Processing Ultrasound Scans of the Inferior Vena Cava: Techniques and Applications
Abstract
1. Introduction
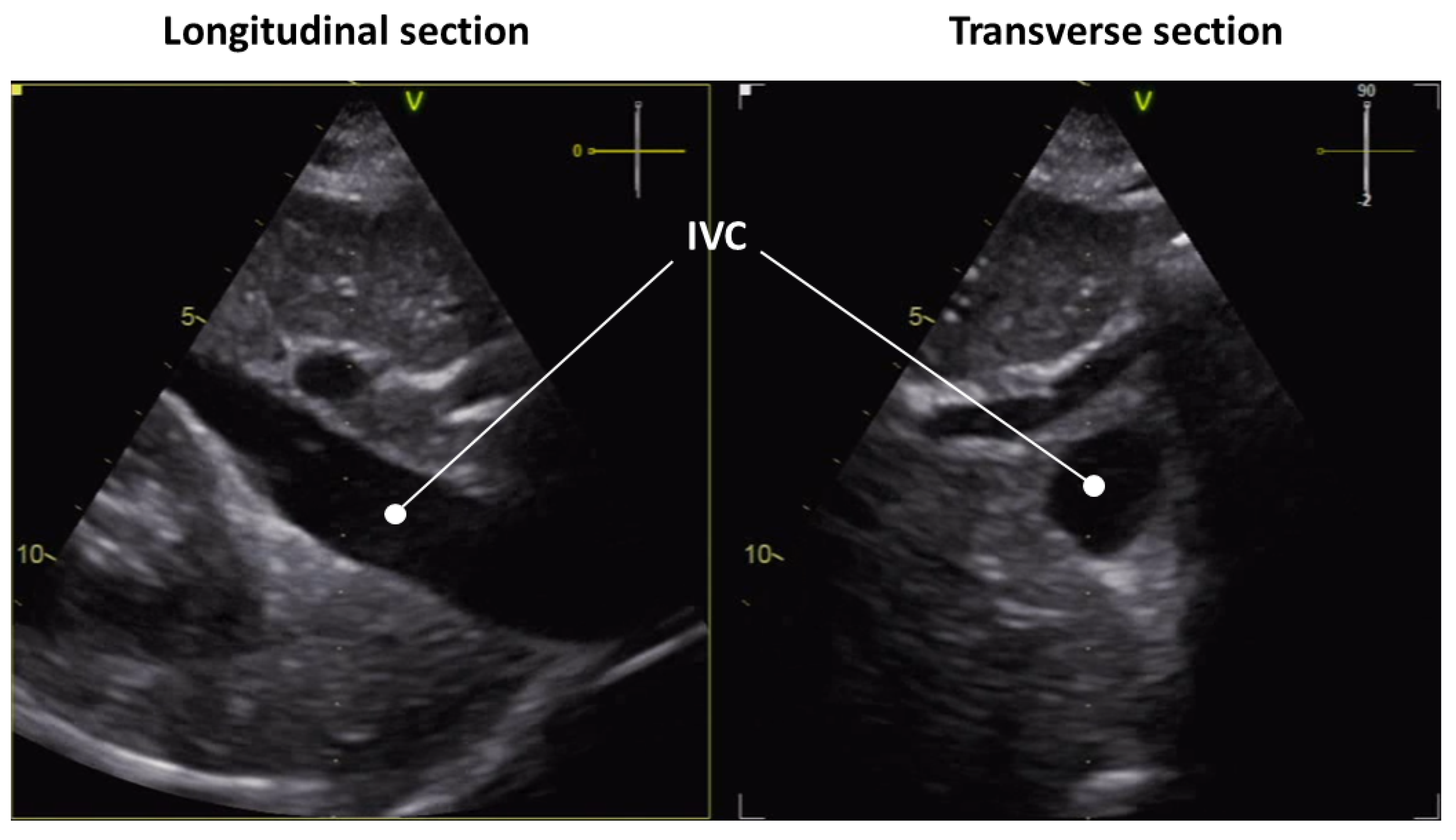
2. Anatomy of the IVC
3. Processing US Scans of IVC
3.1. Longitudinal View
3.1.1. KLT Feature Tracker
3.1.2. IVC Tracking and Detection of Intensity Jump
- Two reference points to be tracked;
- The section along which to estimate the diameter.
- Select a rectangular area on the first frame of the US scan around the IVC;
- Establish two landmarks to track tissue movement;
- Identify the first and last segmentation lines that delimit the vein portion of interest and indicate the edges along the first one.
- The reference points are tracked;
- The segmentation lines are displaced and rotated proportionally to the movement of the reference points;
- Edges are identified by the algorithm described above, searching for local intensity variations close to the border points found in the previous frame.
3.2. Transverse View
3.2.1. Active Contour
3.2.2. Estimation of Jumps of Intensity along Lines
3.2.3. Shape-Based Algorithms
3.3. General Methods
3.3.1. Block-Matching Algorithm
3.3.2. Deep Learning
- A bidirectional LSTM network.
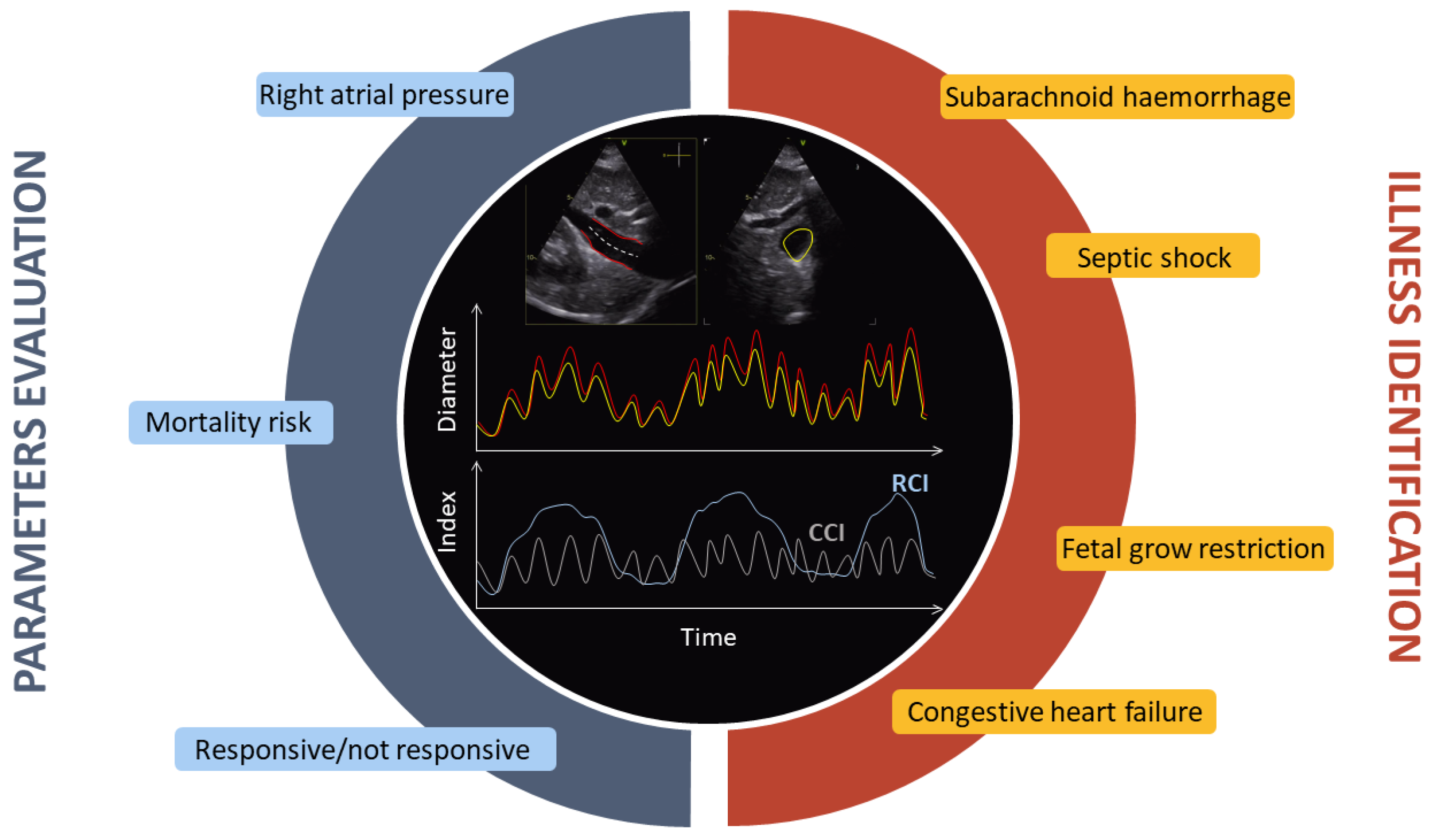
4. Clinical Applications of IVC Diameter Estimation
4.1. Foetal Grow Restriction
4.2. Right Atrial Pressure
4.3. Fluid Administration
4.4. Volume Conditions
4.5. Congestion and Heart Failure
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHF | Acute decompensated heart failure |
| AP | Anterior–posterior |
| CCI | Cardiac caval index |
| CNN | Convolutional neural network |
| CO | Cardiac output |
| CT | Computed tomography |
| CVP | Central venous pressure |
| ESC-HF-LT | European Society of Cardiology Heart Failure Long-Term |
| FGR | Foetal grow restriction |
| HR | Heart rate |
| IVC | Inferior vena cava |
| KLT | Kanade–Lucas–Tomasi |
| LSTM | Long short-term memory |
| PCWP | Pulmonary capillary wedge pressure |
| PLR | Passive leg raise |
| POCUS | Point-of-care ultrasonography |
| RAP | Right atrial pressure |
| RCI | Respiratory caval index |
| SAH | Subarachnoid haemorrhage |
| SV | Stroke volume |
| ROI | Region of interest |
| US | Ultrasound |
| YOLO | You only look once |
| USoP | Ultrasonic-system-on-patch |
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Cismaru, G.; Serban, T.; Tirpe, A. Ultrasound methods in the evaluation of atherosclerosis: From pathophysiology to clinic. Biomedicines 2021, 9, 418. [Google Scholar] [CrossRef]
- Intengan, H.D.; Schiffrin, E.L. Structure and mechanical properties of resistance arteries in hypertension: Role of adhesion molecules and extracellular matrix determinants. Hypertension 2000, 36, 312–318. [Google Scholar] [CrossRef]
- Mesin, L.; Albani, S.; Policastro, P.; Pasquero, P.; Porta, M.; Melchiorri, C.; Leonardi, G.; Albera, C.; Scacciatella, P.; Pellicori, P.; et al. Assessment of phasic changes of vascular size by automated edge tracking-state of the art and clinical perspectives. Front. Cardiovasc. Med. 2021, 8, 775635. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.C. Autonomic control of the venous system in health and disease: Effects of drugs. Pharmacol. Ther. 2001, 90, 179–230. [Google Scholar] [CrossRef] [PubMed]
- Ermini, L.; Chiarello, N.E.; De Benedictis, C.; Ferraresi, C.; Roatta, S. Venous Pulse Wave Velocity variation in response to a simulated fluid challenge in healthy subjects. Biomed. Signal Process. Control 2021, 63, 102177. [Google Scholar] [CrossRef]
- Karami, E.; Shehata, M.S.; Smith, A. Estimation and tracking of AP-diameter of the inferior vena cava in ultrasound images using a novel active circle algorithm. Comput. Biol. Med. 2018, 98, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Albani, S.; Mesin, L.; Roatta, S.; De Luca, A.; Giannoni, A.; Stolfo, D.; Biava, L.; Bonino, C.; Contu, L.; Pelloni, E.; et al. Inferior Vena Cava edge tracking echocardiography: A promising tool with applications in multiple clinical settings. Diagnostics 2022, 12, 427. [Google Scholar] [CrossRef]
- Nakamura, K.; Tomida, M.; Ando, T.; Sen, K.; Inokuchi, R.; Kobayashi, E.; Nakajima, S.; Sakuma, I.; Yahagi, N. Cardiac variation of inferior vena cava: New concept in the evaluation of intravascular blood volume. J. Med. Ultrason. 2013, 40, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B.; Fong, T.; Rhyne, R.; Risko, N. A systematic review of the cost-effectiveness of ultrasound in emergency care settings. Ultrasound J. 2021, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Begum, H.A.; Grewal, H.; Etxeandia-Ikobaltzeta, I.; Morgano, G.P.; Khatib, R.; Nieuwlaat, R.; Ding, C.; Wiercioch, W.; Mustafa, R.A.; et al. Cost-effectiveness of diagnostic strategies for venous thromboembolism: A systematic review. Blood Adv. 2022, 6, 544–567. [Google Scholar] [CrossRef]
- Mesin, L.; Albani, S.; Sinagra, G. Non-invasive estimation of right atrial pressure using inferior Vena Cava echography. Ultrasound Med. Biol. 2019, 45, 1331–1337. [Google Scholar] [CrossRef]
- Kaptein, M.J.; Kaptein, J.S.; Nguyen, C.D.; Oo, Z.; Thwe, P.P.; Thu, M.B.; Kaptein, E.M. Changes in cardiac output with hemodialysis relate to net volume balance and to inferior vena cava ultrasound collapsibility in critically ill patients. Ren. Fail. 2020, 42, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Ermini, L.; Seddone, S.; Policastro, P.; Mesin, L.; Pasquero, P.; Roatta, S. The cardiac Caval Index: Improving noninvasive assessment of cardiac preload: Improving noninvasive assessment of cardiac preload. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2022, 41, 2247–2258. [Google Scholar] [CrossRef]
- Mesin, L.; Pasquero, P.; Albani, S.; Porta, M.; Roatta, S. Semi-automated tracking and continuous monitoring of inferior vena cava diameter in simulated and experimental ultrasound imaging. Ultrasound Med. Biol. 2015, 41, 845–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonoo, T.; Nakamura, K.; Ando, T.; Sen, K.; Maeda, A.; Kobayashi, E.; Sakuma, I.; Doi, K.; Nakajima, S.; Yahagi, N. Prospective analysis of cardiac collapsibility of inferior vena cava using ultrasonography. J. Crit. Care 2015, 30, 945–948. [Google Scholar] [CrossRef]
- Mesin, L.; Pasquero, P.; Roatta, S. Tracking and monitoring pulsatility of a portion of inferior Vena Cava from ultrasound imaging in long axis. Ultrasound Med. Biol. 2019, 45, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Pasquero, P.; Roatta, S. Multi-directional assessment of respiratory and cardiac pulsatility of the inferior Vena Cava from ultrasound imaging in short axis. Ultrasound Med. Biol. 2020, 46, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Spentzouris, G.; Zandian, A.; Cesmebasi, A.; Kinsella, C.R.; Muhleman, M.; Mirzayan, N.; Shirak, M.; Tubbs, R.S.; Shaffer, K.; Loukas, M. The clinical anatomy of the inferior vena cava: A review of common congenital anomalies and considerations for clinicians: Inferior Vena Cava. Clin. Anat. 2014, 27, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Kaura, D.R.; Gray, R.R.; Sadler, D.J.; So, C.B.; Saliken, J.C. Value of frontal caval measurement in the placement of inferior vena cava filter. Can. Assoc. Radiol. J. 1999, 50, 301–305. [Google Scholar] [PubMed]
- Verma, M.; Pandey, N.N.; Ojha, V.; Kumar, S.; Ramakrishnan, S. Developmental anomalies of the inferior Vena Cava and its tributaries: What the radiologist needs to know? Indian J. Radiol. Imaging 2022, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Shin, H.C.; Hwang, J.A.; Jou, S.S.; Lee, W.H.; Choi, S.Y.; Park, C.H. Various congenital anomalies of the inferior vena cava: Review of cross-sectional imaging findings and report of a new variant. Abdom. Radiol. 2018, 43, 2130–2149. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Lee, J.; Hall, J.; Sutherland, T.R. The inferior vena cava: Anatomical variants and acquired pathologies. Insights Imaging 2021, 12, 123. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. (Eds.) Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Shin, D.S.; Sandstrom, C.K.; Ingraham, C.R.; Monroe, E.J.; Johnson, G.E. The inferior vena cava: A pictorial review of embryology, anatomy, pathology, and interventions. Abdom. Radiol. 2019, 44, 2511–2527. [Google Scholar] [CrossRef]
- Karami, E.; Shehata, M.; Smith, A. Segmentation and tracking of inferior vena cava in ultrasound images using a novel polar active contour algorithm. In Proceedings of the 2017 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Montreal, QC, Canada, 14–16 November 2017. [Google Scholar]
- Karami, E.; Shehata, M.S.; Smith, A. Semi-automatic algorithms for estimation and tracking of AP-diameter of the IVC in ultrasound images. J. Imaging 2019, 5, 12. [Google Scholar] [CrossRef]
- Policastro, P.; Chiarion, G.; Ponzio, F.; Ermini, L.; Civera, S.; Albani, S.; Musumeci, G.; Roatta, S.; Mesin, L. Detection of inferior Vena Cava in ultrasound scans through a deep learning model. Electronics 2023, 12, 1725. [Google Scholar] [CrossRef]
- Belmont, B.; Kessler, R.; Theyyunni, N.; Fung, C.; Huang, R.; Cover, M.; Ward, K.R.; Shih, A.J.; Tiba, M. Continuous inferior Vena Cava diameter tracking through an iterative Kanade-Lucas-Tomasi-based algorithm. Ultrasound Med. Biol. 2018, 44, 2793–2801. [Google Scholar] [CrossRef]
- Blaivas, M.; Blaivas, L. Are all deep learning architectures alike for point-of-care ultrasound?: Evidence from a cardiac image classification model suggests otherwise. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2020, 39, 1187–1194. [Google Scholar] [CrossRef]
- Lucas, B.D.; Kanade, T. An Iterative Image Registration Technique with an Application to Stereo Vision. In Proceedings of the International Joint Conference on Artificial Intelligence, Vancouver, BC, Canada, 24–28 August 1981. [Google Scholar]
- Wallace, D.J.; Allison, M.; Stone, M.B. Inferior vena cava percentage collapse during respiration is affected by the sampling location: An ultrasound study in healthy volunteers. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2010, 17, 96–99. [Google Scholar] [CrossRef]
- Mesin, L.; Giovinazzo, T.; D’Alessandro, S.; Roatta, S.; Raviolo, A.; Chiacchiarini, F.; Porta, M.; Pasquero, P. Improved repeatability of the estimation of pulsatility of inferior vena cava. Ultrasound Med. Biol. 2019, 45, 2830–2843. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.; Witkin, A.; Terzopoulos, D. Snakes: Active contour models. Int. J. Comput. Vis. 1988, 1, 321–331. [Google Scholar] [CrossRef]
- Guerrero, J.; Salcudean, S.E.; McEwen, J.A.; Masri, B.A.; Nicolaou, S. Real-time vessel segmentation and tracking for ultrasound imaging applications. IEEE Trans. Med. Imaging 2007, 26, 1079–1090. [Google Scholar] [CrossRef]
- Zong, J.J.; Qiu, T.S.; Li, W.D.; Guo, D.M. Automatic ultrasound image segmentation based on local entropy and active contour model. Comput. Math. Appl. 2019, 78, 929–943. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, W.; Qin, J.; Wong, K.H.; Choi, K.S.; Heng, P.A. Fast feature-preserving speckle reduction for ultrasound images via phase congruency. Signal Process. 2017, 134, 275–284. [Google Scholar] [CrossRef]
- Pal, S.K.; Bhardwaj, A.; Shukla, A.P. A Review on Despeckling Filters in Ultrasound Images for Speckle Noise Reduction. In Proceedings of the 2021 International Conference on Advance Computing and Innovative Technologies in Engineering (ICACITE), Greater Noida, India, 4–5 March 2021; pp. 973–978. [Google Scholar]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S. Assessment of Myocardial Mechanics Using Speckle Tracking Echocardiography: Fundamentals and Clinical Applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Hinton, G.E.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef]
- Singh, M.; Pujar, G.V.; Kumar, S.A.; Bhagyalalitha, M.; Akshatha, H.S.; Abuhaija, B.; Alsoud, A.R.; Abualigah, L.M.; Beeraka, N.M.; Gandomi, A.H. Evolution of Machine Learning in Tuberculosis Diagnosis: A Review of Deep Learning-Based Medical Applications. Electronics 2022, 11, 2634. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Ma, L. Detection of Thyroid Nodules with Ultrasound Images Based on Deep Learning. Curr. Med. Imaging Rev. 2020, 162, 174–180. [Google Scholar] [CrossRef]
- Shokoohi, H.; LeSaux, M.A.; Roohani, Y.H.; Liteplo, A.; Huang, C.; Blaivas, M. Enhanced point-of-care ultrasound applications by integrating automated feature-learning systems using deep learning: Deep learning in point-of-care ultrasound. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2019, 38, 1887–1897. [Google Scholar] [CrossRef]
- An, Q.; Wang, H.; Chen, X. EPSDNet: Efficient Campus Parking Space Detection via Convolutional Neural Networks and Vehicle Image Recognition for Intelligent Human–Computer Interactions. Sensors 2022, 22, 9835. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.P.; Prakash, A.J.; Plawiak, P.; Samantray, S. Real-Time Hand Gesture Recognition Using Fine-Tuned Convolutional Neural Network. Sensors 2022, 22, 706. [Google Scholar] [CrossRef] [PubMed]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A novel explainable machine learning approach for EEG-based brain-computer interface systems. Neural Comput. Appl. 2021, 34, 11347–11360. [Google Scholar] [CrossRef]
- Kundu, S.; Ari, S. MsCNN: A Deep Learning Framework for P300-Based Brain–Computer Interface Speller. IEEE Trans. Med. Robot. Bionics 2020, 2, 86–93. [Google Scholar] [CrossRef]
- Desai, S.; Goh, G.; Babu, A.; Aly, A. Lightweight Convolutional Representations for On-Device Natural Language Processing. arXiv 2020, arXiv:2002.01535. [Google Scholar]
- Ombabi, A.H.; Ouarda, W.; Alimi, A.M. Deep learning CNN–LSTM framework for Arabic sentiment analysis using textual information shared in social networks. Soc. Netw. Anal. Min. 2020, 10, 53. [Google Scholar] [CrossRef]
- Ponzio, F.; Macii, E.; Ficarra, E.; Di Cataldo, S. Colorectal cancer classification using deep convolutional networks—An experimental study. In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies. SCITEPRESS, Madeira, Portugal, 1–21 January 2018. [Google Scholar]
- Zhan, X.; Long, H.; Gou, F.; Duan, X.; Kong, G.; Wu, J. A Convolutional Neural Network-Based Intelligent Medical System with Sensors for Assistive Diagnosis and Decision-Making in Non-Small Cell Lung Cancer. Sensors 2021, 21, 7996. [Google Scholar] [CrossRef]
- López-Linares, K.; García, I.; García-Familiar, A.; Macía, I.; Ballester, M.Á.G. 3D convolutional neural network for abdominal aortic aneurysm segmentation. arXiv 2019, arXiv:1903.00879. [Google Scholar]
- Urbanos, G.; Martín, A.; Vázquez, G.; Villanueva, M.; Villa, M.; Jimenez-Roldan, L.; Chavarrías, M.; Lagares, A.; Juárez, E.; Sanz, C. Supervised Machine Learning Methods and Hyperspectral Imaging Techniques Jointly Applied for Brain Cancer Classification. Sensors 2021, 21, 3827. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Blaivas, M.; Blaivas, L.; Philips, G.; Merchant, R.; Levy, M.; Abbasi, A.; Eickhoff, C.; Shapiro, N.; Corl, K. Development of a deep learning network to classify inferior Vena Cava collapse to predict fluid responsiveness. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2021, 40, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Nisar, H.; Carnahan, P.K.; Fakim, D.; Akhuanzada, H.; Hocking, D.; Peters, T.M.; Chen, E.C.S. Towards ultrasound-based navigation: Deep learning based IVC lumen segmentation from intracardiac echocardiography. In Medical Imaging 2022: Image-Guided Procedures, Robotic Interventions, and Modeling; Linte, C.A., Siewerdsen, J.H., Eds.; SPIE: Bellingham, WA, USA, 2022. [Google Scholar]
- Yang, J.; Tong, L.; Faraji, M.; Basu, A. IVUS-Net: An Intravascular Ultrasound Segmentation Network. In Smart Multimedia. ICSM 2018; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2018; pp. 367–377. [Google Scholar]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef]
- Society for Maternal-Foetal Medicine Publications Committee; Berkley, E.; Chauhan, S.P.; Abuhamad, A. Doppler assessment of the foetus with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2012, 206, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Denizli, R.; Tanaçan, A.; Sakcak, B.; Farisogulları, N.; Agaolu, Z.; Turgut, E.; Kara, O.; Sahin, D. Evaluation of the Caval aortic index in fetal growth restriction: A case-control study in a tertiary center. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2023. [Google Scholar] [CrossRef]
- Zur, R.L.; Kingdom, J.C.; Parks, W.T.; Hobson, S.R. The placental basis of fetal growth restriction. Obstet. Gynecol. Clin. N. Am. 2020, 47, 81–98. [Google Scholar] [CrossRef]
- Lichtblau, M.; Bader, P.R.; Saxer, S.; Berlier, C.; Schwarz, E.I.; Hasler, E.D.; Furian, M.; Grünig, E.; Bloch, K.E.; Ulrich, S. Right atrial pressure during exercise predicts survival in patients with pulmonary hypertension. J. Am. Heart Assoc. 2020, 9, e018123. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Albani, S.; Pinamonti, B.; Giovinazzo, T.; de Scordilli, M.; Fabris, E.; Stolfo, D.; Perkan, A.; Gregorio, C.; Barbati, G.; Geri, P.; et al. Accuracy of right atrial pressure estimation using a multi-parameter approach derived from inferior vena cava semi-automated edge-tracking echocardiography: A pilot study in patients with cardiovascular disorders. Int. J. Cardiovasc. Imaging 2020, 36, 1213–1225. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 786–788. [Google Scholar] [CrossRef]
- Milan, A.; Magnino, C.; Veglio, F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2010, 23, 225–239; quiz 332–334. [Google Scholar] [CrossRef] [PubMed]
- Vourvouri, E.C.; Schinkel, A.F.L.; Roelandt, J.R.T.C.; Boomsma, F.; Sianos, G.; Bountioukos, M.; Sozzi, F.B.; Rizzello, V.; Bax, J.J.; Karvounis, H.I.; et al. Screening for left ventricular dysfunction using a hand-carried cardiac ultrasound device. Eur. J. Heart Fail. 2003, 5, 767–774. [Google Scholar] [CrossRef]
- Magnino, C.; Omedé, P.; Avenatti, E.; Presutti, D.; Iannaccone, A.; Chiarlo, M.; Moretti, C.; Gaita, F.; Veglio, F.; Milan, A. Inaccuracy of right atrial pressure estimates through inferior Vena Cava indices. Am. J. Cardiol. 2017, 120, 1667–1673. [Google Scholar] [CrossRef]
- Ermini, L.; Ferraresi, C.; De Benedictis, C.; Roatta, S. Objective assessment of venous pulse wave velocity in healthy humans. Ultrasound Med. Biol. 2020, 46, 849–854. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef]
- Messmer, A.S.; Zingg, C.; Müller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Critical Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef]
- Tan, G.F.L.; Du, T.; Liu, J.S.; Chai, C.C.; Nyein, C.M.; Liu, A.Y.L. Automated lung ultrasound image assessment using artificial intelligence to identify fluid overload in dialysis patients. BMC Nephrol. 2022, 23, 410. [Google Scholar] [CrossRef]
- Bentzer, P.; Griesdale, D.E.; Boyd, J.; MacLean, K.; Sirounis, D.; Ayas, N.T. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA J. Am. Med. Assoc. 2016, 316, 1298. [Google Scholar] [CrossRef]
- Atallah, H.; Gaballah, K.M.; Khattab, A. Fluid responsiveness in hemodynamically unstable patients: A systematic review. Menoufia Med. J. 2019, 32, 397–404. [Google Scholar]
- Musu, M.; Guddelmoni, L.; Murgia, F.; Mura, S.; Bonu, F.; Mura, P.; Finco, G. Prediction of fluid responsiveness in ventilated critically ill patients. J. Emerg. Crit. Care Med. 2020, 4, 26. [Google Scholar] [CrossRef]
- Marik, P.E.; Lemson, J. Fluid responsiveness: An evolution of our understanding. Br. J. Anaesth. 2014, 112, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Hofer, C.; Teboul, J.L.; Pettila, V.; Wilkman, E.; Molnar, Z.; Della Rocca, G.; Aldecoa, C.; Artigas, A.; Jog, S.; et al. Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med. 2015, 41, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.C.; Kory, P.D.; Arntfield, R.T. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J. Crit. Care 2016, 31, 96–100. [Google Scholar] [CrossRef]
- Pourmand, A.; Pyle, M.; Yamane, D.; Sumon, K.; Frasure, S.E. The utility of point-of-care ultrasound in the assessment of volume status in acute and critically ill patients. World J. Emerg. Med. 2019, 10, 232–238. [Google Scholar] [CrossRef]
- Ilyas, A.; Ishtiaq, W.; Assad, S.; Ghazanfar, H.; Mansoor, S.; Haris, M.; Qadeer, A.; Akhtar, A. Correlation of IVC diameter and collapsibility index with central venous pressure in the assessment of intravascular volume in critically ill patients. Cureus 2017, 9, e1025. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Marx, G. Fluid therapy in sepsis with capillary leakage. Eur. J. Anaesthesiol. 2003, 20, 429–442. [Google Scholar] [CrossRef]
- Alonso, J.V.; Turpie, J.; Farhad, I.; Ruffino, G. Protocols for point-of-care-ultrasound (POCUS) in a patient with sepsis; An algorithmic approach. Bull. Emerg. Trauma 2019, 7, 67–71. [Google Scholar] [CrossRef]
- Barbier, C.; Loubières, Y.; Schmit, C.; Hayon, J.; Ricome, J.L.; Jardin, F.; Vieillard-Baron, A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004, 30, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Schefold, J.C.; Storm, C.; Bercker, S.; Pschowski, R.; Oppert, M.; Krüger, A.; Hasper, D. Inferior vena cava diameter correlates with invasive hemodynamic measures in mechanically ventilated intensive care unit patients with sepsis. J. Emerg. Med. 2010, 38, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Roatta, S.; Pasquero, P.; Porta, M. Automated volume status assessment using inferior Vena Cava pulsatility. Electronics 2020, 9, 1671. [Google Scholar] [CrossRef]
- Rahman, N.H.N.; Ahmad, R.; Kareem, M.M.; Mohammed, M.I. Ultrasonographic assessment of inferior vena cava/abdominal aorta diameter index: A new approach of assessing hypovolaemic shock class 1. Int. J. Emerg. Med. 2016, 9, 8. [Google Scholar] [CrossRef]
- Callcut, R.A.; Cotton, B.A.; Muskat, P.; Fox, E.E.; Wade, C.E.; Holcomb, J.B.; Schreiber, M.A.; Rahbar, M.H.; Cohen, M.J.; Knudson, M.M.; et al. Defining when to initiate massive transfusion: A validation study of individual massive transfusion triggers in PROMMTT patients. J. Trauma Acute Care Surg. 2013, 74, 59–65, 67–68; discussion 66–67. [Google Scholar] [CrossRef]
- Moore, K.A.; Arthur, A.S.; Hamm, C.W. Anesthesia management of intracranial aneurysms. In Intracranial Aneurysms; Academic Press: Cambridge, MA, USA, 2018; pp. 191–205. [Google Scholar]
- Celebi Yamanoglu, N.G.; Yamanoglu, A.; Parlak, I.; Pınar, P.; Tosun, A.; Erkuran, B.; Aydınok, G.; Torlak, F. The role of inferior vena cava diameter in volume status monitoring; the best sonographic measurement method? Am. J. Emerg. Med. 2015, 33, 433–438. [Google Scholar] [CrossRef]
- Takada, H.; Hifumi, T.; Yoshioka, H.; Okada, I.; Kiriu, N.; Inoue, J.; Morimoto, K.; Matsumoto, J.; Koido, Y.; Kato, H. Initial inferior vena cava diameter predicts massive transfusion requirements in blunt trauma patients: A retrospective cohort study. Am. J. Emerg. Med. 2018, 36, 1155–1159. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Beltrami, M.; Pirrotta, F.; Tavera, M.C.; Gennari, L.; Ruocco, G. Clinical, Laboratory and Lung Ultrasound Assessment of Congestion in Patients with Acute Heart Failure. J. Clin. Med. 2022, 11, 1642. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.S.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Rocca, H.P.B.L.; Martens, P.; Testani, J.M.; Tang, W.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Pellicori, P.; Shah, P.; Cuthbert, J.; Urbinati, A.; Zhang, J.; Kallvikbacka-Bennett, A.; Clark, A.L.; Cleland, J.G.F. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure: Congestion by ultrasound in heart failure. Eur. J. Heart Fail. 2019, 21, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Khandwalla, R.M.; Birkeland, K.T.; Zimmer, R.; Henry, T.D.; Nazarian, R.; Sudan, M.; Mirocha, J.; Cha, J.; Kedan, I. Usefulness of serial measurements of inferior Vena Cava diameter by VscanTM to identify patients with heart failure at high risk of hospitalization. Am. J. Cardiol. 2017, 119, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Pagnesi, M.; Baldetti, L.; Adamo, M. Inferior vena cava monitoring in heart failure: Don’t wait until the last drop makes the cup run over. Eur. J. Heart Fail. 2023, 25, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Jobs, A.; Brünjes, K.; Katalinic, A.; Babaev, V.; Desch, S.; Reppel, M.; Thiele, H. Inferior vena cava diameter in acute decompensated heart failure as predictor of all-cause mortality. Heart Vessel. 2017, 32, 856–864. [Google Scholar] [CrossRef]
- Griffin, M.D.; Ivey-Miranda, J.B.; McCallum, W.; Sarnak, M.J.; Eder, M.D.; Bellumkonda, L.; Maulion, C.; Wilson, F.P.; Rao, V.; Testani, J.M. Inferior Vena Cava Diameter Measurement Provides Distinct and Complimentary Information to Right Atrial Pressure in Acute Decompensated Heart Failure. J. Card. Fail. 2022, 28, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, Z.; Gao, X.; Bian, Y.; Wu, R.S.; Park, G.; Lou, Z.; Zhang, Z.; Xu, X.; Chen, X.; et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef]
| IVC View | Article | Publication Date | Method |
|---|---|---|---|
| Transverse | [10] | 2013 | Snake |
| [10] | 2013 | Template matching | |
| [28] | 2017 | Snake | |
| [8] | 2018 | Shape-based algorithm | |
| [29] | 2019 | Shape-based algorithm | |
| [19] | 2020 | Jump of intensity | |
| [30] | 2023 | YOLO | |
| Longitudinal | [16] | 2015 | Tracking and jump along a single line |
| [17] | 2015 | Block matching | |
| [31] | 2018 | KLT features | |
| [18] | 2019 | Tracking and jumps on multiple lines | |
| [32] | 2020 | CNN and LSTM | |
| [30] | 2023 | YOLO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policastro, P.; Mesin, L. Processing Ultrasound Scans of the Inferior Vena Cava: Techniques and Applications. Bioengineering 2023, 10, 1076. https://doi.org/10.3390/bioengineering10091076
Policastro P, Mesin L. Processing Ultrasound Scans of the Inferior Vena Cava: Techniques and Applications. Bioengineering. 2023; 10(9):1076. https://doi.org/10.3390/bioengineering10091076
Chicago/Turabian StylePolicastro, Piero, and Luca Mesin. 2023. "Processing Ultrasound Scans of the Inferior Vena Cava: Techniques and Applications" Bioengineering 10, no. 9: 1076. https://doi.org/10.3390/bioengineering10091076
APA StylePolicastro, P., & Mesin, L. (2023). Processing Ultrasound Scans of the Inferior Vena Cava: Techniques and Applications. Bioengineering, 10(9), 1076. https://doi.org/10.3390/bioengineering10091076







