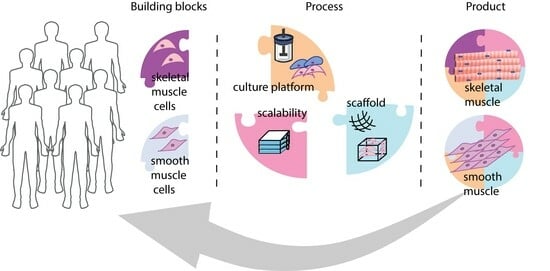
Bioprocessing Considerations towards the Manufacturing of Therapeutic Skeletal and Smooth Muscle Cells
Abstract
:1. Introduction
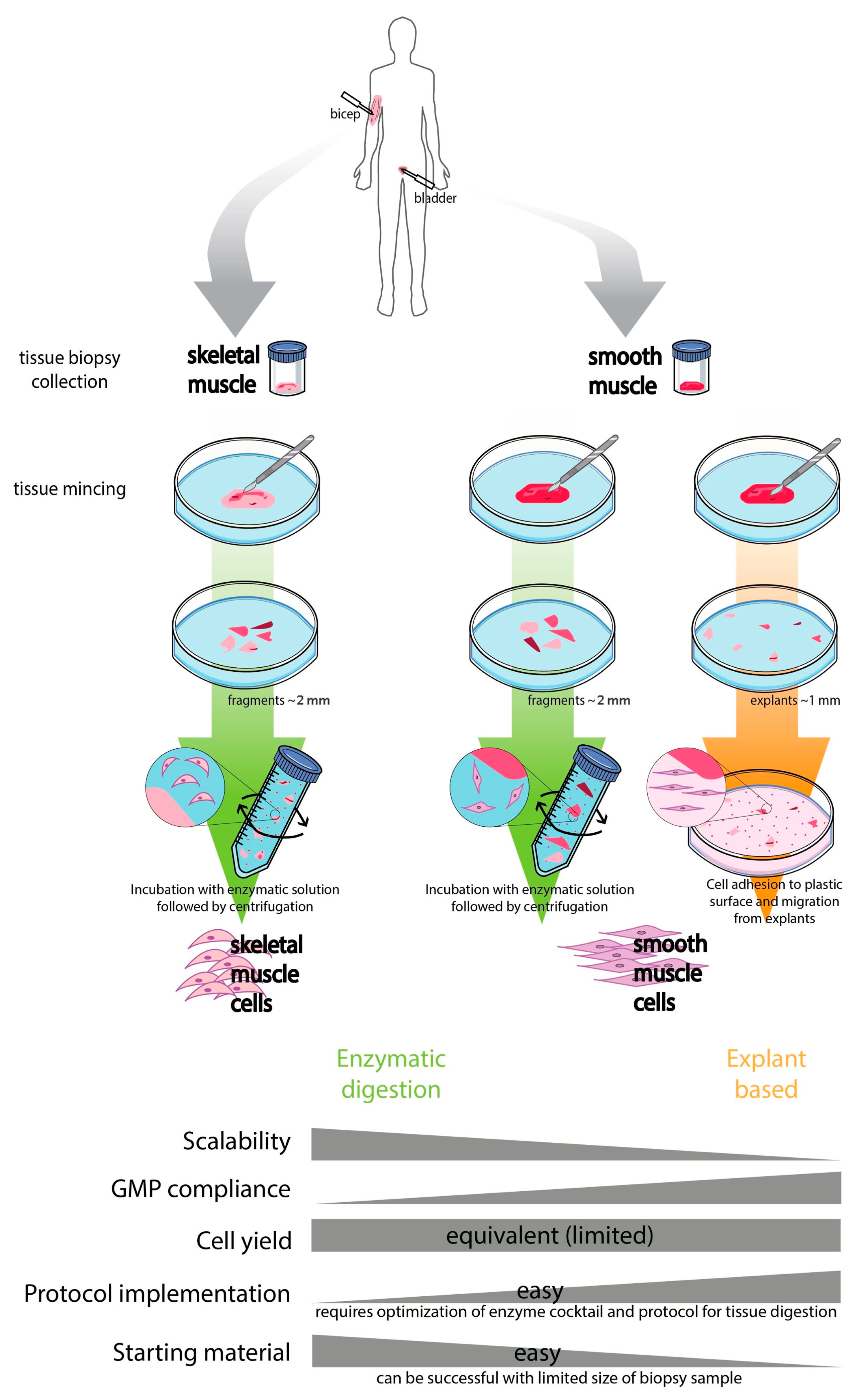
2. Isolation and Ex Vivo Expansion of Smooth and Skeletal Muscle Cells
2.1. Smooth Muscle Cells (SMCs)
2.2. Skeletal Muscle Cells (SkMCs)
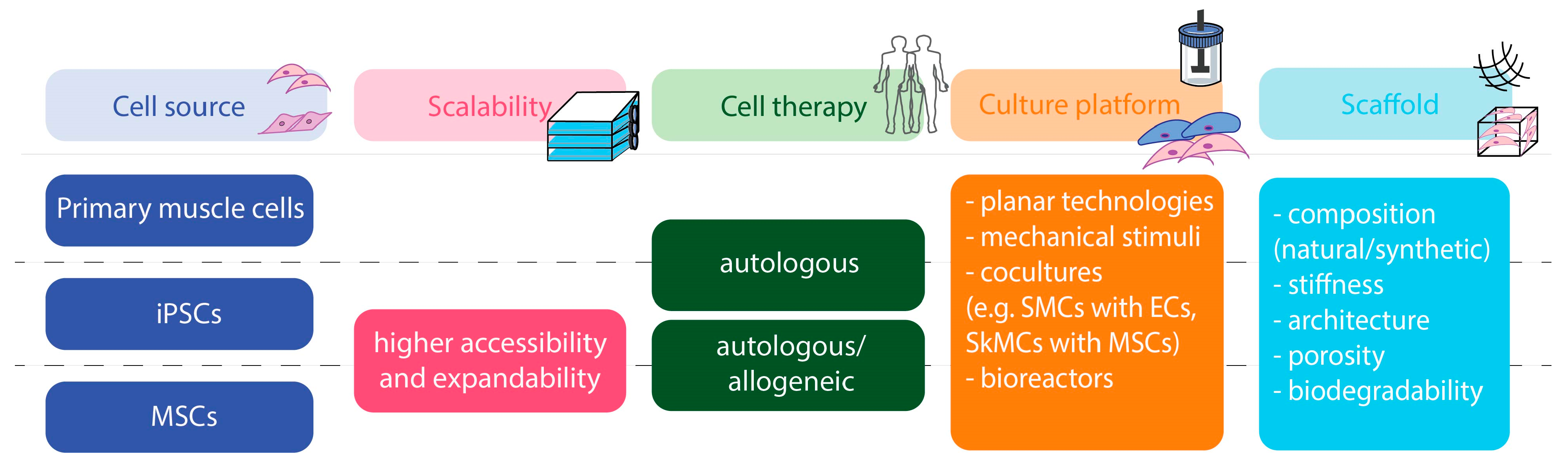
3. Strategies for Advancing SMC and SkMC Manufacturing
3.1. SMCs
| Cell Source | Culture Setup (Scaffold; Cell Sorting; Growth Factors) | Main Outcome(s) | Reference | |
|---|---|---|---|---|
| iPSCs | Alginate | High VSMC purity (>80%) and yield (~5.0 × 108 cells/mL) | [60] | |
| Embryoid-based differentiation Normoxia vs. hypoxia (5%O2) | Higher VSMC viability under hypoxia | [61] | ||
| Human platelet lysate and human serum vs. FBS culture medium supplementation | Mechanical strength comparable to hiPSC-derived VSMCs under FBS-supplemented medium | [58] | ||
| Cell sorting for CD34+ cells PDGF-BB for SMCs differentiation | In vivo injection of SMCs into mouse models of urinary incontinence: tissue remodeling with higher detection of elastin | [65] | ||
| MSCs | ASCs | Low FBS supplementation | Identification of SMCs markers | [72] |
| PLGA microcarriers | SMC-like phenotype, with cell attachment and proliferation on the microcarriers | [69,70] | ||
| Collagen microspheres PDGF-AB and TGF-β1 Mechanical stretch | Identification of SMCs markers | [71] | ||
| ASCs spheroids Bioprinting on gelatin–alginate TGF-β for SMCs induction | Assessment of viability, proliferation and SMC differentiation post-bioprinting | [81] | ||
| Primary SMCs | Polyurethane porous sheet-type scaffold Cyclic mechanical strain | Observation of SMCs contractile capacity | [73] | |
| Poly (lactide-co-caprolactone) electrospun scaffold and collagen coating Pulse bioreactor | Identification of α-SMA, and SMCs presented higher proliferation rates under pulsatile flow than when compared to static culture | [76] | ||
| Electrospun gelatin fiber scaffold in bioreactor | Dynamic setup led to higher cell proliferation than under static conditions | [77] | ||
| Decellularized porcine artery in bioreactor | High VSMC viability, variable levels of cell seeding in the decellularized matrices | [78,80] | ||
| Nanofibrous gelatin–PLLA scaffold | In vivo model of urethral reconstruction exhibited SMC remodeling upon transplantation | [82] | ||
| Bilayer silk scaffold | SMC alignment and proliferation in the scaffold | [83] | ||
| Electrospun PCL and GelMA | High cell viability and proliferation | [84] | ||
| Bioprinting on GelMA and hyaluronic acid | Bilayer of outer SMCs and inner ECs, with 20 mm length and 4 mm lumen diameter | [85] | ||
| Cardiovascular progenitor cells | Decellularized ovine arteries in a pulsatile flow bioreactor | VSMCs by detection of calponin and MYH11 | [79] | |
3.2. SkMCs
| Cell Source | Culture Setup (Scaffold; Cell Sorting; Growth Factors) | Main Outcome(s) | Reference | |
|---|---|---|---|---|
| iPSCs | GSK3 β inhibitor, FGF-2, ascorbic acid, BMP-4, IGF-1, insulin and PDGF [112] | Generation of Pax7+ myogenic progenitors at varying efficiencies | [112,114] | |
| GSK3 β inhibitor, FGF-2 and ITS | 2 × 1016 myogenic progenitors upon 43 days of culture | [120] | ||
| FACS based on different surface markers: ERBB3+NGFR+; CD24−CD10+; CD57−ACHR+cMET+; CD57− NCAM1+ | Enhanced in vitro myotube formation and/or enhanced muscle engraftment in vivo | [115,116,117,118] | ||
| PCL scaffold with decellularized skeletal ECM motifs | In vivo cell integration in murine model of VML | [121] | ||
| MSCs | Adipose tissue | GSK3 β inhibitor and FGF-2 supplementation Cyclic strain | Identification of myogenic transcription factors and multinucleated myofibers | [101,102] |
| BM | FGF-2 and PDGF-AA | Observed differentiation towards myoblasts | [91] | |
| HGF and IGF-1 | HGF and IGF-1 may not be sufficient for myogenic differentiation | [97] | ||
| Umbilical cord | Horse serum-supplemented medium or HGF, IGF-1 and FGF-2 | Enhanced myogenic differentiation potential of MSCs from umbilical cord tissue compared to MSCs from umbilical cord blood | [96] | |
| Primary muscle | Human serum vs. FBS | Enhanced detection of CD56 and myosin proteins under high serum or platelet-rich plasma | [100] | |
| Primary SkMCs | FACS depletion for CD45, CD31, CD11b, Sca1 and enrichment for CD34 and/or integrinα7 Fibrin vs. Matrigel | Higher cell expansion (threefold) on fibrin gel than Matrigel | [122] | |
| Electrospun nanofiber of PMMA | Laminin-coated PMMA facilitated myoblast proliferation in comparison to collagen-coated PMMA | [123] | ||
| Collagen and Matrigel replating steps | Short-time protocol with cell isolation from low amounts of skeletal muscle (0.02 g) | [124] | ||
| Fibrin-based hydrogel | Electrical stimulation led to improved contractility and mature phenotype | [125] | ||
4. Clinical Studies with Expanded SMCs and SkMCs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.; Yi, F.; Pan, H.; Duan, S.; Ding, Z.; Yuan, G.; Qu, J.; Zhang, H. Progress and prospects in stem cell therapy. Nat. Publ. Gr. 2013, 34, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [PubMed]
- Musar, A. Muscle Homeostasis and Regeneration: From Molecular Mechanisms to Therapeutic Opportunities. Cells 2020, 9, 2033. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef]
- Edenfield, A.; Patnam, R.; Swift, S. A narrative review of the epidemiology, diagnosis, and treatment of latent stress urinary incontinence. Neurourol. Urodyn. 2019, 38, S7–S11. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Xia, M.C.; Cui, F.; Chen, J.W.; Bian, X.D.; Xie, H.J.; Shuang, W.B. Epidemiological survey of adult female stress urinary incontinence. BMC Women’s Health 2021, 21, 172. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Chung, J.D.; Lynch, G.S.; Ryall, J.G. The Microenvironment Is a Critical Regulator of Muscle Stem Cell Activation and Proliferation. Front. Cell Dev. Biol. 2019, 7, 254. [Google Scholar] [CrossRef]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle 2019, 49, 501–516. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 15, 529–539. [Google Scholar] [CrossRef]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell. Mol. Life Sci. 2014, 71, 2271. [Google Scholar] [CrossRef]
- Donadon, M.; Santoro, M.M. The Origin and Mechanisms of Smooth Muscle Cell Development in Vertebrates, The Company of Biologists Ltd. Development. 2021, 148, dev197384. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Maruhashi, T.; Kajikawa, M.; Oda, N.; Kishimoto, S.; Matsui, S.; Hashimoto, H.; Aibara, Y.; Mohamad, F.; Hidaka, T.; et al. Chronic kidney disease is associated with vascular smooth muscle dysfunction but not with endothelial dysfunction. Int. J. Cardiol. 2018, 254, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.L.; Kim, S.; Helmke, B.P.; Yu, W.W.; Du, K.L.; Lu, M.M.; Strobeck, M.; Yu, Q.-C.; Parmacek, M.S. Analysis of SM22α-Deficient Mice Reveals Unanticipated Insights into Smooth Muscle Cell Differentiation and Function. Mol. Cell. Biol. 2001, 21, 1336. [Google Scholar] [CrossRef]
- Scharner, J.; Zammit, P.S. The muscle satellite cell at 50: The formative years. Skelet. Muscle 2011, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. Biomed Res. Int. 2018, 16, 1984879. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.N.; Rossi, F.M.V. Towards stem cell therapies for skeletal muscle repair. NPJ Regen. Med. 2020, 5, 10. [Google Scholar] [CrossRef]
- Salemi, S.; Prange, J.A.; Baumgartner, V.; Mohr-Haralampieva, D.; Eberli, D. Adult stem cell sources for skeletal and smooth muscle tissue engineering. Stem Cell Res. Ther. 2022, 13, 156. [Google Scholar] [CrossRef]
- Handschin, C.; Mortezavi, A.; Plock, J.; Eberli, D. External physical and biochemical stimulation to enhance skeletal muscle bioengineering. Adv. Drug Deliv. Rev. 2015, 82–83, 168–175. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z.; Day, K.; Vine, A.; Shefer, G. Defining the transcriptional signature of skeletal muscle stem cells. J. Anim. Sci. 2008, 86, E207–E216. [Google Scholar] [CrossRef]
- Oprescu, S.N.; Yue, F.; Qiu, J.; Brito, L.F.; Kuang, S. Temporal Dynamics and Heterogeneity of Cell Populations during Skeletal Muscle Regeneration. iScience 2020, 23, 100993. [Google Scholar] [CrossRef]
- Sacco, A.; Doyonnas, R.; Kraft, P.; Vitorovic, S.; Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008, 456, 502–506. [Google Scholar] [CrossRef]
- Marg, A.; Escobar, H.; Karaiskos, N.; Grunwald, S.A.; Metzler, E.; Kieshauer, J.; Sauer, S.; Pasemann, D.; Malfatti, E.; Mompoint, D.; et al. Human muscle-derived CLEC14A-positive cells regenerate muscle independent of PAX7. Nat. Commun. 2019, 10, 5776. [Google Scholar] [CrossRef] [PubMed]
- Lecourt, S.; Marolleau, J.P.; Fromigué, O.; Vauchez, K.; Andriamanalijaona, R.; Ternaux, B.; Lacassagne, M.N.; Robert, I.; Boumédiene, K.; Chéreau, F.; et al. Characterization of distinct mesenchymal-like cell populations from human skeletal muscle in situ and in vitro. Exp. Cell Res. 2010, 316, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011, 25, 3344. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Manabe, I.; Oishi, Y. Mechanisms of cooperative cell-cell interactions in skeletal muscle regeneration. Inflamm. Regen. 2022, 42, 48. [Google Scholar] [CrossRef]
- Huard, J.; Cao, B.; Qu-Petersen, Z. Muscle-derived stem cells: Potential for muscle regeneration. Birth Defects Res. Part C Embryo Today 2003, 69, 230–237. [Google Scholar] [CrossRef]
- Matthias, N.; Hunt, S.D.; Wu, J.; Lo, J.; Smith Callahan, L.A.; Li, Y.; Huard, J.; Darabi, R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res. 2018, 27, 65–73. [Google Scholar] [CrossRef]
- Liu, M.; Gomez, D. Smooth Muscle Cell phenotypic diversity: At the crossroads of lineage tracing and single-cell transcriptomics. Arterioscler. Thromb. Vasc. Biol. 2020, 39, 1715–1723. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Balcerczyk, D.; Jundzill, A.; Gagat, M.; Czapiewska, M.; Kloskowski, T.; Nowacki, M.; Gastecka, A.M.; Bodnar, M.; Grzanka, A.; et al. Isolation, expansion and characterization of porcine urinary bladder smooth muscle cells for tissue engineering. Biol. Proced. Online 2016, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.; Shekar, K.C.; Staggs, R.; Win, Z.; Steucke, K.; Lin, Y.-W.; Wei, L.-N.; Alford, P.; Hall, J.L. Guidelines for the Isolation and Characterization of Murine Vascular Smooth Muscle Cells. A Report from the International Society of Cardiovascular Translational Research. J. Cardiovasc. Transl. Res. 2016, 8, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; Leach, R.; Herbert, J.; Benson, M. Isolation of vascular smooth msucle cells from a single murine aorta. Methods Cell Sci. 2001, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, M.; Yan, Z.; Zhang, S.; Liu, H. Isolation and culture of vascular smooth muscle cells from rat placenta. J. Cell. Physiol. 2019, 234, 7675–7682. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Gratopp, A.; Akanbi, S.; Rheinlaender, C.; Sallmon, H.; Barikbin, P.; Koehne, P.S. Isolation and Culture of Fibroblasts, Vascular Smooth Muscle, and Endothelial Cells From the Fetal Rat Ductus Arteriosus. Pediatr. Res. 2011, 70, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Metz, R.P.; Patterson, J.L.; Wilson, E. Vascular Smooth Muscle Cells: Isolation, Culture, and Characterization. Cardiovasc. Dev. Methods Protoc. 2012, 843, 169–176. [Google Scholar] [CrossRef]
- Day, K.; Shefer, G.; Shearer, A.; Yablonka-Reuveni, Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 2010, 340, 330–343. [Google Scholar] [CrossRef]
- Hindi, L.; McMillan, J.; Afroze, D.; Hindi, S.; Kumar, A. Isolation, Culturing, and Differentiation of Primary Myoblasts from Skeletal Muscle of Adult Mice. Bio-Protocol 2017, 7, e2248. [Google Scholar] [CrossRef]
- Shahini, A.; Vydiam, K.; Choudhury, D.; Rajabian, N.; Nguyen, T.; Lei, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Ceusters, J.; Lejeune, J.P.; Sandersen, C.; Niesten, A.; Lagneaux, L.; Serteyn, D. From skeletal muscle to stem cells: An innovative and minimally-invasive process for multiple species. Sci. Rep. 2017, 7, 696. [Google Scholar] [CrossRef]
- Smith, L.R.; Meyer, G.A. Skeletal muscle explants: Ex-vivo models to study cellular behavior in a complex tissue environment. Connect. Tissue Res. 2020, 61, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Calve, S.; Odelberg, S.J.; Simon, H.G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 2010, 344, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, K.P.; Snyman, C.; Myburgh, K.H.; Niesler, C.U. Simultaneous isolation of enriched myoblasts and fibroblasts for migration analysis within a novel co-culture assay. Biotechniques 2015, 58, 25–32. [Google Scholar] [CrossRef]
- Wang, Z.; Cheung, D.; Zhou, Y.; Han, C.; Fennelly, C.; Criswell, T.; Soker, S. An in vitro culture system that supports robust expansion and maintenance of in vivo engraftment capabilities for myogenic progenitor cells from adult mice. Biores. Open Access 2014, 3, 79–87. [Google Scholar] [CrossRef]
- Motohashi, N.; Asakura, Y.; Asakura, A. Isolation, Culture, and Transplantation of Muscle Satellite Cells. J. Vis. Exp. 2014, 86, 50846. [Google Scholar] [CrossRef]
- Pasut, A.; Jones, A.E.; Rudnicki, M.A. Isolation and culture of individual myofibers and their satellite cells from adult skeletal muscle. J. Vis. Exp. 2013, 73, e50074. [Google Scholar] [CrossRef]
- Liu, L.; Cheung, T.H.; Charville, G.W.; Rando, T.A. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat. Protoc. 2015, 10, 1612–1624. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Chen, C.; Stoelzel, K.; Kaufmann, A.M.; Albers, A.E. Human skeletal muscle-derived stem cells retain stem cell properties after expansion in myosphere culture. Exp. Cell Res. 2011, 317, 1016–1027. [Google Scholar] [CrossRef]
- Garcia, S.M.; Tamaki, S.; Lee, S.; Wong, A.; Jose, A.; Dreux, J.; Kouklis, G.; Sbitany, H.; Seth, R.; Knott, P.D.; et al. High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Rep. 2018, 10, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.W.; Capel, A.J.; Rimington, R.P.; Wheeler, P.; Leonard, A.N.; Bishop, N.C.; Davies, O.G.; Lewis, M.P. Bioengineered human skeletal muscle capable of functional regeneration. BMC Biol. 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Du, X.; Li, W.; Du, G.; Cho, H.; Yu, M.; Fang, Q.; Lee, L.P.; Fang, J. Droplet Array-Based 3D Coculture System for High-Throughput Tumor Angiogenesis Assay. Anal. Chem. 2018, 90, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Lean, G.; Halloran, M.; Mariscal, O.; Jamet, S.; Lumb, J.-P.; Crist, C. Ex vivo expansion of skeletal muscle stem cells with a novel small compound inhibitor of eIF2α dephosphorylation. Regen Med. Front. 2019, 1, e190003. [Google Scholar] [CrossRef]
- Pantelic, M.N.; Larkin, L.M. Stem Cells for Skeletal Muscle Tissue Engineering. Tissue Eng. Part B Rev. 2018, 24, 373–391. [Google Scholar] [CrossRef]
- Jonah, D.; Lee, B.C.S.; Lisa, M.; Larkin, K.W.V. Isolation and Purification of Satellite Cells for Skeletal Muscle Tissue Engineering. J. Regen. Med. 2015, 3, 117. [Google Scholar] [CrossRef]
- Luo, J.; Lin, Y.; Shi, X.; Li, G.; Kural, M.H.; Anderson, C.W.; Ellis, M.W.; Riaz, M.; Tellides, G.; Niklason, L.E.; et al. Xenogeneic-free Generation of Vascular Smooth Muscle Cells from Human Induced Pluripotent Stem Cells for Vascular Tissue Engineering. Acta Biomater. 2022, 119, 155–168. [Google Scholar] [CrossRef]
- Hockemeyer, D.; Jaenisch, R.; Biology, C. Induced pluripotent stem cells meet genome editing. Cell Stem Cell 2017, 18, 573–586. [Google Scholar] [CrossRef]
- Lin, H.; Qiu, X.; Du, Q.; Li, Q.; Wang, O.; Akert, L.; Wang, Z.; Anderson, D.; Liu, K.; Gu, L.; et al. Engineered Microenvironment for Manufacturing Human Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells Haishuang. Stem Cell Rep. 2019, 12, 84–97. [Google Scholar] [CrossRef]
- Fang, L.; Mei, J.; Yao, B.; Liu, J.; Liu, P.; Wang, X.; Zhou, J.; Lin, Z. Hypoxia facilitates proliferation of smooth muscle cells derived from pluripotent stem cells for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2022, 16, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Astori, G.; Amati, E.; Bambi, F.; Bernardi, M.; Chieregato, K.; Schäfer, R.; Sella, S.; Rodeghiero, F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem / stromal cells: Present and future. Stem Cell Res. Ther. 2016, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Henschler, R.; Gabriel, C.; Schallmoser, K.; Burnouf, T.; Koh, M.B.C. Human platelet lysate current standards and future developments. Transfusion 2019, 59, 1407–1413. [Google Scholar] [CrossRef]
- Pérez-simon, J.A.; López-villar, O.; Andreu, E.J.; Rifón, J.; Muntion, S.; Campelo, M.D.; Sánchez-guijo, F.M.; Martinez, C.; Valcarcel, D.; Cañizo, C. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft- versus -host disease: Results of a phase I/II clinical trial. Haematologica 2011, 96, 1072–1076. [Google Scholar] [CrossRef]
- Li, Y.; Green, M.; Wen, Y.; Wei, Y.; Wani, P.; Wang, Z.; Pera, R.R.; Chen, B. Efficacy and Safety of Immuno-Magnetically Sorted Smooth Muscle Progenitor Cells Derived from Human-Induced Pluripotent Stem Cells for Restoring Urethral Sphincter Function. Stem Cells Transl. Med. 2017, 6, 1158–1167. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Enhejirigala, B.; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 640388. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.; Basu, J.; Rivera, E.A.; Spencer, T.; Jain, D.; Payne, R. Fabrication of a multi-layer three-dimensional scaffold with controlled porous micro-architecture for application in small intestine tissue engineering. Cell Adhes. Migr. 2013, 7, 267–274. [Google Scholar] [CrossRef]
- Schop, D.; Janssen, F.W.; Borgart, E.; Bruijn, J.D. De Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: Growth and metabolism. J. Tissue Eng. Regen. Med. 2008, 2, 126–135. [Google Scholar] [CrossRef]
- Parmar, N.; Ahmadi, R.; Day, R.M. A Novel Method for Differentiation of Human Mesenchymal Stem Cells into Smooth Muscle-Like Cells on Clinically Deliverable Thermally Induced. Tissue Eng. 2015, 21, 404–412. [Google Scholar] [CrossRef]
- Ahmadi, R.; Mordan, N.; Forbes, A.; Day, R.M. Acta Biomaterialia Enhanced attachment, growth and migration of smooth muscle cells on microcarriers produced using thermally induced phase separation. Acta Biomater. 2011, 7, 1542–1549. [Google Scholar] [CrossRef]
- Walters, B.; Turner, P.A.; Rolauffs, B.; Hart, M.L.; Stegemann, J.P. Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells. Cells 2021, 10, 3123. [Google Scholar] [CrossRef]
- Salem, S.A.; Hwie, A.N.M.; Saim, A.; Kong, C.H.C.; Sagap, I.; Singh, R.; Yusof, M.R.; Zainuddin, Z.M.; Idrus, R.H. Human Adipose Tissue Derived Stem Cells as a Source of Smooth Muscle Cells in the Regeneration of Muscular Layer of Urinary Bladder Wall. MJMS 2013, 20, 80. [Google Scholar] [PubMed]
- Cha, J.M.; Park, S.N.; Park, G.O.; Kim, J.K.; Suh, H. Construction of functional soft tissues from premodulated smooth muscle cells using a bioreactor system. Artif. Organs 2006, 30, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Meza, D.; Musmacker, B.; Steadman, E.; Stransky, T.; Rubenstein, D.A.; Yin, W. Endothelial Cell Biomechanical Responses are Dependent on Both Fluid Shear Stress and Tensile Strain. Cell. Mol. Bioeng. 2019, 12, 311–325. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.F.; Van Blitterswijk, C.A.; Dhert, W.J.; Malda, J. Supply of Nutrients to Cells in Engineered Tissues Supply of Nutrients to Cells in Engineered. Biotechnol. Genet. Eng. Rev. 2013, 8725, 2046–5556. [Google Scholar] [CrossRef]
- Mun, C.H.; Jung, Y.; Kim, S.; Kim, H.C.; Kim, S.H. Effects of Pulsatile Bioreactor Culture on Vascular Smooth Muscle Cells Seeded on Electrospun Poly (lactide-co-ε-caprolactone) Scaffold. Artif. Organs 2013, 8, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, Y.; Lekakou, C.; Labeed, F.; Tomlins, P. Smooth muscle tissue engineering in crosslinked electrospun gelatin scaffolds. Soc. Biomater. 2015, 8, 313–321. [Google Scholar] [CrossRef]
- McFetridge, P.S.; Daniel, J.W.; Bodamyali, T.; Horrocks, M.; Chaudhuri, J.B. Preparation of porcine carotid arteries for vascular tissue engineering applications. J. Biomed. Mater. Res.—Part A 2004, 70, 224–234. [Google Scholar] [CrossRef]
- Knox, C.; Garcia, K.; Tran, J.; Wilson, S.M.; Blood, A.B.; Kearns-Jonker, M.; Martens, T.P. A Biomimetic Approach Utilizing Pulsatile Perfusion Generates Contractile Vascular Grafts. Tissue Eng. Part A 2023, 29, 358–371. [Google Scholar] [CrossRef]
- Yazdani, S.K.; Watts, B.; Machingal, M.; Jarajapu, Y.P.; Van Dyke, M.E.; Christ, G.J.; Amiel, G.E.; Komura, M.; Shapira, O.; Yoo, J.J.; et al. Smooth Muscle Cell Seeding of Decellularized Scaffolds: The Importance of Bioreactor Preconditioning to Development of a More Native Architecture for Tissue-Engineered Blood Vessels. Tissue Eng. 2009, 15, 827–840. [Google Scholar] [CrossRef]
- Yipeng, J.; Yongde, X.; Yuanyi, W.; Jilei, S.; Jiaxiang, G.; Jiangping, G.; Yong, Y. Microtissues enhance smooth muscle differentiation and cell viability of hADSCs for three dimensional bioprinting. Front. Physiol. 2017, 8, 534. [Google Scholar] [CrossRef]
- Liu, G.; Fu, M.; Li, F.; Fu, W.; Zhao, Z.; Xia, H.; Niu, Y. Tissue-engineered PLLA/gelatine nanofibrous scaffold promoting the phenotypic expression of epithelial and smooth muscle cells for urethral reconstruction. Mater. Sci. Eng. C 2020, 111, 110810. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, C.; Manousiouthakis, E.; Wang, X.; Cairns, D.M.; Roh, T.T.; Du, C.; Kaplan, D.L. Bi-Layered Tubular Microfiber Scaffolds as Functional Templates for Engineering Human Intestinal Smooth Muscle Tissue. Adv. Funct. Mater. 2020, 30, 2000543. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Fu, Y.; Zhang, G.; Xu, L.; Jin, G.; Tang, L.; Ju, J.; Zhao, W.; Hou, R. Nanofiber electrospinning combined with rotary bioprinting for fabricating small-diameter vessels with endothelium and smooth muscle. Compos. Part B Eng. 2022, 234, 109691. [Google Scholar] [CrossRef]
- Xu, L.; Varkey, M.; Jorgensen, A.; Ju, J.; Jin, Q.; Park, J.H.; Fu, Y.; Zhang, G.; Ke, D.; Zhao, W.; et al. Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step. Biofabrication 2020, 12, 045012. [Google Scholar] [CrossRef] [PubMed]
- Méndez-barbero, N.; Gutiérrez-muñoz, C.; Blanco-Colio, L.M. Cellular crosstalk between endothelial and smooth muscle cells in vascular wall remodeling. Int. J. Mol. Sci. 2021, 22, 7284. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Helenius, G.; Johansson, B.R.; Li, J.Y.; Mattsson, E.; Risberg, B. Co-Culture of Endothelial Cells and Smooth Muscle Cells Affects Gene Expression of Angiogenic Factors. J. Cell. Biochem. 2003, 1259, 1250–1259. [Google Scholar] [CrossRef]
- Williams, C.; Wick, T. Endothelial Cell—Smooth Muscle Cell Co-Culture in a Perfusion Bioreactor System. Ann. Biomed. Eng. 2005, 33, 920–928. [Google Scholar] [CrossRef]
- Saunders, S.K.; Cole, S.Y.; Sierra, V.A.; Bracamonte, J.H.; Toldo, S.; Soares, J.S. Evaluation of perfusion-driven cell seeding of small diameter engineered tissue vascular grafts with a custom-designed seed-and-culture bioreactor. PLoS ONE 2022, 17, e0269499. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Yang, T.; Lu, T.; Du, P.; Jing, C.; Chen, Z.; Lin, F.; Zhao, G.; Zhao, L. Construction of spider silk protein small-caliber tissue engineering vascular grafts based on dynamic culture and its performance evaluation. J. Biomed. Mater. Res. Part A 2023, 111, 71–87. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.I.; Ide, C.; Nabeshima, Y.I. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- de la Garza-Rodea, A.S.; van der Velde, I.; Boersma, H.; Gonçalves, M.A.F.V.; van Bekkum, D.W.; de Vries, A.A.F.; Knaän-Shanzer, S. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant. 2011, 20, 217–231. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Vandenabeele, F.; Vermeesch, J.R.; Raymackers, J.M.; Luyten, F.P. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J. Cell Biol. 2003, 160, 909–918. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Pisani, D.; Dechesne, C.A.; Turc-Carel, C.; Kurzenne, J.Y.; Wdziekonski, B.; Villageois, A.; Bagnis, C.; Breittmayer, J.P.; Groux, H.; et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005, 201, 1397–1405. [Google Scholar] [CrossRef]
- Meligy, F.Y.; Shigemura, K.; Behnsawy, H.M.; Fujisawa, M.; Kawabata, M.; Shirakawa, T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sevak, J.K.; Das, A.; Arimbasseri, G.A.; Bhatnagar, S.; Gopinath, S.D. Umbilical cord tissue is a robust source for mesenchymal stem cells with enhanced myogenic differentiation potential compared to cord blood. Sci. Rep. 2020, 10, 18978. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef]
- Linard, C.; Brachet, M.; L’Homme, B.; Strup-Perrot, C.; Busson, E.; Bonneau, M.; Lataillade, J.J.; Bey, E.; Benderitter, M. Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: A proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res. Ther. 2018, 9, 299. [Google Scholar] [CrossRef]
- Pereira, A.R.S.; Mendes, T.F.; Ministro, A.; Teixeira, M.; Filipe, M.; Santos, J.M.; Bárcia, R.N.; Goyri-O’Neill, J.; Pinto, F.; Cruz, P.E.; et al. Therapeutic angiogenesis induced by human umbilical cord tissue-derived mesenchymal stromal cells in a murine model of hindlimb ischemia. Stem Cell Res. Ther. 2016, 7, 145. [Google Scholar] [CrossRef]
- Testa, S.; Riera, C.S.; Fornetti, E.; Riccio, F.; Fuoco, C.; Bernardini, S.; Baldi, J.; Costantini, M.; Foddai, M.L.; Cannata, S.; et al. Skeletal Muscle-Derived Human Mesenchymal Stem Cells: Influence of Different Culture Conditions on Proliferative and Myogenic Capabilities. Front. Physiol. 2020, 11, 553198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Li, Y.; Cao, J.; Zhang, H.; Chen, M.; Wang, L.; Zhang, C. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Hum. Mol. Genet. 2015, 24, 6029–6040. [Google Scholar] [CrossRef] [PubMed]
- Yilgor Huri, P.; Cook, C.A.; Hutton, D.L.; Goh, B.C.; Gimble, J.M.; DiGirolamo, D.J.; Grayson, W.L. Biophysical cues enhance myogenesis of human adipose derived stem/stromal cells. Biochem. Biophys. Res. Commun. 2013, 438, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, R.; Bagno, L.D.L.E.S.; Ribeiro, M.B.; Ferreira, A.B.R.; Moraes, M.O.; Zapata-Sudo, G.; Kasai-Brunswick, T.H.; Campos-de-Carvalho, A.C.; Goldenberg, R.C.D.S.; Werneck-de-Castro, J.P.S. Adipose-derived stem-cell treatment of skeletal muscle injury. J. Bone Jt. Surg. Am. 2012, 94, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Goudenege, S.; Pisani, D.F.; Wdziekonski, B.; Di Santo, J.P.; Bagnis, C.; Dani, C.; Dechesne, C.A. Enhancement of myogenic and muscle repair capacities of human adipose-derived stem cells with forced expression of MyoD. Mol. Ther. 2009, 17, 1064–1072. [Google Scholar] [CrossRef]
- Pourquié, O.; Al Tanoury, Z.; Chal, J. The Long Road to Making Muscle In Vitro. Curr. Top. Dev. Biol. 2018, 129, 123–142. [Google Scholar] [CrossRef]
- Iberite, F.; Gruppioni, E.; Ricotti, L. Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: Perspectives and challenges. NPJ Regen. Med. 2022, 7, 23. [Google Scholar] [CrossRef]
- Darabi, R.; Santos, F.N.C.; Filareto, A.; Pan, W.; Koene, R.; Rudnicki, M.A.; Kyba, M.; Perlingeiro, R.C.R. Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors. Stem Cells 2011, 29, 777–790. [Google Scholar] [CrossRef]
- Young, C.S.; Hicks, M.R.; Ermolova, N.V.; Nakano, H.; Jan, M.; Younesi, S.; Karumbayaram, S.; Kumagai-Cresse, C.; Wang, D.; Zack, J.A.; et al. A Single CRISPR-Cas9 Deletion Strategy that Targets the Majority of DMD Patients Restores Dystrophin Function in hiPSC-Derived Muscle Cells. Cell Stem Cell 2016, 18, 533–540. [Google Scholar] [CrossRef]
- Shelton, M.; Kocharyan, A.; Liu, J.; Skerjanc, I.S.; Stanford, W.L. Robust generation and expansion of skeletal muscle progenitors and myocytes from human pluripotent stem cells. Methods 2016, 101, 73–84. [Google Scholar] [CrossRef]
- Hicks, M.; Pyle, A. The Path from Pluripotency to Skeletal Muscle: Developmental Myogenesis Guides the Way. Cell Stem Cell 2015, 17, 255–257. [Google Scholar] [CrossRef]
- Chal, J.; Al Tanoury, Z.; Hestin, M.; Gobert, B.; Aivio, S.; Hick, A.; Cherrier, T.; Nesmith, A.P.; Parker, K.K.; Pourquié, O. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat. Protoc. 2016, 11, 1833–1850. [Google Scholar] [CrossRef]
- Fang, J.; Sia, J.; Soto, J.; Wang, P.; Li, L.A.K.; Hsueh, Y.Y.; Sun, R.; Faull, K.F.; Tidball, J.G.; Li, S. Skeletal muscle regeneration via the chemical induction and expansion of myogenic stem cells in situ or in vitro. Nat. Biomed. Eng. 2021, 5, 864–879. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Oginuma, M.; Al Tanoury, Z.; Gobert, B.; Sumara, O.; Hick, A.; Bousson, F.; Zidouni, Y.; Mursch, C.; Moncuquet, P.; et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 2015, 33, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.R.; Hiserodt, J.; Paras, K.; Fujiwara, W.; Eskin, A.; Jan, M.; Xi, H.; Young, C.S.; Evseenko, D.; Nelson, S.F.; et al. ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol. 2017, 20, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Matthias, N.; Lo, J.; Ortiz-Vitali, J.L.; Shieh, A.W.; Wang, S.H.; Darabi, R. A Myogenic Double-Reporter Human Pluripotent Stem Cell Line Allows Prospective Isolation of Skeletal Muscle Progenitors. Cell Rep. 2018, 25, 1966–1981. [Google Scholar] [CrossRef]
- Borchin, B.; Chen, J.; Barberi, T. Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells. Stem Cell Rep. 2013, 1, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Lim, H.T.; Estrellas, K.; Mula, J.; Cohen, T.V.; Zhang, Y.; Donnelly, C.J.; Richard, J.P.; Kim, Y.J.; Kim, H.; et al. Concordant but Varied Phenotypes among Duchenne Muscular Dystrophy Patient-Specific Myoblasts Derived using a Human iPSC-Based Model. Cell Rep. 2016, 15, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Magli, A.; Chan, S.S.K.; Oliveira, V.K.P.; Wu, J.; Darabi, R.; Kyba, M.; Perlingeiro, R.C.R. Expansion and Purification Are Critical for the Therapeutic Application of Pluripotent Stem Cell-Derived Myogenic Progenitors. Stem Cell Rep. 2017, 9, 12–22. [Google Scholar] [CrossRef]
- van der Wal, E.; Herrero-Hernandez, P.; Wan, R.; Broeders, M.; in ’t Groen, S.L.M.; van Gestel, T.J.M.; van IJcken, W.F.J.; Cheung, T.H.; van der Ploeg, A.T.; Schaaf, G.J.; et al. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Rep. 2018, 10, 1975–1990. [Google Scholar] [CrossRef]
- Jin, Y.; Shahriari, D.; Jeon, E.J.; Park, S.; Choi, Y.S.; Back, J.; Lee, H.; Anikeeva, P.; Cho, S.W. Functional Skeletal Muscle Regeneration with Thermally Drawn Porous Fibers and Reprogrammed Muscle Progenitors for Volumetric Muscle Injury. Adv. Mater. 2021, 33, 2007946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhou, Y.; Wu, F.; Hong, Y.; Wang, X.; Shekhawat, G.; Mosenson, J.; Wu, W.S. Selective Expansion of Skeletal Muscle Stem Cells from Bulk Muscle Cells in Soft Three-Dimensional Fibrin Gel. Stem Cells Transl. Med. 2017, 6, 1412. [Google Scholar] [CrossRef] [PubMed]
- Zahari, N.K.; Idrus, R.B.H.; Chowdhury, S.R. Laminin-coated poly(Methyl methacrylate) (PMMA) nanofiber scaffold facilitates the enrichment of skeletal muscle myoblast population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kitajima, Y.; Okazaki, N.; Chiba, K.; Yonekura, A.; Ono, Y. A Modified Pre-plating Method for High-Yield and High-Purity Muscle Stem Cell Isolation From Human/Mouse Skeletal Muscle Tissues. Front. Cell Dev. Biol. 2020, 8, 793. [Google Scholar] [CrossRef]
- Khodabukus, A.; Madden, L.; Prabhu, N.K.; Koves, T.R.; Jackman, C.P.; Muoio, D.M.; Bursac, N. Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle. Biomaterials 2019, 198, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, A.; Burdzinska, A.; Szczepanska, I.; Zarychta-Wisniewska, W.; Pajak, B.; Bojarczuk, K.; Dybowski, B.; Paczek, L. The Mutual Interactions between Mesenchymal Stem Cells and Myoblasts in an Autologous Co-Culture Model. PLoS ONE 2016, 11, e0161693. [Google Scholar] [CrossRef]
- Cai, A.; Hardt, M.; Schneider, P.; Schmid, R.; Lange, C.; Dippold, D.; Schubert, D.W.; Boos, A.M.; Weigand, A.; Arkudas, A.; et al. Myogenic differentiation of primary myoblasts and mesenchymal stromal cells under serum-free conditions on PCL-collagen I-nanoscaffolds. BMC Biotechnol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Juhas, M.; Abutaleb, N.; Wang, J.T.; Ye, J.; Shaikh, Z.; Sriworarat, C.; Qian, Y.; Bursac, N. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng. 2018, 2, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.T.V.; Palla, A.R.; Blake, M.R.; Yucel, N.D.; Wang, Y.X.; Magnusson, K.E.G.; Holbrook, C.A.; Kraft, P.E.; Delp, S.L.; Blau, H.M. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl. Acad. Sci. USA 2017, 114, 6675–6684. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 2015, 25, 655–673. [Google Scholar] [CrossRef]
- Le Grand, F.; Jones, A.E.; Seale, V.; Scimè, A.; Rudnicki, M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 2009, 4, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kino-Oka, M.; Chowdhury, S.R.; Muneyuki, Y.; Manabe, M.; Saito, A.; Sawa, Y.; Taya, M. Automating the Expansion Process of Human Skeletal Muscle Myoblasts with Suppression of Myotube Formation. Tissue Eng. Part C Methods 2009, 15, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Sakurai, H.; Suzuki, N.; Mabuchi, Y.; Sekiya, I.; Sekiguchi, K.; Akazawa, C. Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro. Stem Cell Rep. 2018, 10, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.G.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef]
- Han, W.M.; Anderson, S.E.; Mohiuddin, M.; Barros, D.; Nakhai, S.A.; Shin, E.; Amaral, I.F.; Pêgo, A.P.; García, A.J.; Jang, Y.C. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci. Adv. 2018, 4, eaar4008. [Google Scholar] [CrossRef]
- Bouchentouf, M.; Skuk, D.; Tremblay, J.P. Early and massive death of myoblasts transplanted into skeletal muscle: Responsible factors and potential solutions. Curr. Opin. Organ Transplant. 2007, 12, 664–667. [Google Scholar] [CrossRef]
- Peters, K.M.; Dmochowski, R.R.; Carr, L.K.; Robert, M.; Kaufman, M.R.; Sirls, L.T.; Herschorn, S.; Birch, C.; Kultgen, P.L.; Chancellor, M.B. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J. Urol. 2014, 192, 469–476. [Google Scholar] [CrossRef]
- Kuismanen, K.; Sartoneva, R.; Haimi, S.; Mannerström, B.; Tomás, E.; Miettinen, S.; Nieminen, K. Autologous Adipose Stem Cells in Treatment of Female Stress Urinary Incontinence: Results of a Pilot Study. Stem Cells Transl. Med. 2014, 3, 936. [Google Scholar] [CrossRef]
- Gotoh, M.; Yamamoto, T.; Kato, M.; Majima, T.; Toriyama, K.; Kamei, Y.; Matsukawa, Y.; Hirakawa, A.; Funahashi, Y. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int. J. Urol. 2014, 21, 294–300. [Google Scholar] [CrossRef]
- Yamamoto, T.; Gotoh, M.; Kato, M.; Majima, T.; Toriyama, K.; Kamei, Y.; Iwaguro, H.; Matsukawa, Y.; Funahashi, Y. Periurethral injection of autologous adipose-derived regenerative cells for the treatment of male stress urinary incontinence: Report of three initial cases. Int. J. Urol. 2012, 19, 652–659. [Google Scholar] [CrossRef]
- Gussoni, E.; Pavlath, G.K.; Lanctot, A.M.; Sharma, K.R.; Miller, R.G.; Steinman, L.; Blau, H.M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature 1992, 356, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Law, P.K.; Goodwin, T.G.; Fang, Q.; Duggirala, V.; Larkin, C.; Florendo, J.A.; Kirby, D.S.; Deering, M.B.; Li, H.J.; Chen, M.; et al. Feasibility, safety, and efficacy of myoblast transfer therapy on Duchenne muscular dystrophy boys. Cell Transplant. 1992, 1, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Périé, S.; Trollet, C.; Mouly, V.; Vanneaux, V.; Mamchaoui, K.; Bouazza, B.; Marolleau, J.P.; Laforêt, P.; Chapon, F.; Eymard, B.; et al. Autologous Myoblast Transplantation for Oculopharyngeal Muscular Dystrophy: A Phase I/Iia Clinical Study. Mol. Ther. 2014, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.K.; Robert, M.; Kultgen, P.L.; Herschorn, S.; Birch, C.; Murphy, M.; Chancellor, M.B. Autologous muscle derived cell therapy for stress urinary incontinence: A prospective, dose ranging study. J. Urol. 2013, 189, 595–601. [Google Scholar] [CrossRef]
- Sharifiaghdas, F.; Zohrabi, F.; Moghadasali, R.; Shekarchian, S.; Jaroughi, N.; Bolurieh, T.; Baharvand, H.; Aghdami, N. Autologous Muscle-derived Cell Injection for Treatment of Female Stress Urinary Incontinence: A Single- Arm Clinical Trial with 24-months Follow-Up. Urol. J. 2019, 16, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Gräs, S.; Klarskov, N.; Lose, G. Intraurethral injection of autologous minced skeletal muscle: A simple surgical treatment for stress urinary incontinence. J. Urol. 2014, 192, 850–855. [Google Scholar] [CrossRef]
- Gerullis, H.; Eimer, C.; Georgas, E.; Homburger, M.; El-Baz, A.G.; Wishahi, M.; Borós, M.; Ecke, T.H.; Otto, T. Muscle-derived cells for treatment of iatrogenic sphincter damage and urinary incontinence in men. Sci. World J. 2012, 2012, 898535. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.; Marksteiner, R.; Margreiter, E.; Pinggera, G.M.; Frauscher, F.; Ulmer, H.; Fussenegger, M.; Bartsch, G.; Strasser, H. Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J. Urol. 2008, 179, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Sèbe, P.; Doucet, C.; Cornu, J.N.; Ciofu, C.; Costa, P.; De Medina, S.G.D.; Pinset, C.; Haab, F. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: A prospective study. Int. Urogynecol. J. 2011, 22, 183–189. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.H.; Park, W.J.; Lee, J.E.; Kim, B.; Han, D.W. Advanced techniques for skeletal muscle tissue engineering and regeneration. Bioengineering 2020, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Volpi, M.; Paradiso, A.; Costantini, M.; Świȩszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, D.; Yang, Y.; Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Xiao, W.; Li, Y. The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia. Stem Cell Res. Ther. 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Eugenis, I.; Wu, D.; Rando, T.A. Cells, scaffolds, and bioactive factors: Engineering strategies for improving regeneration following volumetric muscle loss. Biomaterials 2021, 278, 121173. [Google Scholar] [CrossRef]
| Cell Type | Culture Conditions | Disease/Application | Number of Patients | Main Observations | Year | Reference |
|---|---|---|---|---|---|---|
| Myoblasts | - | Duchenne muscular dystrophy | 8 | No dystrophin expression restored; No significant strength recovery. | 1992 | [142] |
| Myoblasts | DMEM with 10% horse serum | Duchenne muscular dystrophy | 21 | No significant strength recovery. | 1992 | [143] |
| Myoblasts | FBS and FGF-2 | Oculopharyngeal muscular dystrophy | 12 | Safety tested; Quality of life improved. | 2014 | [144] |
| SkM-derived cells | Proprietary method | Urinary incontinence | 38 | Safety tested; No major adverse events. | 2013 | [145] |
| SkM-derived cells | Proprietary method | Urinary incontinence | 80 | Safety profile tested; Improvement in stress incontinence symptoms. | 2014 | [138] |
| SkM-derived cells | Ham’s F10 with 20% FBS | Urinary incontinence | 20 | Partial response; Symptoms relapse after 2 years in 50% of the initial responders. | 2019 | [146] |
| SkM (minced tissue) | Without ex vivo culture | Urinary incontinence | 35 | Symptoms improved in 25–63% of patients; Minor adverse events. | 2014 | [147] |
| SkM-derived cells | DMEM/F12 and 10% fetal calf serum | Damaged urethral sphincter | 222 | After 1 year, in 46% patients, no therapeutic effect; 42% reported improvement of symptoms and in 12% urinary continence was restored. | 2012 | [148] |
| Myoblasts (and fibroblasts) | DMEM/F12 with 20% autologous serum | Urinary incontinence after prostatectomy | 63 | 1 year follow-up shows restored continence in 41 patients; 5 patients did not show improvement. | 2008 | [149] |
| Progenitor SkM cells | - | Urinary incontinence | 12 | Quality of life improvement reported. | 2010 | [150] |
| SMCs (and urothelial cells) | DMEM with 10% fetal calf serum | Cystoplasty | 7 | 56% of patients showed signs of enhanced bladder function. | 2006 | [151] |
| SMCs (and epithelial cells) | DMEM with EGF | Urethral reconstruction | 5 | Histology showed that engineered constructs integrated patient’s tissue. | 2011 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchi-Mendes, T.; Silva, M.; Cartaxo, A.L.; Fernandes-Platzgummer, A.; Cabral, J.M.S.; da Silva, C.L. Bioprocessing Considerations towards the Manufacturing of Therapeutic Skeletal and Smooth Muscle Cells. Bioengineering 2023, 10, 1067. https://doi.org/10.3390/bioengineering10091067
Franchi-Mendes T, Silva M, Cartaxo AL, Fernandes-Platzgummer A, Cabral JMS, da Silva CL. Bioprocessing Considerations towards the Manufacturing of Therapeutic Skeletal and Smooth Muscle Cells. Bioengineering. 2023; 10(9):1067. https://doi.org/10.3390/bioengineering10091067
Chicago/Turabian StyleFranchi-Mendes, Teresa, Marília Silva, Ana Luísa Cartaxo, Ana Fernandes-Platzgummer, Joaquim M. S. Cabral, and Cláudia L. da Silva. 2023. "Bioprocessing Considerations towards the Manufacturing of Therapeutic Skeletal and Smooth Muscle Cells" Bioengineering 10, no. 9: 1067. https://doi.org/10.3390/bioengineering10091067
APA StyleFranchi-Mendes, T., Silva, M., Cartaxo, A. L., Fernandes-Platzgummer, A., Cabral, J. M. S., & da Silva, C. L. (2023). Bioprocessing Considerations towards the Manufacturing of Therapeutic Skeletal and Smooth Muscle Cells. Bioengineering, 10(9), 1067. https://doi.org/10.3390/bioengineering10091067