Tunable Double-Network GelMA/Alginate Hydrogels for Platelet Lysate-Derived Protein Delivery
Abstract
:1. Introduction
2. Materials and Methods
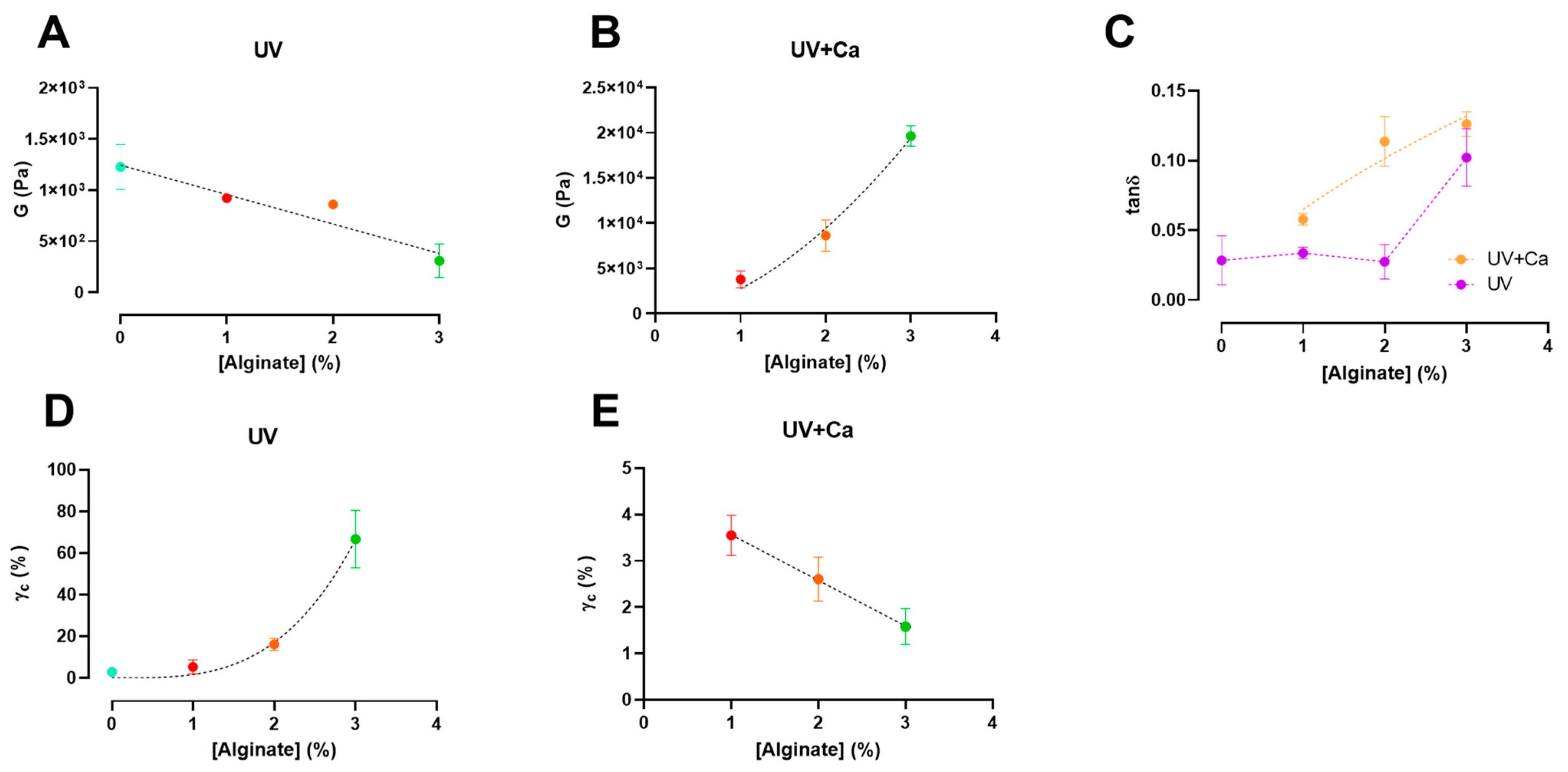
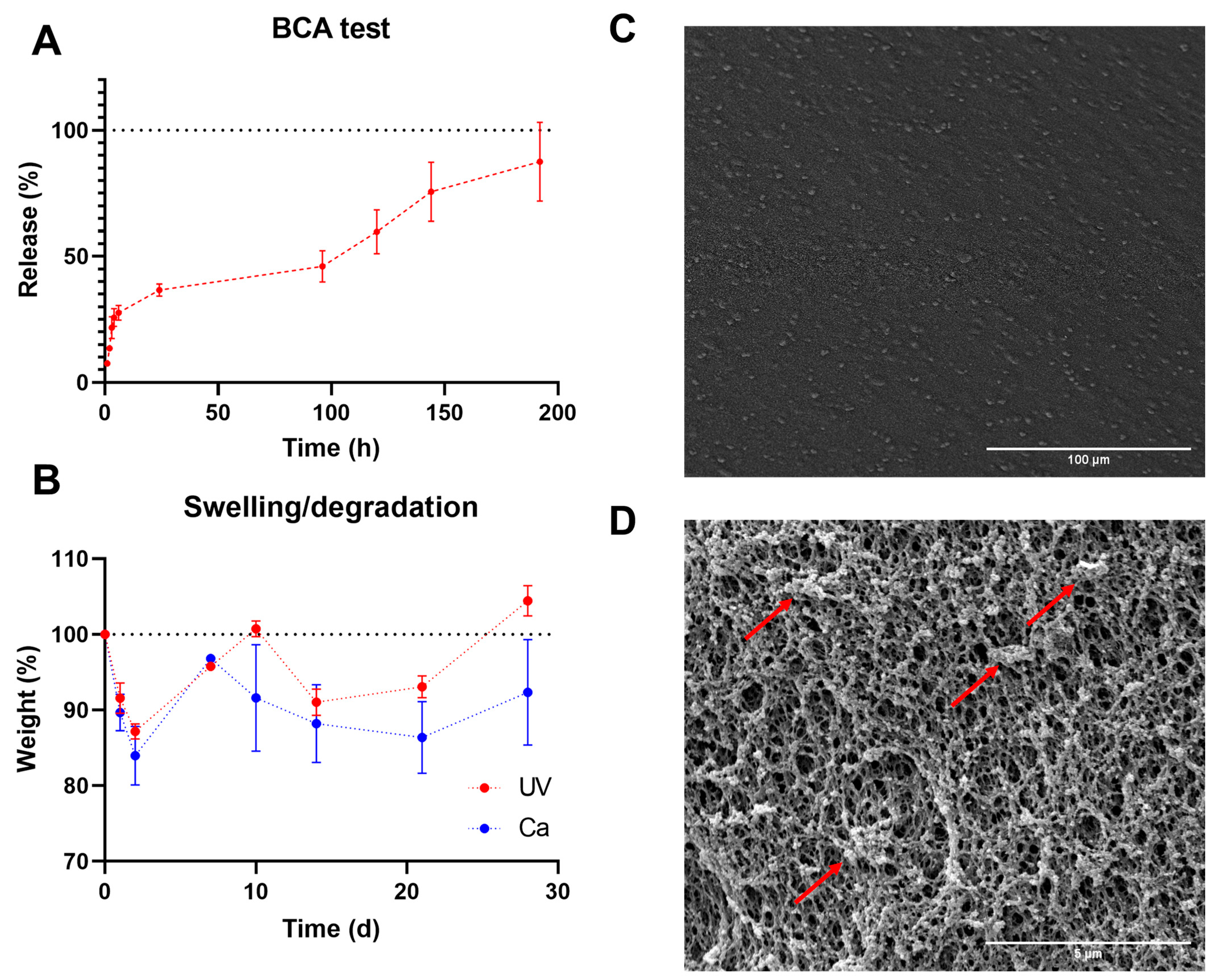
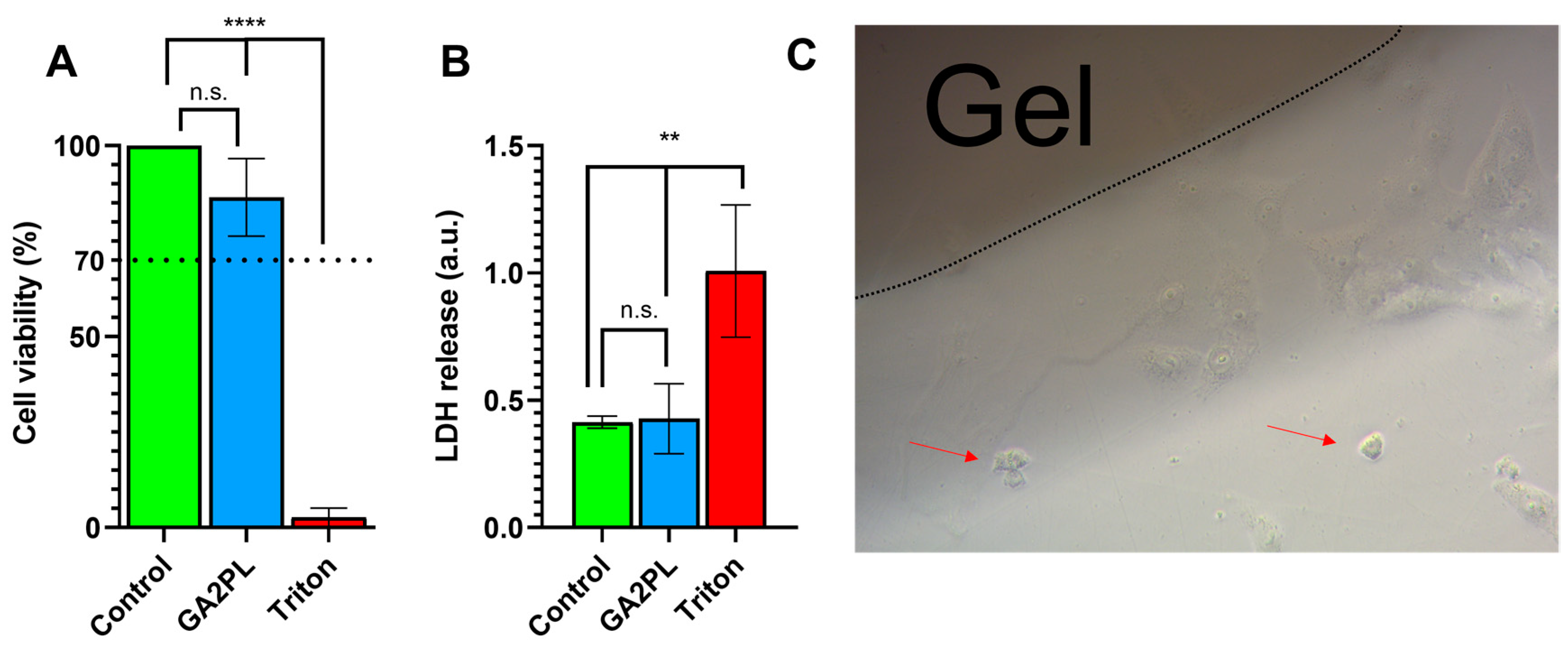
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco Da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, C.; Scognamiglio, F.; Sacco, P.; Lipari, S.; Romano, M.; Donati, I.; Marsich, E. Immediate stress dissipation in dual cross-link hydrogels controls osteogenic commitment of mesenchymal stem cells. Carbohydr. Polym. 2023, 302, 120369. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Tamura, A.; Arisaka, Y.; Seo, J.H.; Yui, N. Mechanically reinforced gelatin hydrogels by introducing slidable supramolecular cross-linkers. Polymers 2019, 11, 1787. [Google Scholar] [CrossRef]
- Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Zeiger, E.; Gollapudi, B.; Spencer, P. Genetic toxicity and carcinogenicity studies of glutaraldehyde—A review. Mutat. Res. Rev. Mutat. Res. 2005, 589, 136–151. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef]
- Tigner, T.J.; Rajput, S.; Gaharwar, A.K.; Alge, D.L. Comparison of Photo Cross Linkable Gelatin Derivatives and Initiators for Three-Dimensional Extrusion Bioprinting. Biomacromolecules 2020, 21, 454–463. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Aldana, A.A.; Malatto, L.; Ur Rehman, M.A.; Boccaccini, A.R.; Abraham, G.A. Fabrication of gelatin methacrylate (GelMA) scaffolds with nano-and micro-topographical and morphological features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Ichanti, H.; Sladic, S.; Kalies, S.; Haverich, A.; Andrée, B.; Hilfiker, A. Characterization of tissue engineered endothelial cell networks in composite collagen-agarose hydrogels. Gels 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Chen, Y.X.; Cain, B.; Soman, P. Gelatin methacrylate-alginate hydrogel with tunable viscoelastic properties. AIMS Mater. Sci. 2017, 4, 363–369. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Ling, J.Y.; Chen, W.C.; Lin, H.H.; Tang, M.J. Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: Reciprocal regulation of caveolin-1 and β1 integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef]
- Wei, W.; Liu, W.; Kang, H.; Zhang, X.; Yu, R.; Liu, J.; Huang, K.; Zhang, Y.; Xie, M.; Hu, Y.; et al. A One-Stone-Two-Birds Strategy for Osteochondral Regeneration Based on a 3D Printable Biomimetic Scaffold with Kartogenin Biochemical Stimuli Gradient. Adv. Healthc. Mater. 2023, 12, 2300108. [Google Scholar] [CrossRef] [PubMed]
- Juhl, O.; Zhao, N.; Merife, A.B.; Cohen, D.; Friedman, M.; Zhang, Y.; Schwartz, Z.; Wang, Y.; Donahue, H. Aptamer-Functionalized Fibrin Hydrogel Improves Vascular Endothelial Growth Factor Release Kinetics and Enhances Angiogenesis and Osteogenesis in Critically Sized Cranial Defects. ACS Biomater. Sci. Eng. 2019, 5, 6152–6160. [Google Scholar] [CrossRef]
- Shao, N.; Guo, J.; Guan, Y.; Zhang, H.; Li, X.; Chen, X.; Zhou, D.; Huang, Y. Development of Organic/Inorganic Compatible and Sustainably Bioactive Composites for Effective Bone Regeneration. Biomacromolecules 2018, 19, 3637–3648. [Google Scholar] [CrossRef]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.F.; Santos, S.C.; Custódio, C.A.; Mano, J.F. Human Platelet Lysates-Based Hydrogels: A Novel Personalized 3D Platform for Spheroid Invasion Assessment. Adv. Sci. 2020, 7, 1902398. [Google Scholar] [CrossRef]
- Fortunato, T.M.; Beltrami, C.; Emanueli, C.; De Bank, P.A.; Pula, G. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: Tissue engineering implications. Sci. Rep. 2016, 6, 25326. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Kirsch, M.; Birnstein, L.; Pepelanova, I.; Handke, W.; Rach, J.; Seltsam, A.; Scheper, T. Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties. Bioengineering 2019, 6, 76. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhang, Y. Hydrogels and hydrogel composites for 3D and 4D printing applications. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes, Applications, and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 427–465. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef]
- Lima, A.C.; Mano, J.F.; Concheiro, A.; Alvarez-Lorenzo, C. Fast and Mild Strategy, Using Superhydrophobic Surfaces, to Produce Collagen/Platelet Lysate Gel Beads for Skin Regeneration. Stem Cell Rev. Rep. 2014, 11, 161–179. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. 3rd ed. ISO: Geneva, Switzerland, 2009.
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci. Rep. 2021, 11, 23276. [Google Scholar] [CrossRef]
- Martyniak, K.; Lokshina, A.; Cruz, M.A.; Karimzadeh, M.; Kemp, R.; Kean, T.J. Biomaterial composition and stiffness as decisive properties of 3D bioprinted constructs for type II collagen stimulation. Acta Biomater. 2022, 152, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Brunel, L.G.; Cai, B.; Kilian, D.; Roth, J.G.; Seymour, A.J.; Heilshorn, S.C. Gelation of Uniform Interfacial Diffusant in Embedded 3D Printing. Adv. Funct. Mater. 2023, 2307435. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Q.; Yuan, Z.; Guo, Q.; Liao, X.; Han, F.; Feng, T.; Liu, G.; Zhao, R.; Zhu, Z.; et al. Engineering the viscoelasticity of gelatin methacryloyl (GelMA) hydrogels via small “dynamic bridges” to regulate BMSC behaviors for osteochondral regeneration. Bioact. Mater. 2023, 25, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Marfoglia, A.; Marsich, E.; Donati, I.; Sacco, P. Strain Hardening in Highly Acetylated Chitosan Gels. Biomacromolecules 2021, 22, 2902–2909. [Google Scholar] [CrossRef]
- Janzen, D.; Bakirci, E.; Wieland, A.; Martin, C.; Dalton, P.D.; Villmann, C. Cortical Neurons form a Functional Neuronal Network in a 3D Printed Reinforced Matrix. Adv. Healthc. Mater. 2020, 9, 1901630. [Google Scholar] [CrossRef]
- Borries, M.; Barooji, Y.F.; Yennek, S.; Grapin-Botton, A.; Berg-Sørensen, K.; Oddershede, L.B. Quantification of Visco-Elastic Properties of a Matrigel for Organoid Development as a Function of Polymer Concentration. Front. Phys. 2020, 8, 579168. [Google Scholar] [CrossRef]
- Moreno-Manzano, V.; Zaytseva-Zotova, D.; López-Mocholí, E.; Briz-Redón, Á.; Strand, B.L.; Serrano-Aroca, Á. Injectable gel form of a decellularized bladder induces adipose-derived stem cell differentiation into smooth muscle cells in vitro. Int. J. Mol. Sci. 2020, 21, 8608. [Google Scholar] [CrossRef]
- Sacco, P.; Baj, G.; Asaro, F.; Marsich, E.; Donati, I. Substrate Dissipation Energy Regulates Cell Adhesion and Spreading. Adv. Funct. Mater. 2020, 30, 2001977. [Google Scholar] [CrossRef]
- Turco, G.; Donati, I.; Grassi, M.; Marchioli, G.; Lapasin, R.; Paoletti, S. Mechanical spectroscopy and relaxometry on alginate hydrogels: A comparative analysis for structural characterization and network mesh size determination. Biomacromolecules 2011, 12, 1272–1282. [Google Scholar] [CrossRef]
- Soskey, P.R.; Henning Winter, H. Large Step Shear Strain Experiments with Parallel-Disk Rotational Rheometers. Cit. J. Rheol. 1984, 28, 625. [Google Scholar] [CrossRef]
- Sacco, P.; Piazza, F.; Pizzolitto, C.; Baj, G.; Brun, F.; Marsich, E.; Donati, I. Regulation of Substrate Dissipation via Tunable Linear Elasticity Controls Cell Activity. Adv. Funct. Mater. 2022, 32, 2200309. [Google Scholar] [CrossRef]
- Van Dijk-Wolthuis, W.N.E.; Hoogeboom, J.A.M.; Van Steenbergen, M.J.; Tsang, S.K.Y.; Hennink, W.E. Degradation and release behavior of dextran-based hydrogels. Macromolecules 1997, 30, 4639–4645. [Google Scholar] [CrossRef]
- Van De Wetering, P.; Metters, A.T.; Schoenmakers, R.G.; Hubbell, J.A. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J. Control. Release 2005, 102, 619–627. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marfoglia, A.; Tibourtine, F.; Pilloux, L.; Cazalbou, S. Tunable Double-Network GelMA/Alginate Hydrogels for Platelet Lysate-Derived Protein Delivery. Bioengineering 2023, 10, 1044. https://doi.org/10.3390/bioengineering10091044
Marfoglia A, Tibourtine F, Pilloux L, Cazalbou S. Tunable Double-Network GelMA/Alginate Hydrogels for Platelet Lysate-Derived Protein Delivery. Bioengineering. 2023; 10(9):1044. https://doi.org/10.3390/bioengineering10091044
Chicago/Turabian StyleMarfoglia, Andrea, Fahd Tibourtine, Ludovic Pilloux, and Sophie Cazalbou. 2023. "Tunable Double-Network GelMA/Alginate Hydrogels for Platelet Lysate-Derived Protein Delivery" Bioengineering 10, no. 9: 1044. https://doi.org/10.3390/bioengineering10091044
APA StyleMarfoglia, A., Tibourtine, F., Pilloux, L., & Cazalbou, S. (2023). Tunable Double-Network GelMA/Alginate Hydrogels for Platelet Lysate-Derived Protein Delivery. Bioengineering, 10(9), 1044. https://doi.org/10.3390/bioengineering10091044






