Binding of Pentagalloyl Glucose to Aortic Wall Proteins: Insights from Peptide Mapping and Simulated Docking Studies
Abstract
:1. Introduction
1.1. Extracellular Matrix (ECM) in the Normal Aorta
1.2. Aneurysmal Aorta Components
1.3. Intraluminal Thrombus in AAA
2. Materials and Methods
2.1. Treatment of Pure Elastin with PGG and Sample Preparation for Peptide Fingerprinting
2.2. Peptide Fingerprinting
2.3. Molecular Docking
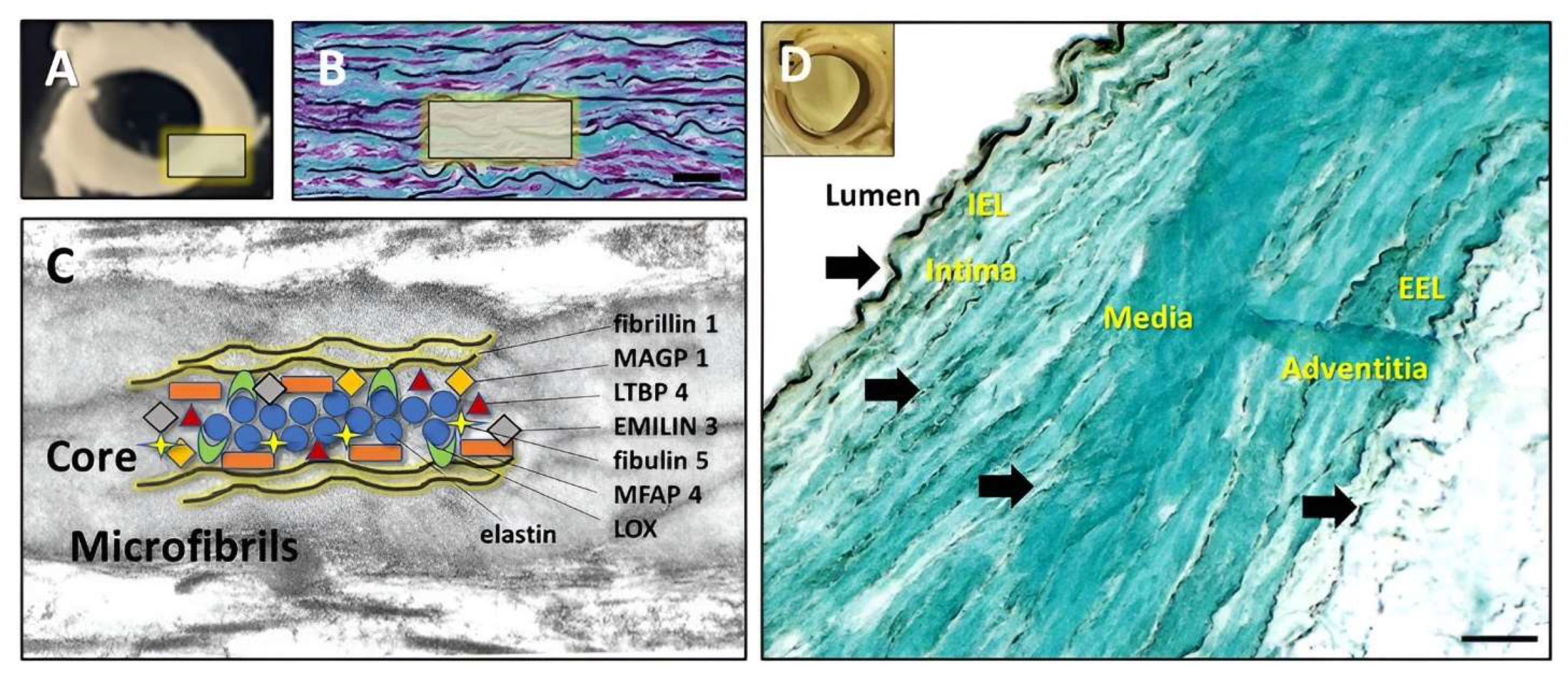
2.4. PGG Binding Patterns Analysis in Fresh Aorta
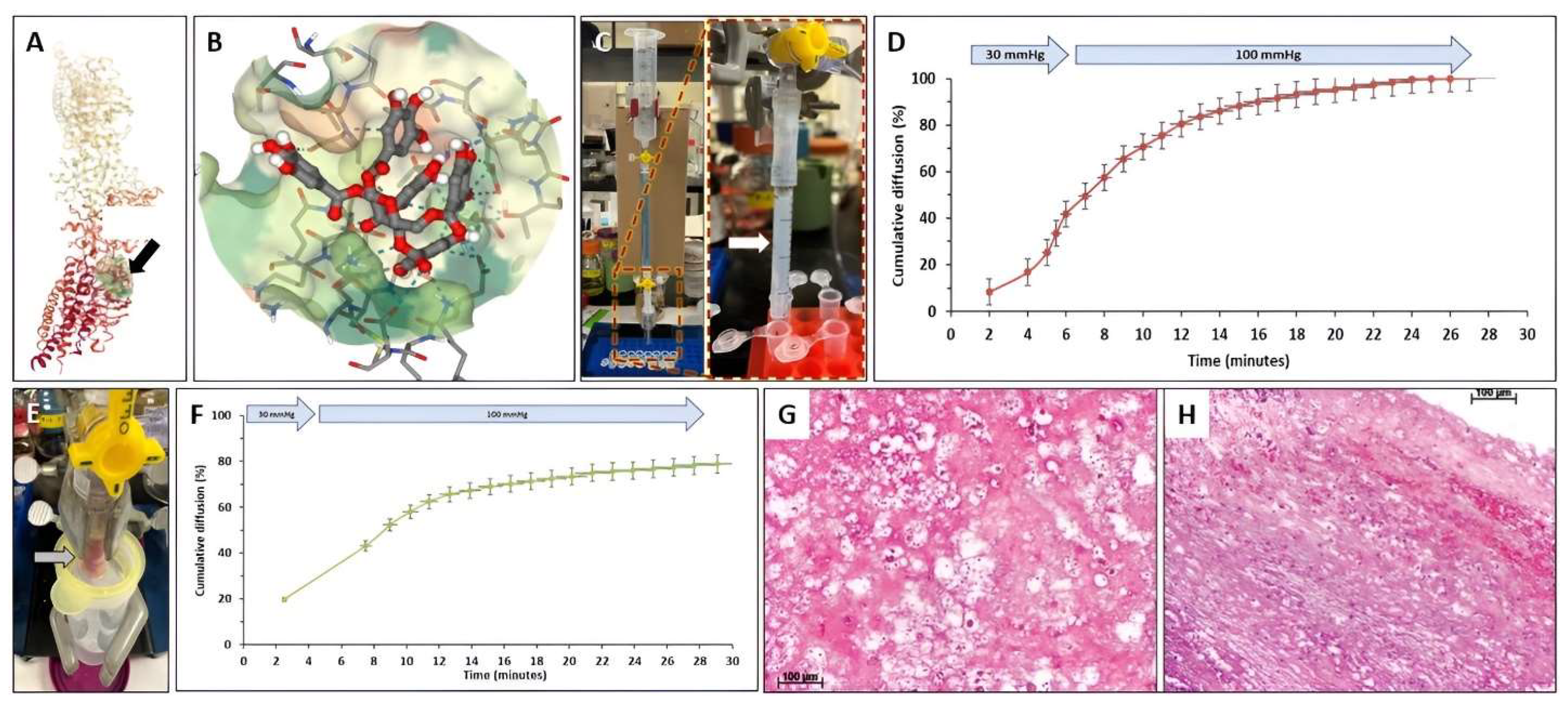
2.5. Diffusion of PGG through Pure Fibrin Gels and Intra-Luminal Thrombus (ILT)
3. Results
3.1. Peptide Fingerprinting
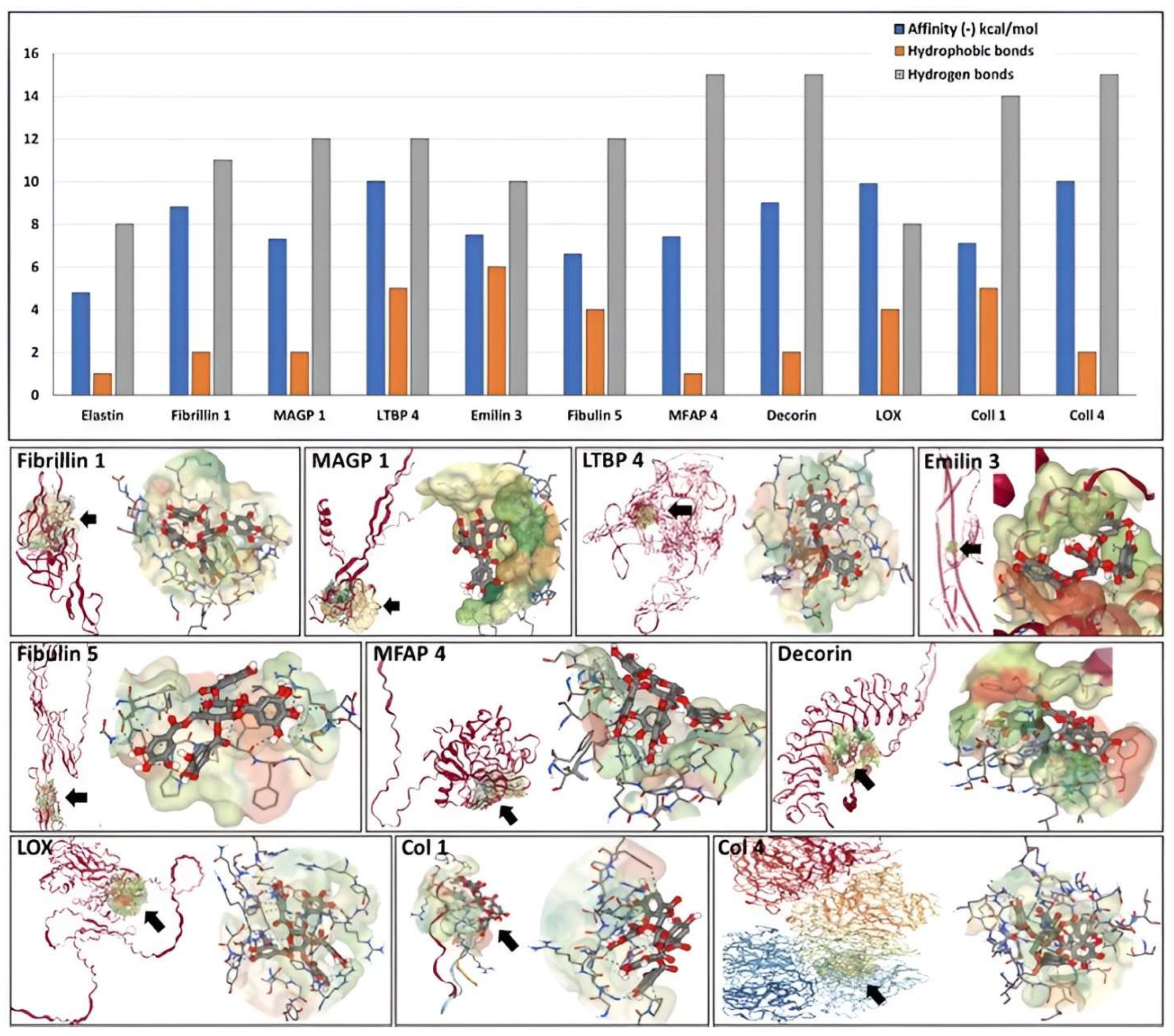
3.2. Molecular Docking of PGG onto Elastin
3.3. Molecular Docking of PGG onto Other Normal Vascular Extracellular Matrix Proteins
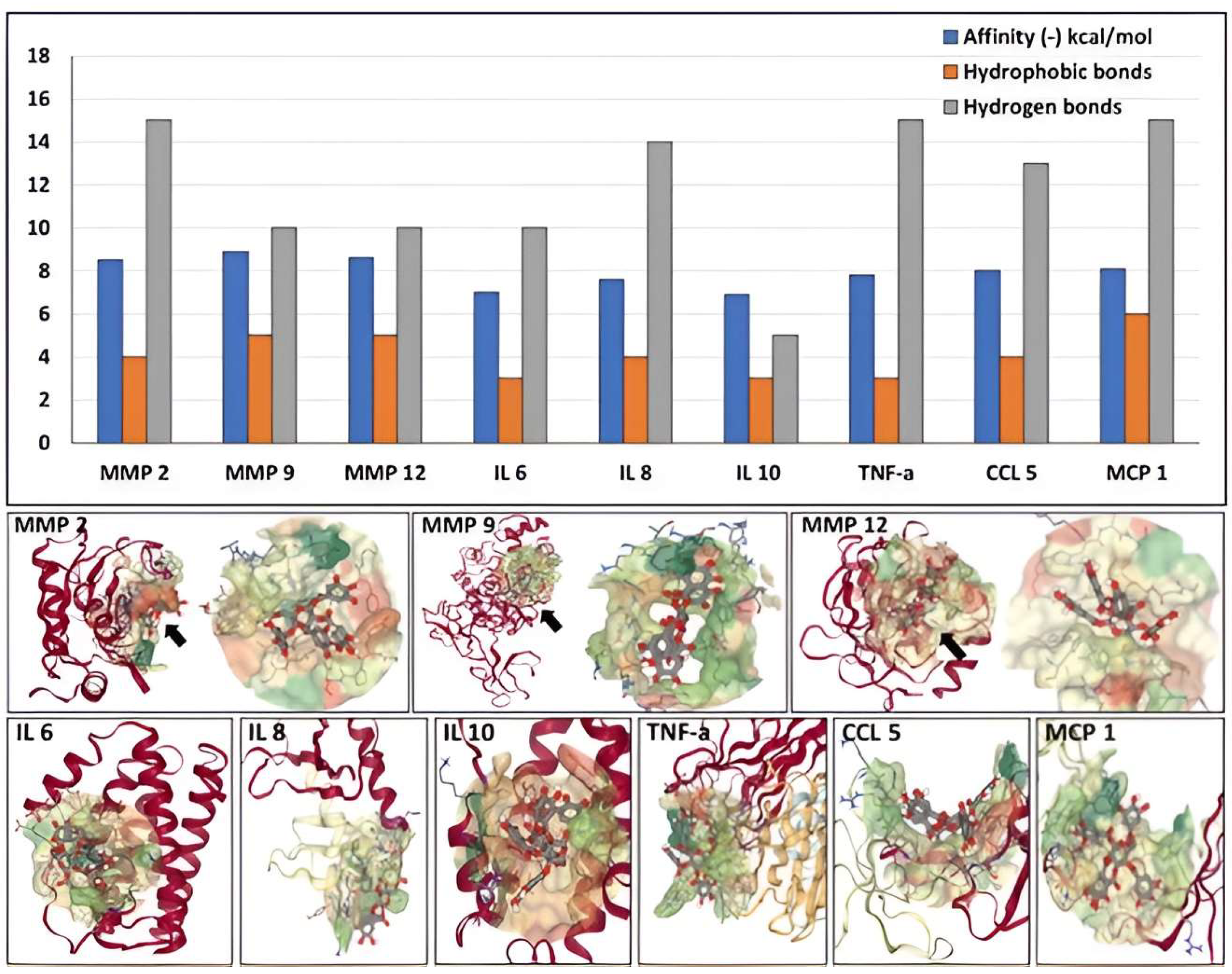
3.4. Molecular Docking of PGG onto Proteins Present in the Aneurysmal Aorta
3.5. Diffusion of PGG through Fibrin Gels and Intra-Luminal Thrombus
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simionescu, N.; Simionescu, M. Galloylglucoses of low molecular weight as mordant in electron microscopy. II. The moiety and functional groups possibly involved in the mordanting effect. J. Cell Biol. 1976, 70, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, N.; Simionescu, M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J. Cell Biol. 1976, 70, 608–621. [Google Scholar] [CrossRef]
- Kajikawa, K.; Yamaguchi, T.; Katsuda, S.; Miwa, A. An improved electron stain for elastic fibers using tannic acid. J. Electron Microsc. 1975, 24, 287–289. [Google Scholar]
- Cotta-Pereira, G.; Rodrigo, F.G.; David-Ferreira, J.F. The use of tannic acid-glutaraldehyde in the study of elastic and elastic-related fibers. Stain. Technol. 1976, 51, 7–11. [Google Scholar] [CrossRef]
- Tsuji, T.; Hamada, T. Elastotic material and elastic fibers in aged skin: An ultrastructural study with conventional and tannic acid stain. Acta Derm. Venereol. 1981, 61, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Elastin stabilization in cardiovascular implants: Improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials 2004, 25, 3293–3302. [Google Scholar] [CrossRef]
- Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials 2005, 26, 1237–1245. [Google Scholar] [CrossRef]
- Isenburg, J.C.; Karamchandani, N.V.; Simionescu, D.T.; Vyavahare, N.R. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006, 27, 3645–3651. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Simionescu, D.T.; Goergen, C.J.; Hoyt, K.; Sirsi, S.; Finol, E.A. Pentagalloyl Glucose and Its Functional Role in Vascular Health: Biomechanics and Drug-Delivery Characteristics. Ann. Biomed. Eng. 2019, 47, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.S.; Piskin, S.; Pillalamarri, N.R.; Romero, G.; Escobar, G.P.; Sprague, E.; Finol, E.A. Biomechanical Restoration Potential of Pentagalloyl Glucose after Arterial Extracellular Matrix Degeneration. Bioengineering 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Isenburg, J.C.; Simionescu, D.T.; Starcher, B.C.; Vyavahare, N.R. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 2007, 115, 1729–1737. [Google Scholar] [CrossRef] [Green Version]
- Kloster, B.O.; Lund, L.; Lindholt, J.S. Inhibition of early AAA formation by aortic intraluminal pentagalloyl glucose (PGG) infusion in a novel porcine AAA model. Ann. Med. Surg. 2016, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Schack, A.S.; Stubbe, J.; Steffensen, L.B.; Mahmoud, H.; Laursen, M.S.; Lindholt, J.S. Intraluminal infusion of Penta-Galloyl Glucose reduces abdominal aortic aneurysm development in the elastase rat model. PLoS ONE 2020, 15, e0234409. [Google Scholar] [CrossRef]
- Simionescu, D.; Casco, M.; Turner, J.; Rierson, N.; Yue, J.; Ning, K. Chemical stabilization of the extracellular matrix attenuates growth of experimentally induced abdominal aorta aneurysms in a large animal model. JVS Vasc. Sci. 2020, 1, 69–80. [Google Scholar] [CrossRef]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Schrader, C.U.; Baud, S.; Keeley, F.W.; Mithieux, S.M.; Weiss, A.S.; Neubert, R.H.; Schmelzer, C.E. Molecular-level characterization of elastin-like constructs and human aortic elastin. Matrix Biol. 2014, 38, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hedtke, T.; Schrader, C.U.; Heinz, A.; Hoehenwarter, W.; Brinckmann, J.; Groth, T.; Schmelzer, C.E.H. A comprehensive map of human elastin cross-linking during elastogenesis. FEBS J. 2019, 286, 3594–3610. [Google Scholar] [CrossRef]
- Kielty, C.M.; Shuttleworth, C.A. Microfibrillar elements of the dermal matrix. Microsc. Res. Tech. 1997, 38, 413–427. [Google Scholar] [CrossRef]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Sun, B.; Zhang, X.; Li, F.; Liu, Z.; Zheng, Y. Discovery of crucial cytokines associated with abdominal aortic aneurysm formation by protein array analysis. Exp. Biol. Med. 2019, 244, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Puchenkova, O.A.; Soldatov, V.O.; Belykh, A.E.; Bushueva, O.; Piavchenko, G.A.; Venediktov, A.A.; Shakhpazyan, N.K.; Deykin, A.V.; Korokin, M.V.; Pokrovskiy, M.V. Cytokines in Abdominal Aortic Aneurysm: Master Regulators with Clinical Application. Biomark. Insights 2022, 17, 11772719221095676. [Google Scholar] [CrossRef]
- Nishihara, M.; Aoki, H.; Ohno, S.; Furusho, A.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Imaizumi, T.; Fukumoto, Y. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS ONE 2017, 12, e0185923. [Google Scholar] [CrossRef] [Green Version]
- Golledge, J. Is there a new target in the renin-angiotensin system for aortic aneurysm therapy? Arter. Thromb. Vasc. Biol. 2013, 33, 1456–1457. [Google Scholar] [CrossRef] [Green Version]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [CrossRef] [PubMed]
- Nosoudi, N.; Nahar-Gohad, P.; Sinha, A.; Chowdhury, A.; Gerard, P.; Carsten, C.G.; Gray, B.H.; Vyavahare, N.R. Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metalloproteinase activity with batimastat-loaded nanoparticles. Circ. Res. 2015, 117, e80–e89. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Matsumura, J.; Curci, J.A.; McBride, R.; Larson, L.; Blackwelder, W.; Lam, D.; Wijesinha, M.; Terrin, M.; Investigators, N.T.C. Effect of Doxycycline on Aneurysm Growth among Patients with Small Infrarenal Abdominal Aortic Aneurysms: A Randomized Clinical Trial. JAMA 2020, 323, 2029–2038. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Jozkowicz, A.; Nowak, W.; Eilenberg, W.; Neumayer, C.; Malinski, T.; Huk, I.; Brostjan, C. The Abdominal Aortic Aneurysm and Intraluminal Thrombus: Current Concepts of Development and Treatment. Front. Cardiovasc. Med. 2015, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Hans, S.S.; Jareunpoon, O.; Balasubramaniam, M.; Zelenock, G.B. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2005, 41, 584–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whaley, Z.L.; Cassimjee, I.; Novak, Z.; Rowland, D.; Lapolla, P.; Chandrashekar, A.; Pearce, B.J.; Beck, A.W.; Handa, A.; Lee, R.; et al. The Spatial Morphology of Intraluminal Thrombus Influences Type II Endoleak after Endovascular Repair of Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2020, 66, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Adolph, R.; Vorp, D.A.; Steed, D.L.; Webster, M.W.; Kameneva, M.V.; Watkins, S.C. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J. Vasc. Surg. 1997, 25, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Murail, S.; de Vries, S.J.; Rey, J.; Moroy, G.; Tuffery, P. SeamDock: An Interactive and Collaborative Online Docking Resource to Assist Small Compound Molecular Docking. Front. Mol. Biosci. 2021, 8, 716466. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.F.; Nabil, M.; Hasan, R.A.; El-Shazly, A.M.; El-Ansari, M.A.; Sobeh, M. Pentagalloyl Glucose, a Major Compound in Mango Seed Kernel, Exhibits Distinct Gastroprotective Effects in Indomethacin-Induced Gastropathy in Rats via Modulating the NO/eNOS/iNOS Signaling Pathway. Front. Pharmacol. 2022, 13, 800986. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Dharmalingam, V.; Durairaj, S.; Sharma, P.; Jayaraman, S.; Choudhary, S. Molecular docking analysis of penta galloyl glucose with the bcl-2 family of anti-apoptotic targets. Bioinformation 2021, 17, 861–865. [Google Scholar] [CrossRef]
- Thirugnanasambandam, M.; Simionescu, D.T.; Escobar, P.G.; Sprague, E.; Goins, B.; Clarke, G.D.; Han, H.C.; Amezcua, K.L.; Adeyinka, O.R.; Goergen, C.J.; et al. The Effect of Pentagalloyl Glucose on the Wall Mechanics and Inflammatory Activity of Rat Abdominal Aortic Aneurysms. J. Biomech. Eng. 2018, 140, 0845021–0845029. [Google Scholar] [CrossRef]
- Chen, R.H.; Yang, L.J.; Hamdoun, S.; Chung, S.K.; Lam, C.W.; Zhang, K.X.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. 1,2,3,4,6-Pentagalloyl Glucose, a RBD-ACE2 Binding Inhibitor to Prevent SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 634176. [Google Scholar] [CrossRef]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Parasaram, V.; Karamched, S.; Vyavahare, N. Systemic Delivery of Nanoparticles Loaded with Pentagalloyl Glucose Protects Elastic Lamina and Prevents Abdominal Aortic Aneurysm in Rats. J. Cardiovasc. Transl. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef]
- Dhital, S.; Vyavahare, N.R. Nanoparticle-based targeted delivery of pentagalloyl glucose reverses elastase-induced abdominal aortic aneurysm and restores aorta to the healthy state in mice. PLoS ONE 2020, 15, e0227165. [Google Scholar] [CrossRef] [Green Version]
- Arnold, F.; Muzzio, N.; Patnaik, S.S.; Finol, E.A.; Romero, G. Pentagalloyl Glucose-Laden Poly(lactide-co-glycolide) Nanoparticles for the Biomechanical Extracellular Matrix Stabilization of an In Vitro Abdominal Aortic Aneurysm Model. ACS Appl. Mater. Interfaces 2021, 13, 25771–25782. [Google Scholar] [CrossRef]
- Dhital, S.; Rice, C.D.; Vyavahare, N.R. Reversal of elastase-induced abdominal aortic aneurysm following the delivery of nanoparticle-based pentagalloyl glucose (PGG) is associated with reduced inflammatory and immune markers. Eur. J. Pharmacol. 2021, 910, 174487. [Google Scholar] [CrossRef]
- Kennamer, A.; Sierad, L.; Pascal, R.; Rierson, N.; Albers, C.; Harpa, M.; Cotoi, O.; Harceaga, L.; Olah, P.; Terezia, P.; et al. Bioreactor Conditioning of Valve Scaffolds Seeded Internally with Adult Stem Cells. Tissue Eng. Regen. Med. 2016, 13, 507–515. [Google Scholar] [CrossRef]
- Deborde, C.; Simionescu, D.T.; Wright, C.; Liao, J.; Sierad, L.N.; Simionescu, A. Stabilized Collagen and Elastin-Based Scaffolds for Mitral Valve Tissue Engineering. Tissue Eng. Part A 2016, 22, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Sierad, L.N.; Shaw, E.L.; Bina, A.; Brazile, B.; Rierson, N.; Patnaik, S.S.; Kennamer, A.; Odum, R.; Cotoi, O.; Terezia, P.; et al. Functional Heart Valve Scaffolds Obtained by Complete Decellularization of Porcine Aortic Roots in a Novel Differential Pressure Gradient Perfusion System. Tissue Eng. Part C Methods 2015, 21, 1284–1296. [Google Scholar] [CrossRef] [Green Version]
- Pennel, T.; Fercana, G.; Bezuidenhout, D.; Simionescu, A.; Chuang, T.H.; Zilla, P.; Simionescu, D. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. Biomaterials 2014, 35, 6311–6322. [Google Scholar] [CrossRef] [Green Version]
- Parasaram, V.; Wang, X.; Krisanarungson, P.; Vyavahare, N. Targeted delivery of pentagalloyl glucose inhibits matrix metalloproteinase activity and preserves elastin in emphysematous lungs. Respir. Res. 2021, 22, 249. [Google Scholar] [CrossRef]
- Parasaram, V.; Nosoudi, N.; Chowdhury, A.; Vyavahare, N. Pentagalloyl glucose increases elastin deposition, decreases reactive oxygen species and matrix metalloproteinase activity in pulmonary fibroblasts under inflammatory conditions. Biochem. Biophys. Res. Commun. 2018, 499, 24–29. [Google Scholar] [CrossRef]


| Traditional Abbreviation | Protein Full Name | Classification and Properties | ID Code or Accession Number | Interaction Box Coordinates |
|---|---|---|---|---|
| NORMAL COMPONENTS | ||||
| ELN | Elastin | Matrix protein | * UniProt P15502; ** AlphaFold AF-P15502 | 6 separate areas |
| FLN 1 | Fibrillin 1 | Elastin-associated microfibrillar protein | *** RCSB 2W86; UniProt P35555 | Whole molecule |
| MAGP 1 (MFAP 2) | Microfibril-associated glycoprotein 1 | Elastin-associated microfibrillar protein | UniProt P55001; AlphaFold AF- P55001 | Whole molecule |
| LTBP 4 | Latent-transforming growth factor beta-binding protein 4 | Elastin-associated microfibrillar protein | UniProt Q8N2S1; AlphaFold AF- Q8N2S1 | Central core |
| EMILIN 3 | Emilin 3 | Elastin-associated microfibrillar protein | UniProt Q9NT22; AlphaFold AF- Q9NT22 | Central core |
| FBLN 5 | Fibulin 5 | Elastin-associated microfibrillar protein | UniProt Q9UBX5; AlphaFold AF- Q9UBX5 | Central core |
| MFAP 4 | Microfibril-associated glycoprotein 4 | Elastin-associated microfibrillar protein | UniProt P55083; AlphaFold AF-P55083 | Central core |
| DCN | Decorin | Proteoglycan core protein | UniProt P07585; AlphaFold AF-P07585 | Whole molecule |
| LOX 1 | Protein-lysine Oxidase | Crosslinking enzyme | UniProt P28300; AlphaFold AF-P28300 | Central core |
| COL 1 | Collagen type I | Matrix protein; Fibrillar collagen | RCSB 7CWK | Central core (repetitive sequence) |
| COL 4 | Collagen type IV, NC1 region | Basement membrane collagen | RCSB 1M3D | Central core |
| PATHOLOGIC COMPONENTS | ||||
| MMP 2 | Matrix metalloproteinase 2 | Enzyme involved in matrix degradation | RCSB 1QIB | Catalytic site (whole molecule) |
| MMP 9 | Matrix metalloproteinase 9 | Enzyme involved in matrix degradation | RCSB 1L6J | Catalytic site (whole molecule) |
| MMP 12 | Matrix metalloproteinase 2 | Enzyme involved in matrix degradation | RCSB 1JK3 | Catalytic site (whole molecule) |
| IL 6 | Interleukin-6 | Cytokine | RCSB 1ALU | Whole molecule |
| IL 8 | Interleukin-8 | Cytokine | RCSB 1IL8 | Whole molecule |
| IL 10 | Interleukin-10 | Cytokine | RCSB 2H24 | Whole molecule |
| TNF-α | Tumor necrosis factor alpha | Cytokine | RCSB 1TNF | Whole molecule |
| CCL 5 | CC chemokine 5 | Cytokine | RCSB 5COY | Whole molecule |
| MCP 1 | Monocyte chemoattractant protein 1 | Cytokine | RCSB 1DOK | |
| Fibrin | D-dimer from cross-linked fibrin | Coagulation factor | RCSB 1N86 | Whole molecule |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionescu, D.; Tharayil, N.; Leonard, E.; Carlyle, W.; Schwarz, A.; Ning, K.; Carsten, C.; Garcia, J.C.C.; Carter, A.; Owens, C.; et al. Binding of Pentagalloyl Glucose to Aortic Wall Proteins: Insights from Peptide Mapping and Simulated Docking Studies. Bioengineering 2023, 10, 936. https://doi.org/10.3390/bioengineering10080936
Simionescu D, Tharayil N, Leonard E, Carlyle W, Schwarz A, Ning K, Carsten C, Garcia JCC, Carter A, Owens C, et al. Binding of Pentagalloyl Glucose to Aortic Wall Proteins: Insights from Peptide Mapping and Simulated Docking Studies. Bioengineering. 2023; 10(8):936. https://doi.org/10.3390/bioengineering10080936
Chicago/Turabian StyleSimionescu, Dan, Nishanth Tharayil, Elizabeth Leonard, Wenda Carlyle, Alex Schwarz, Kelvin Ning, Christopher Carsten, Juan Carlos Carrillo Garcia, Alexander Carter, Collin Owens, and et al. 2023. "Binding of Pentagalloyl Glucose to Aortic Wall Proteins: Insights from Peptide Mapping and Simulated Docking Studies" Bioengineering 10, no. 8: 936. https://doi.org/10.3390/bioengineering10080936








