Functionalized 3D-Printed PLA Biomimetic Scaffold for Repairing Critical-Size Bone Defects
Abstract
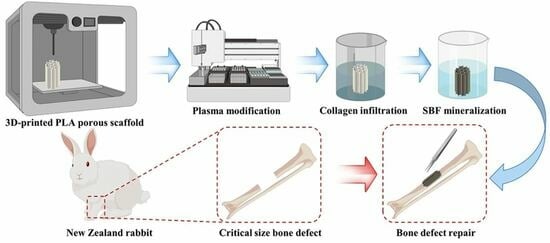
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D-Printed PLA Porous Scaffold
2.2. Functionalization of PLA Porous Scaffolds
2.2.1. Plasma Modification (PI Group)
2.2.2. Collagen Infiltration (PC Group)
2.2.3. Simulation of Body Fluid Mineralization (PS Group)
2.2.4. Simulate Body Fluid Mineralization Following Collagen Infiltration (PCS Group)
2.3. Characterization Analysis
2.3.1. Gel Permeation Chromatography (GPC)
2.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
2.3.5. X-ray Photoelectron Spectroscopy (XPS)
2.3.6. Porosity Testing
2.4. In Vitro Cytology Testing
2.4.1. Cell Culture
2.4.2. Methyl Thiazolyl Tetrazolium Assay (MTT)
2.4.3. Alkaline Phosphatase (ALP)
2.4.4. Alizarin Red Staining and Quantification
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Osteoblast Differentiation-Related Gene Testing
2.5. In Vivo Biological Testing
2.5.1. Modeling of Critical Size Bone Defects
2.5.2. X-ray Testing
2.5.3. Micro-CT Testing
2.5.4. Postoperative Mechanical Testing
2.5.5. Histological Testing
2.6. Statistical Analysis
3. Result
3.1. Characterization Analysis
3.2. In Vitro Cytology Testing
3.3. In Vivo Biological Testing
3.3.1. X-ray Testing
3.3.2. Micro-CT and Postoperative Mechanical Testing
3.3.3. Histological Testing
4. Discussion
5. Conclusions
6. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Zhou, Y.; Ji, X.; Cheng, S.; Bian, D.; Fan, L.; Zhou, L.; Ning, C.; Zhang, Y. Biomimetic Ti-6Al-4V alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration. Bioact. Mater. 2021, 6, 3437–3448. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Li, Z.; Liu, H.; Fu, C. Biomaterial scaffolds regulate macrophage activity to accelerate bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1140393. [Google Scholar] [CrossRef]
- Kanakaris, N.K.; Giannoudis, P.V. The health economics of the treatment of long-bone non-unions. Injury 2007, 38 (Suppl. S2), S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Linder, H.R.; Glass, A.A.; Day, D.E.; Sell, S.A. Manipulating Air-Gap Electrospinning to Create Aligned Polymer Nanofiber-Wrapped Glass Microfibers for Cortical Bone Tissue Engineering. Bioengineering 2020, 7, 165. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N. Natural Medicinal Compounds in Bone Tissue Engineering. Trends Biotechnol. 2020, 38, 404–417. [Google Scholar] [CrossRef]
- Frölich, A.M.; Spallek, J.; Brehmer, L.; Buhk, J.H.; Krause, D.; Fiehler, J.; Kemmling, A. 3D Printing of Intracranial Aneurysms Using Fused Deposition Modeling Offers Highly Accurate Replications. AJNR Am. J. Neuroradiol. 2016, 37, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lin, K.; He, S.; Wang, C.; Zhang, S.; Li, D.; Wang, J.; Cao, T.; Bi, L.; Pei, G. Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin. Int. J. Nanomed. 2018, 13, 505–523. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Wang, M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 2017, 9, 025031. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Ke, X.; Liu, A.; Sun, M.; He, Y.; Yang, X.; Fu, J.; Liu, Y.; Zhang, L.; Yang, G.; et al. Bone regeneration in 3D printing bioactive ceramic scaffolds with improved tissue/material interface pore architecture in thin-wall bone defect. Biofabrication 2017, 9, 025003. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Cha, M.; Jin, Y.Z.; Park, J.W.; Lee, K.M.; Han, S.H.; Choi, B.S.; Lee, J.H. Three-dimensional printed polylactic acid scaffold integrated with BMP-2 laden hydrogel for precise bone regeneration. Biomater. Res. 2021, 25, 35. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Nasr, M.; Awad, G.A.; Mansour, S.; Al Shamy, A.; Mortada, N.D. Hydrophilic versus hydrophobic porogens for engineering of poly(lactide-co-glycolide) microparticles containing risedronate sodium. Pharm. Dev. Technol. 2013, 18, 1078–1088. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Beaurain, A.; Payen, E.; Morent, R. Plasma surface modification of polylactic acid to promote interaction with fibroblasts. J. Mater. Sci. Mater. Med. 2013, 24, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Ibrahim, A.; Seifalian, A.M.; Butler, P.E.M.; Kalaskar, D.M.; Ferretti, P. Argon plasma modification promotes adipose derived stem cells osteogenic and chondrogenic differentiation on nanocomposite polyurethane scaffolds; implications for skeletal tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110085. [Google Scholar] [CrossRef]
- Abbasi, N.; Soudi, S.; Hayati-Roodbari, N.; Dodel, M.; Soleimani, M. The Effects of Plasma Treated Electrospun Nanofibrous Poly (ε-caprolactone) Scaffolds with Different Orientations on Mouse Embryonic Stem Cell Proliferation. Cell J. 2014, 16, 245–254. [Google Scholar] [PubMed]
- Yiğiter, Ö.; Yorukoglu, A.C.; Şentürk, N.; Dodurga, Y.; Demirkan, A.F. The effects of type I collagen on bone defects and gene expression changes for osteogenesis: In a rat model. J. Cell. Biochem. 2019, 120, 11525–11530. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, D.; Zhao, J.; Yang, Z.; Kang, P. In vivo evaluation of porous lithium-doped hydroxyapatite scaffolds for the treatment of bone defect. Biomed. Mater. Eng. 2018, 29, 699–721. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Biosilica incorporated 3D porous scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 274–291. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Li, K.; Zhu, S.; Lu, B.; Liu, J.; Tang, Y.; Wei, Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact. Mater. 2021, 6, 137–145. [Google Scholar] [CrossRef]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021, 6, 1491–1511. [Google Scholar] [CrossRef]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.R.; Kellesarian, T.V.; Bashir Ahmed, H.; Javed, F. Does incorporating collagen and chondroitin sulfate matrix in implant surfaces enhance osseointegration? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 241–251. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.J.; Aydin, H.M.; Hu, B.; Konduru, S.; Kuiper, J.H.; Yang, Y. A facile in vitro model to study rapid mineralization in bone tissues. Biomed. Eng. Online 2014, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Arora, A.; Katti, D.S. Surface hydrophilicity of PLGA fibers governs in vitro mineralization and osteogenic differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Cui, W.; Yang, F.; Min, C.; Shen, H.; Bei, J.; Wang, S. The effect of oxygen plasma pretreatment and incubation in modified simulated body fluids on the formation of bone-like apatite on poly(lactide-co-glycolide) (70/30). Biomaterials 2007, 28, 9–18. [Google Scholar] [CrossRef]
- Mohammadalipour, M.; Asadolahi, M.; Mohammadalipour, Z.; Behzad, T.; Karbasi, S. Plasma surface modification of electrospun polyhydroxybutyrate (PHB) nanofibers to investigate their performance in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123167. [Google Scholar] [CrossRef] [PubMed]
- Qhattal, H.S.; Hye, T.; Alali, A.; Liu, X. Hyaluronan polymer length, grafting density, and surface poly(ethylene glycol) coating influence in vivo circulation and tumor targeting of hyaluronan-grafted liposomes. ACS Nano 2014, 8, 5423–5440. [Google Scholar] [CrossRef]
- Wu, J.; Cao, L.; Liu, Y.; Zheng, A.; Jiao, D.; Zeng, D.; Wang, X.; Kaplan, D.L.; Jiang, X. Functionalization of Silk Fibroin Electrospun Scaffolds via BMSC Affinity Peptide Grafting through Oxidative Self-Polymerization of Dopamine for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 8878–8895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, W.; Tang, M.; Tu, M.; Zeng, R.; Liang, Z.; Zhou, C. Structure, morphology and cell affinity of poly(L-lactide) films surface-functionalized with chitosan nanofibers via a solid-liquid phase separation technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1546–1553. [Google Scholar] [CrossRef]
- Liu, Z.; Yamada, S.; Otsuka, Y.; Peñaflor Galindo, T.G.; Tagaya, M. Surface modification of hydroxyapatite nanoparticles for bone regeneration by controlling their surface hydration and protein adsorption states. Dalton Trans. 2022, 51, 9572–9583. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Gao, J.; Xing, F.; Zhang, Y.; Ma, Y.; Zhang, G. Comparative evaluation of the physicochemical properties of nano-hydroxyapatite/collagen and natural bone ceramic/collagen scaffolds and their osteogenesis-promoting effect on MC3T3-E1 cells. Regen. Biomater. 2019, 6, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Zhang, L.; Yin, X.; Guo, Y. In simulated body fluid performance of polymorphic apatite coatings synthesized by pulsed electrodeposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 100–107. [Google Scholar] [CrossRef]
- Hamai, R.; Shirosaki, Y.; Miyazaki, T. Apatite-forming ability of vinylphosphonic acid-based copolymer in simulated body fluid: Effects of phosphate group content. J. Mater. Sci. Mater. Med. 2016, 27, 152. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, L.; Li, L.; Li, Z.; Yang, J.; Wang, X. A TPMS-based method for modeling porous scaffolds for bionic bone tissue engineering. Sci. Rep. 2018, 8, 7395. [Google Scholar] [CrossRef]
- Shen, H.; Zhuang, Y.; Zhang, C.; Zhang, C.; Yuan, Y.; Yu, H.; Si, J.; Shen, G. Osteoclast-Driven Osteogenesis, Bone Remodeling and Biomaterial Resorption: A New Profile of BMP2-CPC-Induced Alveolar Bone Regeneration. Int. J. Mol. Sci. 2022, 23, 12204. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Gao, J.; Cui, X.; Nie, S.; Wu, X.; Zhang, L.; Tang, P.; Liu, J.; Li, M. Functionalized 3D-Printed PLA Biomimetic Scaffold for Repairing Critical-Size Bone Defects. Bioengineering 2023, 10, 1019. https://doi.org/10.3390/bioengineering10091019
Liu X, Gao J, Cui X, Nie S, Wu X, Zhang L, Tang P, Liu J, Li M. Functionalized 3D-Printed PLA Biomimetic Scaffold for Repairing Critical-Size Bone Defects. Bioengineering. 2023; 10(9):1019. https://doi.org/10.3390/bioengineering10091019
Chicago/Turabian StyleLiu, Xiao, Jianpeng Gao, Xiang Cui, Shaobo Nie, Xiaoyong Wu, Licheng Zhang, Peifu Tang, Jianheng Liu, and Ming Li. 2023. "Functionalized 3D-Printed PLA Biomimetic Scaffold for Repairing Critical-Size Bone Defects" Bioengineering 10, no. 9: 1019. https://doi.org/10.3390/bioengineering10091019
APA StyleLiu, X., Gao, J., Cui, X., Nie, S., Wu, X., Zhang, L., Tang, P., Liu, J., & Li, M. (2023). Functionalized 3D-Printed PLA Biomimetic Scaffold for Repairing Critical-Size Bone Defects. Bioengineering, 10(9), 1019. https://doi.org/10.3390/bioengineering10091019