficient Decellularization of the Full-Thickness Rat-Derived Abdominal Wall to Produce Acellular Biologic Scaffolds for Tissue Reconstruction: Promising Evidence Acquired from In Vitro Results. Bioengineering. 2023; 10(8):913. https://doi.org/10.3390/bioengineering10080913
Chicago/Turabian StyleSkepastianos, George, Panagiotis Mallis, Epameinondas Kostopoulos, Efstathios Michalopoulos, Vasileios Skepastianos, Chrysoula Palazi, Lucia Pannuto, and Gerasimos Tsourouflis. 2023. "Efficient Decellularization of the Full-Thickness Rat-Derived Abdominal Wall to Produce Acellular Biologic Scaffolds for Tissue Reconstruction: Promising Evidence Acquired from In Vitro Results" Bioengineering 10, no. 8: 913. https://doi.org/10.3390/bioengineering10080913







