Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging
Abstract
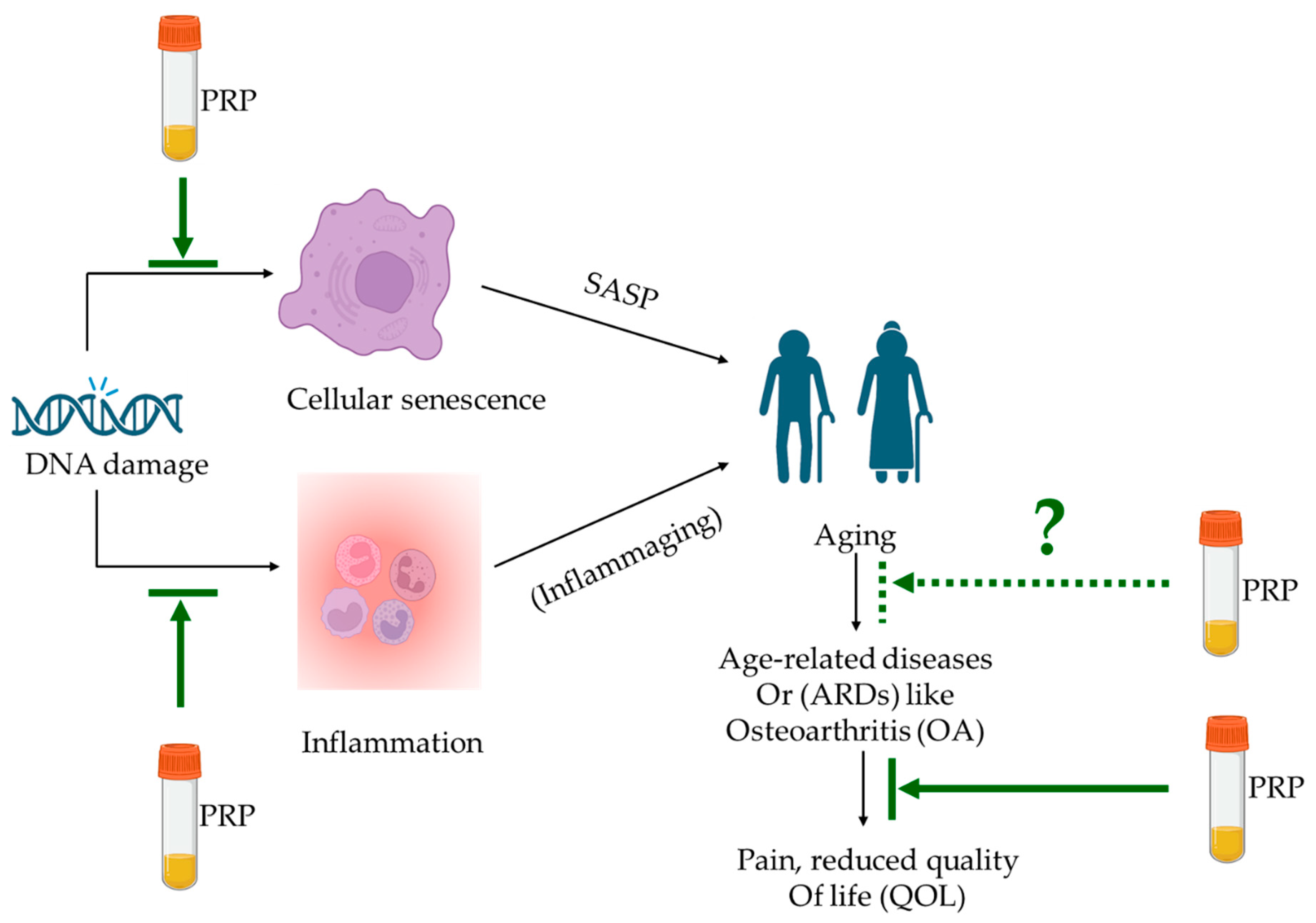
:1. Introduction
2. Senescence and Inflammaging
2.1. Senescence
2.2. Inflammaging
3. Platelet-Rich Plasma (PRP)
3.1. Properties and Contents of PRP and Platelet-Derived Biologics
3.2. Current Applications of PRP
4. Anti-Aging Potential of PRP for ARDs
4.1. Current Evidence in Literature
| PRP Used | Model | PRP Details | Target Disease/Organ | Senescence/Aging-Related Parameters Tested | Main Age-Related Findings | Ref. |
|---|---|---|---|---|---|---|
| Autologous IA PRP | In vivo: prospective, double-blinded, comparative RCT between PRP and HA | Leukocyte poor, single spin, PRP average volume of 4 mL | Knee OA | Inflammatory/catabolic markers by ELISA and pain scores (WOMAC, IKDC, VAS) | PRP indicated significantly better outcomes at weeks 24 and 52. Additionally, PRP treatment decreased the secretion of two pro-inflammatory cytokines, i.e., IL-1β and TNF-α | [12] |
| Human PRP releasate coated onto porous collagen-glycosaminoglycans (GAG) scaffolds | In vitro: human bone marrow mesenchymal stromal cells (MSCs) and human pooled endothelial cells | Platelet concentration of 1 × 106/mL formulated into PRP releasate | Skin wound healing application | Proliferation, migration, angiogenesis, vascularisation, macrophage polarisation | PRP-coated scaffold exhibited enhanced angiogenic and vascularisation potential. It also indicated a lower release of pro-inflammatory markers when exposed to an inflammatory environment | [30] |
| Autologous IA and IO PRP for patient treatment before cell isolation | Ex vivo: human bone marrow MSCs | Liquid PRP IA: 8 mL and IO: 10 mL volumes, respectively | Hip OA | MSC proliferation, SA-β-gal, gene expression | Enhanced MSC proliferation and stress resistance capacities in older MSC donors treated with PRP | [32] |
| Healthy male New Zealand white rabbit PRP; cell culture treated | In vitro: murine dermal fibroblasts | 1%, 5% and 10% PRP diluted in 1%FBS for in vitro cultures | Senescence associated phenotypes in photo aging | Cell morphology, SA-β-gal, cell cycle arrest, ECM, ROS | PRP prevented cell cycle arrest, reduced SA-β-gal, increased ECM collagen and decreased MMPs. PRP may counteract cell senescence in MSK | [34] |
| Allogenic, IV PRP | In vivo: senescent female BALB/c mice (16–18 months old); n = 46 | Not provided | Cognitive aging in senescent mice | Behavioural and cognitive: open-field, elevated plus maze, tail suspension, and Morris water maze test | PRP increased locomotion, improved memory, reduced anxiety and depression-like behaviour in old mice; enhanced cognitive function with PRP | [35] |
| Enhanced PRP (ePRP) powder prepared by freeze-drying PRP from human patients | In vitro: human dermal fibroblasts | Platelet count: 1 × 109/mL lyophilised powder reconstituted with 1 mL media | Wound healing in fibroblasts in vitro | Proliferation, wound healing, qPCR, intracellular ATP measurement, SA-β-gal, metabolic flux | ePRP stimulates wound healing by releasing growth factors, increased anti-oxidant production, halts the senescence progression of fibroblasts by activating SIRT1 expression | [107] |
| PRP prepared from rabbit/mouse used for the study | In vitro and in vivo: rabbit tendon cells in vitro studies and mice for in vivo studies | In vitro: 10%PRP and in vivo: 10 mL PRP | Injured tendons | In vitro—COX-1, COX-2, mPGES levels by gene expression, Western blotting and immunostaining; in vivo—immunohistochemistry | PRP treatment suppressed tendon cell inflammation in vitro and tendon inflammation in vivo, at least partially mediated by HGF in PRP | [109] |
| Intra-ovarian delivery of autologous PRP | In vivo: women with primary ovarian insufficiency (POI) | 2–4 mL of PRP was injected underneath the ovarian cortex | Ovarian stimulation and IVF outcome in women with POI | Antral follicular count, follicle-stimulating hormone, IVF, clinical pregnancy | PRP treatment aided conception in women suffering from POI, with 7.4% of the women being able to conceive spontaneously after PRP treatment | [110] |
| Human PRP is used to treat NIH3T3 cells to be transplanted in vivo | In vivo: genetically modified NIH3T3 embryonic fibroblasts with an enhanced green fluorescent protein (NIH3T3-G) differentiated into osteoblasts transplanted into variectomized senescence-accelerated mouse prone substrain 8 (OVX-SAMP8 mice) | Not provided | Bone regeneration applications in osteoporosis and conditions associated with accelerated aging | Molecular imaging and immunohistochemistry | (PRP/NIH3T3-G) engraftment prevented the development of osteoporosis, and the life span of OVX-SAMP8 mice receiving PRP/NIH3T3-G transplantation was significantly prolonged | [111] |
4.2. Potential Mechanism of Action (MOA)
5. Limitations, Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ageing and Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 5 April 2023).
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Jeon, O.H.; David, N.; Campisi, J.; Elisseeff, J.H. Senescent cells and osteoarthritis: A painful connection. J. Clin. Investig. 2018, 128, 1229–1237. [Google Scholar] [CrossRef]
- Zhang, X.-X.; He, S.-H.; Liang, X.; Li, W.; Li, T.-F.; Li, D.-F. Aging, Cell Senescence, the Pathogenesis and Targeted Therapies of Osteoarthritis. Front. Pharmacol. 2021, 12, 728100. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, T.; Xu, H.; Zhang, H. Senescence in osteoarthritis: From mechanism to potential treatment. Arthritis Res. Ther. 2022, 24, 174. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Best, T.M.; Huard, J.; Philippon, M.; Hornicek, F.; Duan, Z.; Griswold, A.J.; Kaplan, L.D.; Hare, J.M.; Kouroupis, D. Therapeutic Perspectives for Inflammation and Senescence in Osteoarthritis Using Mesenchymal Stem Cells, Mesenchymal Stem Cell-Derived Extracellular Vesicles and Senolytic Agents. Cells 2023, 12, 1421. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm. 2018, 2018, 9076485. [Google Scholar] [CrossRef]
- Sánchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Recalde, E.A.; Gutierrez, A.G.; Albillos, J.; Gárate, R.; Aguirre, J.J.; Padilla, S.; et al. A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1070–1078. [Google Scholar] [CrossRef]
- Cole, B.J.; Karas, V.; Hussey, K.; Merkow, D.B.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Beitia, M.; Pompei, O.; Jorquera, C.; Sánchez, P.; Knörr, J.; Soldado, F.; López, L.; Oraa, J.; Bilbao, A.M.; et al. Isolation, Activation, and Mechanism of Action of Platelet-Rich Plasma and Its Applications for Joint Repair. In Regenerative Medicine; Intechopen: London, UK, 2019. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-Rich Plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Du, R.; Lei, T. Effects of autologous platelet-rich plasma injections on facial skin rejuvenation. Exp. Ther. Med. 2020, 19, 3024–3030. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Wang, H.L.; Avila, G. Platelet rich plasma: Myth or reality? Eur. J. Dent. 2007, 1, 192–194. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- NICE. Platelet-Rich Plasma Injections for Knee Osteoarthritis. Available online: https://www.nice.org.uk/guidance/ipg637 (accessed on 5 April 2023).
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021–2030. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef]
- Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Dev. Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review—The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Akeda, K.; Yamada, J.; Takegami, N.; Fujiwara, T.; Fujita, N.; Sudo, A. Advances in Platelet-Rich Plasma Treatment for Spinal Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7677. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; Garate, A.; Sánchez, P.; Bilbao, A.M.; del Caño, G.G.; Salles, J.; Sánchez, M. Biological and structural effects after intraosseous infiltrations of age-dependent platelet-rich plasma: An in vivo study. J. Orthop. Res. 2020, 38, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, R.J.F.C.; Zayed, N.M.A.; Pascu, E.I.; Cavanagh, B.; Hobbs, C.; Santarella, F.; Simpson, C.R.; Murphy, C.M.; Sridharan, R.; González-Vázquez, A.; et al. Functionalising Collagen-Based Scaffolds With Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lei, X.X.; Xuan, L.; Tang, J.B.; Cheng, B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets 2019, 30, 773–792. [Google Scholar] [CrossRef]
- Ganguly, P.; Fiz, N.; Beitia, M.; Owston, H.E.; Delgado, D.; Jones, E.; Sánchez, M. Effect of Combined Intraosseous and Intraarticular Infiltrations of Autologous Platelet-Rich Plasma on Subchondral Bone Marrow Mesenchymal Stromal Cells from Patients with Hip Osteoarthritis. J. Clin. Med. 2022, 11, 3891. [Google Scholar] [CrossRef]
- Ruan, D.; Tang, C.; Fei, Y.; Qian, S.; Xiang, X.; Xu, J.; Huang, Z.; Chen, X.; Heng, B.C.; Yin, Z.; et al. Early-Stage Primary Anti-inflammatory Therapy Enhances the Regenerative Efficacy of Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am. J. Sports Med. 2021, 49, 3357–3371. [Google Scholar] [CrossRef]
- Jia, C.; Lu, Y.; Bi, B.; Chen, L.; Yang, Q.; Yang, P.; Guo, Y.; Zhu, J.; Zhu, N.; Liu, T. Platelet-rich plasma ameliorates senescence-like phenotypes in a cellular photoaging model. RSC Adv. 2017, 7, 3152–3160. [Google Scholar] [CrossRef]
- Demir, E.A.; Karagoz, M. Platelet-Rich Plasma (PRP) is a Potential Self-Sourced Cognition Booster in Elderly Mice. Exp. Aging Res. 2020, 46, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Zhai, W.; Tan, J.; Russell, T.; Chen, S.; McGonagle, D.; Naing, M.W.; Yong, D.; Jones, E. Multi-pronged approach to human mesenchymal stromal cells senescence quantification with a focus on label-free methods. Sci. Rep. 2021, 11, 1054. [Google Scholar] [CrossRef]
- Olivieri, F.; Albertini, M.C.; Orciani, M.; Ceka, A.; Cricca, M.; Procopio, A.D.; Bonafè, M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget 2015, 6, 35509–35521. [Google Scholar] [CrossRef]
- D’adda Di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- Rodier, F.; Coppé, J.-P.; Patil, C.K.; Hoeijmakers, W.A.M.; Muñoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45lowCD271+ Phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef]
- Schultz, M.B.; Sinclair, D.A. When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Development 2016, 143, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Drummond-Barbosa, D. Stem Cells, Their Niches and the Systemic Environment: An Aging Network. Genetics 2008, 180, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Xue, W.; Zender, L.; Yon, M.; Hernando, E.; Lowe, S. Implications of Cellular Senescence in Tissue Damage Response, Tumor Suppression, and Stem Cell Biology. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Y.; Lu, L.; Liu, L.; Yu, X.; Pei, F. Cellular senescence in knee osteoarthritis: Molecular mechanisms and therapeutic implications. Ageing Res. Rev. 2021, 70, 101413. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.-M.; DeMaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; DeMaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Walaszczyk, A.; Dookun, E.; Redgrave, R.; Tual-Chalot, S.; Victorelli, S.; Spyridopoulos, I.; Owens, A.; Arthur, H.M.; Passos, J.F.; Richardson, G.D. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 2019, 18, e12945. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.-O.; Lee, Y.-K.; Kim, J.-M.; Yoon, G. From cell senescence to age-related diseases: Differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2015, 48, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflammation markers predicting frailty and mortality in the elderly. Exp. Mol. Pathol. 2006, 80, 219–227. [Google Scholar] [CrossRef]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Heal. 2013, 2, 8. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.-D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.H.M.; Schajowicz, F.; Trueta, J. Osteoarthritis of the hip: A study of the nature and evolution of the disease. J. Bone Jt. Surg. 1953, 35-B, 598–626. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res. Rev. 2020, 64, 101142. [Google Scholar] [CrossRef]
- Sánchez, M.; Delgado, D.; Sánchez, P.; Muiños-López, E.; Paiva, B.; Granero-Moltó, F.; Prósper, F.; Pompei, O.; Pérez, J.C.; Azofra, J.; et al. Combination of Intra-Articular and Intraosseous Injections of Platelet Rich Plasma for Severe Knee Osteoarthritis: A Pilot Study. BioMed Res. Int. 2016, 2016, 4868613. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.; Han, Y.; Yang, W.; Xu, N.; Luo, M.; Pan, J.; Liu, J.; Zeng, L.-F. Combination of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) in the treatment of knee osteoarthritis: A meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e061008. [Google Scholar] [CrossRef]
- Su, K.; Bai, Y.; Wang, J.; Zhang, H.; Liu, H.; Ma, S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin. Rheumatol. 2018, 37, 1341–1350. [Google Scholar] [CrossRef]
- Paterson, K.L.; Hunter, D.J.; Metcalf, B.R.; Eyles, J.; Duong, V.; Kazsa, J.; Wang, Y.; Buchbinder, R.; Cicuttini, F.; Forbes, A.; et al. Efficacy of intra-articular injections of platelet-rich plasma as a symptom- and disease-modifying treatment for knee osteoarthritis—The RESTORE trial protocol. BMC Musculoskelet. Disord. 2018, 19, 272. [Google Scholar] [CrossRef] [PubMed]
- Mojica, E.S.; Markus, D.H.; Hurley, E.T.; Blaeser, A.M.; Jazrawi, L.M.; Campbell, K.A.; Strauss, E.J. Estimated Time to Maximum Medical Improvement of Intra-articular Injections in the Treatment of Knee Osteoarthritis—A Systematic Review. Arthroscopy 2022, 38, 980–988.e4. [Google Scholar] [CrossRef] [PubMed]
- Roffi, A.; Di Matteo, B.; Krishnakumar, G.S.; Kon, E.; Filardo, G. Platelet-rich plasma for the treatment of bone defects: From pre-clinical rational to evidence in the clinical practice. A systematic review. Int. Orthop. 2017, 41, 221–237. [Google Scholar] [CrossRef]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilising platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef]
- Soares, C.S.; Babo, P.S.; Reis, R.L.; Carvalho, P.P.; Gomes, M.E. Platelet-Derived Products in Veterinary Medicine: A New Trend or an Effective Therapy? Trends Biotechnol. 2020, 39, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Vun, J.; Panteli, M.; Jones, E.; Giannoudis, P. The in vitro effects of platelet products on the biophysiological functions of human bone marrow mesenchymal stromal cells: A systematic review. Eur. Cells Mater. 2021, 41, 269–315. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Mautner, K.; Malanga, G.A.; Smith, J.; Shiple, B.; Ibrahim, V.; Sampson, S.; Bowen, J.E. A Call for a Standard Classification System for Future Biologic Research: The Rationale for New PRP Nomenclature. PMR 2015, 7 (Suppl. 4), S53–S59. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L., Jr.; Luzo, C.M.; Belangero, W.D.; et al. Contributions for classification of platelet rich plasma–proposal of a new classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The paw classification system. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Mishra, A.; Harmon, K.; Woodall, J.; Vieira, A. Sports Medicine Applications of Platelet Rich Plasma. Curr. Pharm. Biotechnol. 2012, 13, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, H.; Wu, B.; Huang, C.; Liu, Q.; Chen, B. Efficacy of Platelet-Rich Plasma in the Treatment of Fractures: A Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 5105725. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Liang, W.; Chen, G.; Ma, Y.; Zhou, Y.; Fen, R.; Jiang, K. Efficacy of adjuvant treatment for fracture nonunion/delayed union: A network meta-analysis of randomised controlled trials. BMC Musculoskelet. Disord. 2022, 23, 481. [Google Scholar] [CrossRef] [PubMed]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PMR 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Masiello, F.; Pati, I.; Veropalumbo, E.; Pupella, S.; Cruciani, M.; De Angelis, V. Ultrasound-guided injection of platelet-rich plasma for tendinopathies: A systematic review and meta-analysis. Blood Transfus. 2023, 21, 119–136. [Google Scholar] [CrossRef]
- Gong, H.; Li, K.; Xie, R.; Du, G.; Li, L.; Wang, S.; Yin, J.; Gu, J.; Wang, P.; Chen, M.; et al. Clinical therapy of platelet-rich plasma vs hyaluronic acid injections in patients with knee osteoarthritis: A systematic review and meta-analysis of randomised double-blind controlled trials. Medicine 2021, 100, e25168. [Google Scholar] [CrossRef] [PubMed]
- Laver, d.G.; Lior, L. ESSKA Orthobiologic Initiative (ORBIT): Consensus Process on Blood Derived Products. European Society of Sports Traumatology, Knee Surgery and Arthroscopy. 2022. Available online: https://www.esska.org/news/593282/ESSKA-Orthobiologic-Initiative-ORBIT-Consensus-Process-on-Blood-Derived-Products-.htm (accessed on 1 June 2023).
- Boffa, A.; Salerno, M.; Merli, G.; De Girolamo, L.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G. Platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4100–4121. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.-J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 11024. [Google Scholar] [CrossRef]
- Enomoto, H.; Inoki, I.; Komiya, K.; Shiomi, T.; Ikeda, E.; Obata, K.-I.; Matsumoto, H.; Toyama, Y.; Okada, Y. Vascular Endothelial Growth Factor Isoforms and Their Receptors Are Expressed in Human Osteoarthritic Cartilage. Am. J. Pathol. 2003, 162, 171–181. [Google Scholar] [CrossRef]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Thromb. Haemost. 2014, 16, 427. [Google Scholar] [CrossRef]
- Lee, J.S.; Guo, P.; Klett, K.; Hall, M.; Sinha, K.; Ravuri, S.; Huard, J.; Murphy, W.L. VEGF-attenuated platelet-rich plasma improves therapeutic effect on cartilage repair. Biomater. Sci. 2022, 10, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Wang, H.; Yuan, F.; Ding, Y. Platelet-Rich Plasma Combined with Alendronate Reduces Pain and Inflammation in Induced Osteoarthritis in Rats by Inhibiting the Nuclear Factor-Kappa B Signaling Pathway. BioMed Res. Int. 2020, 2020, 8070295. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Yazdanpanah, M.J.; Alamdari, D.H.; Banihashemi, M.; Salehi, M. The effect of platelet-rich plasma injection in the treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.T. Editorial Commentary: Platelet-Rich Plasma for Rotator Cuff Repairs: No Evidence for Improved Long-Term Outcomes … Yet! Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 62–64. [Google Scholar] [CrossRef]
- Dubin, D.P.; Lin, M.J.; Leight, H.M.; Farberg, A.S.; Torbeck, R.L.; Burton, W.B.; Khorasani, H. The effect of platelet-rich plasma on female androgenetic alopecia: A randomised controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1294–1297. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Montalvão, S.A.; Cancela, R.B.; Silva, F.A.; Urban, A.; Huber, S.C.; Júnior, J.L.R.; Lana, J.F.S.; Annichinno-Bizzacchi, J.M. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2019, 80, 694–700. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, D.-L.; Zhao, Y.-Q.; Li, Z.-Y. Effectiveness of platelet rich plasma in burn wound healing: A systematic review and meta-analysis. J. Dermatol. Treat. 2022, 33, 131–137. [Google Scholar] [CrossRef]
- Yao, D.; Feng, G.; Zhao, F.; Hao, D. Effects of platelet-rich plasma on the healing of sternal wounds: A meta-analysis. Wound Repair Regen. 2021, 29, 153–167. [Google Scholar] [CrossRef]
- Capion, S.C.; Jørgensen, H.B.L.; Ågren, M.S.; Daugaard, H.; Ribel-Madsen, S.; Marando, D.; Johansson, P.I.; Salado, J.; Halschou-Jensen, P.M.; Borgwardt, A.; et al. The wound healing effect of local leukocyte platelet-rich plasma after total hip arthroplasty: A randomised controlled trial. Wound Repair Regen. 2021, 29, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Maisel-Campbell, A.L.; Ismail, A.; Reynolds, K.A.; Poon, E.; Serrano, L.; Grushchak, S.; Farid, C.; West, D.P.; Alam, M. A systematic review of the safety and effectiveness of platelet-rich plasma (PRP) for skin aging. Arch. Dermatol. Res. 2020, 312, 301–315. [Google Scholar] [CrossRef]
- Weng, H.-P.; Cheng, Y.-Y.; Lee, H.-L.; Hsu, T.-Y.; Chang, Y.-T.; Shen, Y.-A. Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts. Int. J. Mol. Sci. 2021, 22, 12623. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Anitua, E.; Delgado, D.; Sanchez, P.; Prado, R.; Goiriena, J.J.; Prosper, F.; Orive, G.; Padilla, S. A new strategy to tackle severe knee osteoarthritis: Combination of intra-articular and intraosseous injections of Platelet Rich Plasma. Expert Opin. Biol. Ther. 2016, 16, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Middleton, K.K.; Fu, F.H.; Im, H.-J.; Wang, J.H.-C. HGF Mediates the Anti-inflammatory Effects of PRP on Injured Tendons. PLoS ONE 2013, 8, e67303. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef]
- Lo, W.-C.; Chiou, J.-F.; Gelovani, J.G.; Cheong, M.-L.; Lee, C.-M.; Liu, H.-Y.; Wu, C.-H.; Wang, M.-F.; Lin, C.-T.; Deng, W.-P. Transplantation of Embryonic Fibroblasts Treated with Platelet-Rich Plasma Induces Osteogenesis in SAMP8 Mice Monitored by Molecular Imaging. J. Nucl. Med. 2009, 50, 765–773. [Google Scholar] [CrossRef]
- El-Domyati, M.; Abdel-Wahab, H.; Hossam, A. Combining microneedling with other minimally invasive procedures for facial rejuvenation: A split-face comparative study. Int. J. Dermatol. 2018, 57, 1324–1334. [Google Scholar] [CrossRef]
- Charles-De-Sá, L.; Gontijo-De-Amorim, N.F.; Takiya, C.M.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Effect of Use of Platelet-Rich Plasma (PRP) in Skin with Intrinsic Aging Process. Aesthetic Surg. J. 2018, 38, 321–328. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef]
- Jain, N.K.; Gulati, M. Platelet-rich plasma: A healing virtuoso. BLOOD Res. 2016, 51, 3–5. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Vainchenker, W.; James, C. Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases. Int. J. Mol. Sci. 2020, 21, 7591. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Monzel, A.S.; Karan, K.R.; Michelson, J.; Ware, S.A.; Cardenas, A.; Lin, J.; Bris, C.; Santhanam, B.; Murphy, M.P.; et al. A multi-omics longitudinal aging dataset in primary human fibroblasts with mitochondrial perturbations. Sci. Data 2022, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 2010, 21, 369–374. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef] [PubMed]
- Andia, I. Platelet rich plasma therapies: A great potential to be harnessed. Muscles Ligaments Tendons J. 2014, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Karin, O.; Alon, U. Senescent cell accumulation mechanisms inferred from parabiosis. Geroscience 2021, 43, 329–341. [Google Scholar] [CrossRef]
- Liu, A.; Guo, E.; Yang, J.; Yang, Y.; Liu, S.; Jiang, X.; Hu, Q.; Dirsch, O.; Dahmen, U.; Zhang, C.; et al. Young plasma reverses age-dependent alterations in hepatic function through the restoration of autophagy. Aging Cell 2018, 17, e12708. [Google Scholar] [CrossRef]
- Ganguly, P.; Toghill, B.; Pathak, S. Aging, Bone Marrow and Next-Generation Sequencing (NGS): Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vun, J.; Iqbal, N.; Jones, E.; Ganguly, P. Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging. Bioengineering 2023, 10, 987. https://doi.org/10.3390/bioengineering10080987
Vun J, Iqbal N, Jones E, Ganguly P. Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging. Bioengineering. 2023; 10(8):987. https://doi.org/10.3390/bioengineering10080987
Chicago/Turabian StyleVun, James, Neelam Iqbal, Elena Jones, and Payal Ganguly. 2023. "Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging" Bioengineering 10, no. 8: 987. https://doi.org/10.3390/bioengineering10080987
APA StyleVun, J., Iqbal, N., Jones, E., & Ganguly, P. (2023). Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging. Bioengineering, 10(8), 987. https://doi.org/10.3390/bioengineering10080987







