Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats
Abstract
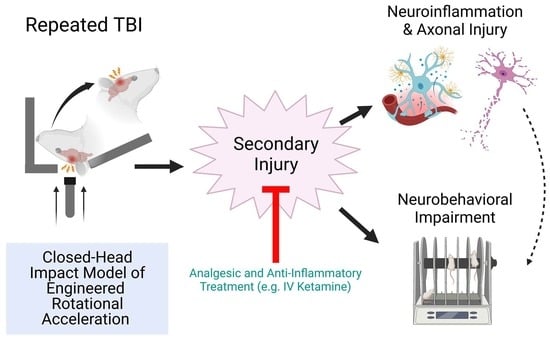
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CHIMERA Injury
2.3. Ketamine Infusion
Blood Sampling
2.4. Cytokine Multiplex Immunoassay
2.5. Behavioral Tests
2.5.1. Rotarod
2.5.2. Locomotor Activity
2.5.3. ASR/PPI
2.6. Brain Tissue Collection
2.7. Histology
2.7.1. Immunohistochemistry
2.7.2. Silver Staining
2.7.3. Microscope Imaging and Quantification
2.8. Statistical Analysis
3. Results
3.1. Body Weight Changes following CHIMERA Injury
3.2. Ketamine-Induced Locomotor Activity Changes
3.3. Time Course of Plasma Cytokines
3.4. Behavioral Outcome Measures
3.5. Neuroinflammation and Axonal Damage
4. Discussion
5. Transparency, Rigor, and Reproducibility Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faul, M.; Coronado, V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 3–13. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths-United States. 2014. Available online: https://www.cdc.gov/traumaticbraininjury/get_the_facts.html (accessed on 15 January 2023).
- Kim, K.; Priefer, R. Evaluation of current post-concussion protocols. Biomed. Pharmacother. 2020, 129, 110406. [Google Scholar] [CrossRef]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Primers 2016, 2, 16084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Naalt, J.; Jacobs, B. Highlights mild traumatic brain injury 2021. Curr. Opin. Anaesthesiol. 2022, 35, 77–582. [Google Scholar] [CrossRef] [PubMed]
- Chase, R.P.; Nevin, R.L. Population estimates of undocumented incident traumatic brain injuries among combat-deployed US military personnel. J. Head Trauma Rehabil. 2015, 30, E57–E64. [Google Scholar] [CrossRef]
- Flanagan, S.R. Invited Commentary on “Centers for Disease Control and Prevention Report to Congress: Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation”. Arch. Phys. Med. Rehabil. 2015, 96, 1753–1755. [Google Scholar] [CrossRef]
- Levin, H.S.; Diaz-Arrastia, R.R. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015, 14, 506–517. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatri, N.; Sumadhura, B.; Kumar, S.; Kaundal, R.K.; Sharma, S.; Datusalia, A.K. The Complexity of Secondary Cascade Consequent to Traumatic Brain Injury: Pathobiology and Potential Treatments. Curr. Neuropharmacol. 2021, 19, 1984. [Google Scholar] [CrossRef]
- Devoto, C.; Arcurio, L.; Fetta, J.; Ley, M.; Rodney, T.; Kanefsky, R.; Gill, J. Inflammation Relates to Chronic Behavioral and Neurological Symptoms in Military Personnel with Traumatic Brain Injuries. Cell Transplant. 2017, 26, 1169–1177. [Google Scholar] [CrossRef]
- McKee, C.A.; Lukens, J.R. Emerging Roles for the Immune System in Traumatic Brain Injury. Front. Immunol. 2016, 7, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaban, V.; Clarke, G.; Skandsen, T.; Islam, R.; Einarsen, C.; Vik, A.; Damas, J.K.; Mollnes, T.E.; Haberg, A.K.; Pischke, S.E. Systemic Inflammation Persists the First Year after Mild Traumatic Brain Injury: Results from the Prospective Trondheim Mild TBI Study. J. Neurotrauma 2020, 37, 2120–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licastro, F.; Hrelia, S.; Porcellini, E.; Malaguti, M.; Di Stefano, C.; Angeloni, C.; Carbone, I.; Simoncini, L.; Piperno, R. Peripheral Inflammatory Markers and Antioxidant Response during the Post-Acute and Chronic Phase after Severe Traumatic Brain Injury. Front. Neurol. 2016, 7, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippa, S.M.; Werner, J.K.; Miller, M.C.; Gill, J.M.; Diaz-Arrastia, R.; Kenney, K. Recent Advances in Blood-Based Biomarkers of Remote Combat-Related Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2020, 20, 54. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, L.; Niu, X.; Wang, Z.; Yin, B.; Bai, G.; Zhang, D.; Gan, S.; Sun, C.; Wang, S.; et al. Elevated Serum Levels of Inflammation-Related Cytokines in Mild Traumatic Brain Injury Are Associated with Cognitive Performance. Front. Neurol. 2019, 10, 1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, K.A.; Gill, J.M.; Pattinson, C.L.; Lai, C.; Brière, M.; Rogers, N.J.; Milhorn, D.; Elliot, J.; Carr, W. Interleukin-6 is associated with acute concussion in military combat personnel. BMC Neurol. 2020, 20, 209. [Google Scholar] [CrossRef]
- Dalgard, C.L.; Cole, J.T.; Kean, W.S.; Lucky, J.J.; Sukumar, G.; McMullen, D.C.; Pollard, H.B.; Watson, W.D. The cytokine temporal profile in rat cortex after controlled cortical impact. Front. Mol. Neurosci. 2012, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Helmy, A.; Carpenter, K.L.; Menon, D.K.; Pickard, J.D.; Hutchinson, P.J. The cytokine response to human traumatic brain injury: Temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow. Metab. 2011, 31, 658–670. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.C.; Liao, Y.E.; Yang, L.Y.; Wang, J.Y.; Tweedie, D.; Karnati, H.K.; Greig, N.H.; Wang, J.Y. Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Methods 2016, 272, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef]
- Bergold, P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016, 275 Pt 3, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Petz, L.N.; Tyner, S.; Barnard, E.; Ervin, A.; Mora, A.; Clifford, J.; Fowler, M.; Bebarta, V.S. Prehospital and en route analgesic use in the combat setting: A prospectively designed, multicenter, observational study. Mil. Med. 2015, 180, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, S.G.; Mora, A.G.; Maddry, J.K.; Bebarta, V.S. Multicenter, prospective study of prehospital administration of analgesia in the US combat theater of Afghanistan. Prehospital Emerg. Care 2017, 21, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.; Pittman, E.; Drew, B.; Walrath, B. Ketamine Use in Operation Enduring Freedom. Mil. Med. 2021, 186, e720–e725. [Google Scholar] [CrossRef]
- Wedmore, I.S.; Butler, F.K., Jr. Battlefield Analgesia in Tactical Combat Casualty Care. Wilderness Environ. Med. 2017, 28, S109–S116. [Google Scholar] [CrossRef] [Green Version]
- Pamplin, L.J.; Riesberg, L.J.; Powell, M.D.; Keenan, C.S.; Shackelford, C.S. Analgesia and Sedation Management during Prolonged Field Care. J. Spec. Oper. Med. A Peer Rev. J. SOF Med. Prof. 2017, 17, 106–120. [Google Scholar] [CrossRef]
- Bebarta, V.S.; Mora, A.G.; Bebarta, E.K.; Reeves, L.K.; Maddry, J.K.; Schauer, S.G.; Lairet, J.R. Prehospital Use of Ketamine in the Combat Setting: A Sub-Analysis of Patients with Head Injuries Evaluated in the Prospective Life Saving Intervention Study. Mil. Med. 2020, 185, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Zeiler, F.A.; Teitelbaum, J.; West, M.; Gillman, L.M. The ketamine effect on ICP in traumatic brain injury. Neurocrit. Care 2014, 21, 163–173. [Google Scholar] [CrossRef]
- Zanza, C.; Piccolella, F.; Racca, F.; Romenskaya, T.; Longhitano, Y.; Franceschi, F.; Savioli, G.; Bertozzi, G.; De Simone, S.; Cipolloni, L. Ketamine in Acute Brain Injury: Current Opinion Following Cerebral Circulation and Electrical Activity. Healthcare 2022, 10, 566. [Google Scholar] [CrossRef]
- Torres, A.C.; Bebarta, V.S.; April, M.D.; Maddry, J.K.; Herson, P.S.; Bebarta, E.K.; Schauer, S. Ketamine Administration in Prehospital Combat Injured Patients with Traumatic Brain Injury: A 10-Year Report of Survival. Cureus 2020, 12, e9248. [Google Scholar] [CrossRef]
- Wang, C.Q.; Ye, Y.; Chen, F.; Han, W.C.; Sun, J.M.; Lu, X.; Guo, R.; Cao, K.; Zheng, M.J.; Liao, L.C. Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 2017, 343, 30–38. [Google Scholar] [CrossRef]
- Liang, J.; Wu, S.; Xie, W.; He, H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des. Dev. Ther. 2018, 12, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Farrell, D.; Bendo, A.A. Perioperative Management of Severe Traumatic Brain Injury: What Is New? Curr. Anesthesiol. Rep. 2018, 8, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kock, M.; Loix, S.; Lavand’homme, P. Ketamine and peripheral inflammation. CNS Neurosci. Ther. 2013, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Loix, S.; De Kock, M.; Henin, P. The anti-inflammatory effects of ketamine: State of the art. Acta Anaesthesiol. Belg. 2011, 62, 47–58. [Google Scholar]
- Bell, J.D. In Vogue: Ketamine for Neuroprotection in Acute Neurologic Injury. Anesth. Analg. 2017, 124, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiang, R.Y.; Shen, J.; Hong, T.; Liu, N.; Ding, L.C.; Wang, D.M.; Chen, L.J.; Xu, B.; Zhu, B. Ketamine attenuates the lipopolysaccharide-induced inflammatory response in cultured N2a cells. Mol. Med. Rep. 2013, 8, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Lee, J.-J.; Hsieh, C.-Y.; Hsiao, G.; Chou, D.-S.; Sheu, J.-R. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediat. Inflamm. 2009, 2009, 705379. [Google Scholar] [CrossRef] [Green Version]
- Réus, G.Z.; Simões, L.R.; Colpo, G.D.; Scaini, G.; Oses, J.P.; Generoso, J.S.; Prossin, A.R.; Kaddurah-Daouk, R.; Quevedo, J.; Barichello, T. Ketamine potentiates oxidative stress and influences behavior and inflammation in response to lipolysaccharide (LPS) exposure in early life. Neuroscience 2017, 353, 17–25. [Google Scholar] [CrossRef]
- Ward, J.L.; Harting, M.T.; Cox, C.S., Jr.; Mercer, D.W. Effects of ketamine on endotoxin and traumatic brain injury induced cytokine production in the rat. J. Trauma 2011, 70, 1471–1479. [Google Scholar] [CrossRef] [Green Version]
- DeWalt, G.J.; Mahajan, B.; Foster, A.R.; Thompson, L.D.E.; Marttini, A.A.; Schmidt, E.V.; Mansuri, S.; D’Souza, D.; Patel, S.B.; Tenenbaum, M.; et al. Region-specific alterations in astrocyte and microglia morphology following exposure to blasts in the mouse hippocampus. Neurosci. Lett. 2018, 664, 160–166. [Google Scholar] [CrossRef]
- Peters, A.J.; Villasana, L.E.; Schnell, E. Ketamine Alters Hippocampal Cell Proliferation and Improves Learning in Mice after Traumatic Brain Injury. Anesthesiology 2018, 129, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, D.R.; Cheng, W.H.; Bashir, A.; Wilkinson, A.; Stukas, S.; Martens, K.M.; Whyte, T.; Abebe, Z.A.; McInnes, K.A.; Cripton, P.A.; et al. Defining the biomechanical and biological threshold of murine mild traumatic brain injury using CHIMERA (Closed Head Impact Model of Engineered Rotational Acceleration). Exp. Neurol. 2017, 292, 80–91. [Google Scholar] [CrossRef]
- Namjoshi, D.R.; Cheng, W.H.; McInnes, K.A.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Cripton, P.A.; et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A novel, surgery-free model of traumatic brain injury. Mol. Neurodegener. 2014, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonder Haar, C.; Martens, K.M.; Bashir, A.; McInnes, K.A.; Cheng, W.H.; Cheung, H.; Stukas, S.; Barron, C.; Ladner, T.; Welch, K.A.; et al. Repetitive closed-head impact model of engineered rotational acceleration (CHIMERA) injury in rats increases impulsivity, decreases dopaminergic innervation in the olfactory tubercle and generates white matter inflammation, tau phosphorylation and degeneration. Exp. Neurol. 2019, 317, 87–99. [Google Scholar] [CrossRef]
- Spencer, H.F.; Berman, R.Y.; Boese, M.; Zhang, M.; Kim, S.Y.; Radford, K.D.; Choi, K.H. Effects of an intravenous ketamine infusion on inflammatory cytokine levels in male and female Sprague–Dawley rats. J. Neuroinflamm. 2022, 19, 75. [Google Scholar] [CrossRef]
- Tucker, L.B.; Winston, B.S.; Liu, J.; Velosky, A.G.; Fu, A.H.; Grillakis, A.A.; McCabe, J.T. Sex differences in cued fear responses and parvalbumin cell density in the hippocampus following repetitive concussive brain injuries in C57BL/6J mice. PLoS ONE 2019, 14, e0222153. [Google Scholar] [CrossRef] [Green Version]
- McNamara, E.H.; Knutsen, A.; Korotcov, A.; Bosomtwi, A.; Liu, J.; Fu, A.H.; Kostelnik, C.; Grillakis, A.; Spencer, H.; Dardzinski, B.J. Meningeal and visual pathway MRI analysis after single and repetitive Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA)-induced disruption in male and female mice. J. Neurotrauma 2022, 39, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Radford, K.D.; Park, T.Y.; Lee, B.H.; Moran, S.; Osborne, L.A.; Choi, K.H. Dose-response characteristics of intravenous ketamine on dissociative stereotypy, locomotion, sensorimotor gating, and nociception in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2017, 153, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Butler, F.K.; Kotwal, R.S.; Buckenmaier, C., 3rd; Edgar, E.P.; O’Connor, K.C.; Montgomery, H.R.; Shackelford, S.A.; Gandy, J., 3rd; Wedmore, I.S.; Timby, J.W. A triple-option analgesia plan for tactical combat casualty care: TCCC guidelines change 13-04. J. Spec. Oper. Med. 2014, 14, 13–25. [Google Scholar] [CrossRef]
- Edwards, K.A.; Pattinson, C.L.; Guedes, V.A.; Peyer, J.; Moore, C.; Davis, T.; Devoto, C.; Turtzo, L.C.; Latour, L.; Gill, J.M. Inflammatory Cytokines Associate with Neuroimaging After Acute Mild Traumatic Brain Injury. Front. Neurol. 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, A.; Brennan, J.; Levin, H.; McCarthy, J.J.; Dash, P.K.; Redell, J.B.; Yamal, J.M.; Robertson, C.S. Early versus late profiles of inflammatory cytokines after mild TBI and their association with neuropsychological outcomes. J. Neurotrauma 2020, 38, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef] [Green Version]
- McNamara, E.H.; Grillakis, A.A.; Tucker, L.B.; McCabe, J.T. The closed-head impact model of engineered rotational acceleration (CHIMERA) as an application for traumatic brain injury pre-clinical research: A status report. Exp. Neurol. 2020, 333, 113409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, R.; Wen, G.; Ding, R.; Du, A.; Zhou, J.; Dong, Z.; Ren, X.; Yao, H.; Zhao, R. Effects of ketamine on levels of inflammatory cytokines IL-6, IL-1β, and TNF-α in the hippocampus of mice following acute or chronic administration. Front. Pharmacol. 2017, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Li, K.; Roth, E.; Chao, D.; Mecca, C.M.; Hogan, Q.H.; Pawela, C.; Kwok, W.M.; Camara, A.K.S.; Pan, B. Repetitive Mild Traumatic Brain Injury in Rats Impairs Cognition, Enhances Prefrontal Cortex Neuronal Activity, and Reduces Pre-synaptic Mitochondrial Function. Front. Cell Neurosci. 2021, 15, 689334. [Google Scholar] [CrossRef]
- Edem, E.E.; Anyanwu, C.C.; Nebo, K.E.; Akinluyi, E.T.; Fafure, A.A.; Ishola, A.O.; Enye, L.A. Ketamine abrogates sensorimotor deficits and cytokine dysregulation in a chronic unpredictable mild stress model of depression. Psychopharmacology 2021, 239, 185–200. [Google Scholar] [CrossRef]
- Desai, A.; Chen, H.; Kim, H.Y. Multiple mild closed head injuries lead to visual dysfunction in a mouse model. J. Neurotrauma 2019, 37, 286–294. [Google Scholar] [CrossRef]
- Grillon, C.; Morgan, C.A.; Southwick, S.M.; Davis, M.; Charney, D.S. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996, 64, 169–178. [Google Scholar] [CrossRef]
- Browne, C.A.; Wulf, H.A.; Jacobson, M.L.; Oyola, M.G.; Wu, T.J.; Lucki, I. Long-term increase in sensitivity to ketamine’s behavioral effects in mice exposed to mild blast induced traumatic brain injury. Exp. Neurol. 2022, 350, 113963. [Google Scholar] [CrossRef]
- Johnston, J.N.; Greenwald, M.S.; Henter, I.D.; Kraus, C.; Mkrtchian, A.; Clark, N.G.; Park, L.T.; Gold, P.; Zarate, C.A., Jr.; Kadriu, B. Inflammation, stress and depression: An exploration of ketamine’s therapeutic profile. Drug Discov. Today 2023, 28, 103518. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.C.; Mierzwa, A.J.; Marion, C.M.; Sullivan, G.M. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp. Neurol. 2016, 275, 328–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, L.B.; Fu, A.H.; McCabe, J.T. Hippocampal-Dependent Cognitive Dysfunction Following Repeated Diffuse Rotational Brain Injury in Male and Female Mice. J. Neurotrauma 2021, 38, 1585–1606. [Google Scholar] [CrossRef]
- Späni, C.B.; Braun, D.J.; Van Eldik, L.J. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front. Neuroendocrinol. 2018, 50, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Radford, K.D.; Berman, R.Y.; Zhang, M.; Wu, T.J.; Choi, K.H. Sex-related differences in intravenous ketamine effects on dissociative stereotypy and antinociception in male and female rats. Pharmacol. Biochem. Behav. 2020, 199, 173042. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spencer, H.F.; Boese, M.; Berman, R.Y.; Radford, K.D.; Choi, K.H. Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats. Bioengineering 2023, 10, 941. https://doi.org/10.3390/bioengineering10080941
Spencer HF, Boese M, Berman RY, Radford KD, Choi KH. Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats. Bioengineering. 2023; 10(8):941. https://doi.org/10.3390/bioengineering10080941
Chicago/Turabian StyleSpencer, Haley F., Martin Boese, Rina Y. Berman, Kennett D. Radford, and Kwang H. Choi. 2023. "Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats" Bioengineering 10, no. 8: 941. https://doi.org/10.3390/bioengineering10080941
APA StyleSpencer, H. F., Boese, M., Berman, R. Y., Radford, K. D., & Choi, K. H. (2023). Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats. Bioengineering, 10(8), 941. https://doi.org/10.3390/bioengineering10080941