Guar-Based Injectable Hydrogel for Drug Delivery and In Vitro Bone Cell Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
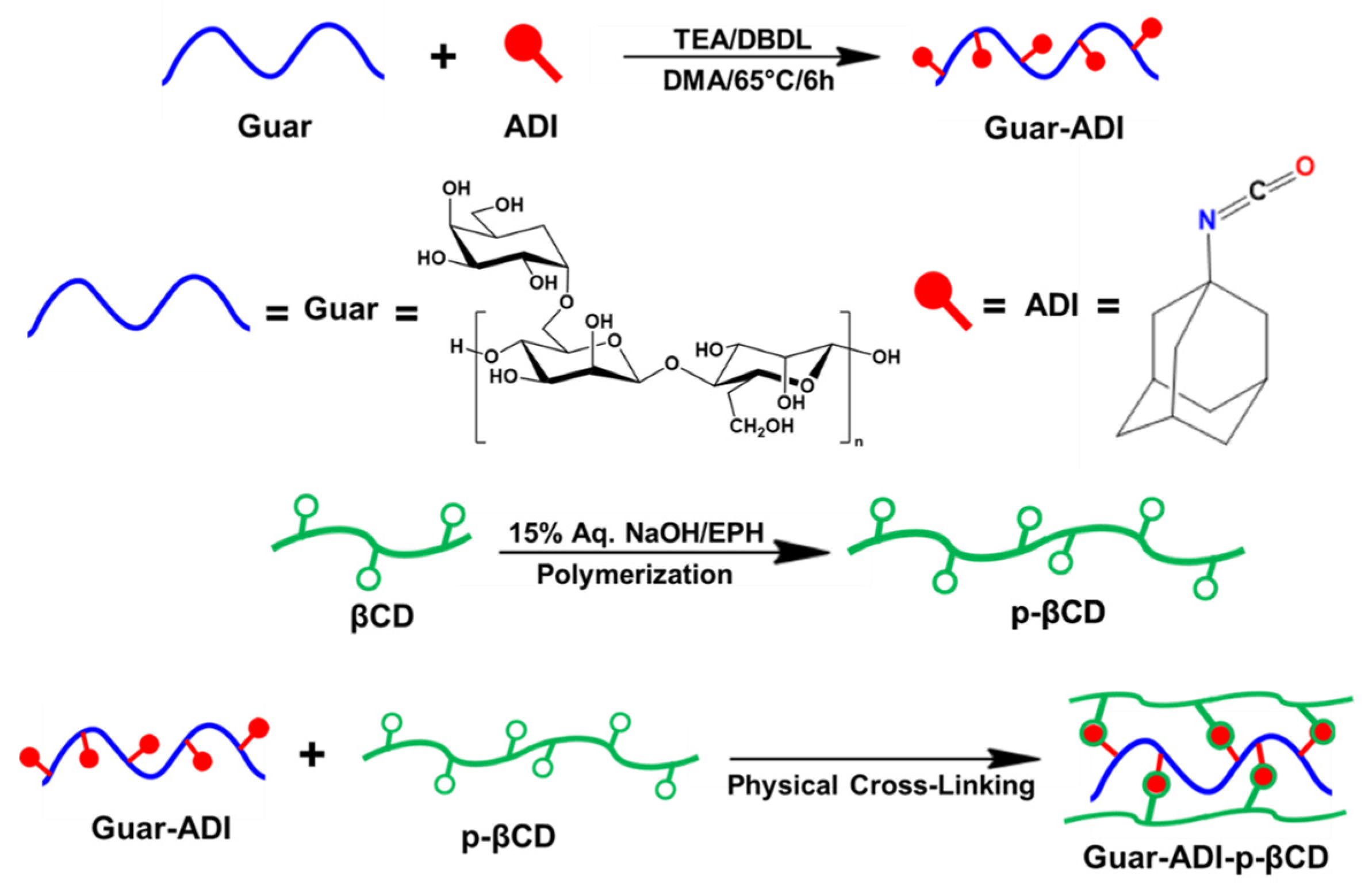
2.2. Synthesis of Guar-ADI Compound
2.3. Polymerization of β-Cyclodextrin
2.4. Preparation of Hydrogel
2.5. Characterizations of Materials
2.6. Measurement of the Swelling Property
2.7. In Vitro Drug Release Study
2.8. Cell Culture
2.9. Biocompatibility Assay
2.10. Statistical Analysis
3. Results and Discussions
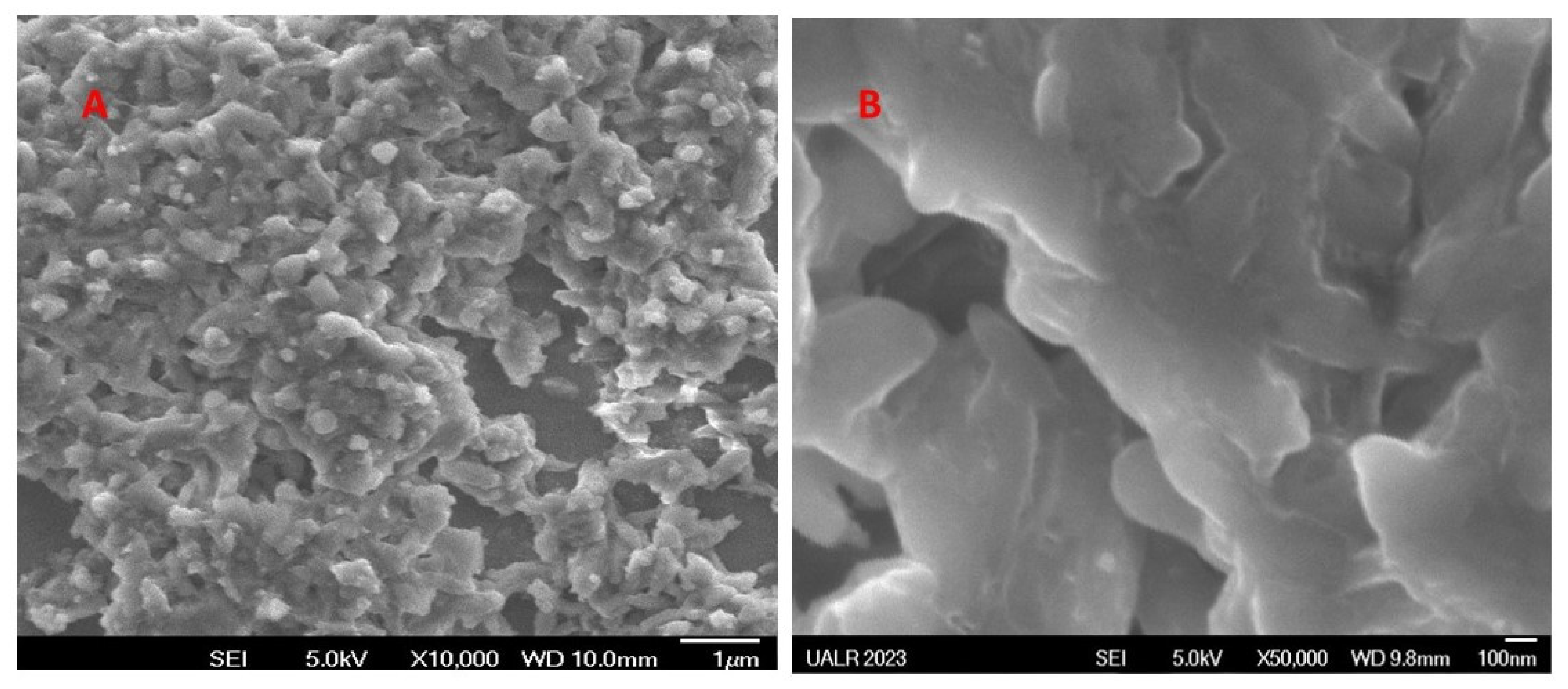
3.1. Characterizations
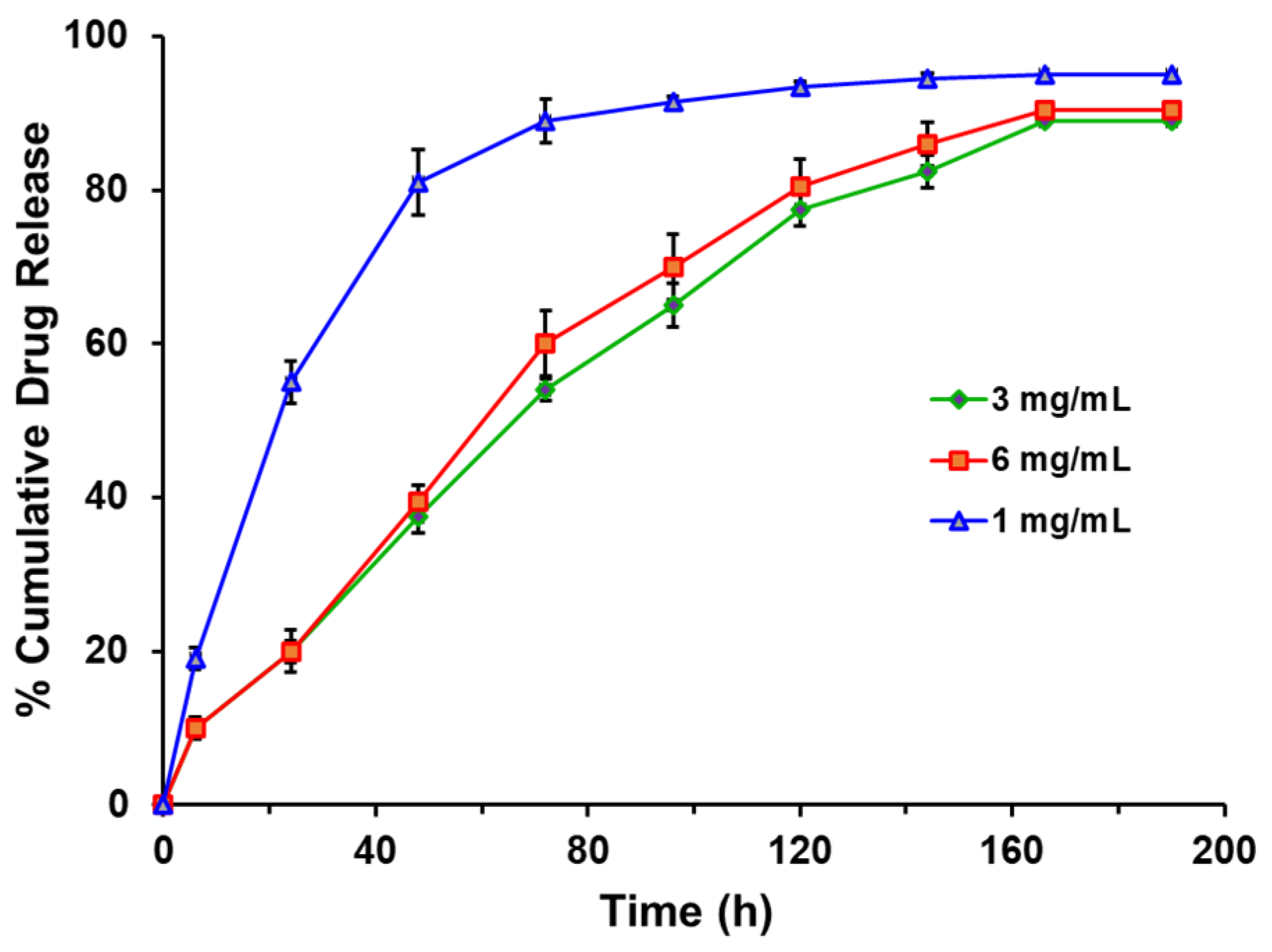
3.2. In Vitro Drug Release Study
3.3. Biocompatibility Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Curvello, R.; Garnier, G. Cationic Cross-Linked Nanocellulose-Based Matrices for the Growth and Recovery of Intestinal Organoids. Biomacromolecules 2021, 22, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Broguiere, N.; Isenmann, L.; Hirt, C.; Ringel, T.; Placzek, S.; Cavalli, E.; Ringnalda, F.; Villiger, L.; Züllig, R.; Lehmann, R.; et al. Growth of Epithelial Organoids in a Defined Hydrogel. Adv. Mater. 2018, 30, e1801621. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Rolandi, M. Soft and Ion-Conducting Materials in Bioelectronics: From Conducting Polymers to Hydrogels. Adv. Healthc. Mater. 2020, 9, e1901372. [Google Scholar] [CrossRef]
- Kandel, R.; Jang, S.R.; Shrestha, S.; Lee, S.Y.; Shrestha, B.K.; Park, C.H.; Kim, C.S. Biomimetic Cell-Substrate of Chitosan-Cross-Linked Polyaniline Patterning on TiO2 Nanotubes Enables HBM-MSCs to Differentiate the Osteoblast Cell Type. ACS Appl. Mater. Interfaces 2021, 13, 47100–47117. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Jochum, F.D.; Theato, P. Temperature- and Light-Responsive Smart Polymer Materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Giang Phan, V.H.; Jeong, J.H.; Lee, D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.C. In Situ-Forming Hydrogels—Review of Temperature-Sensitive Systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, M.; Zhou, Z.; Zhang, W.; Jiang, X.; Lu, X.; Zuo, B.; Lu, Q.; Kaplan, D.L. Injectable Silk Nanofiber Hydrogels for Sustained Release of Small-Molecule Drugs and Vascularization. ACS Biomat. Sci. Eng. 2019, 5, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Muirhead, B.; Dodd, M.; Liu, L.; Xu, F.; Mangiacotte, N.; Hoare, T.; Sheardown, H. An Injectable Hydrogel Prepared Using a PEG/Vitamin E Copolymer Facilitating Aqueous-Driven Gelation. Biomacromolecules 2016, 17, 3648−3658. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran-Thankam, A.; Parnell, C.M.; Watanabe, F.; Rangumagar, A.B.; Chhetri, B.P.; Szwedo, P.K.; Biris, A.S.; Ghosh, A. Guar-Based Injectable Thermoresponsive Hydrogel as a Scaffold for Bone Cell Growth and Controlled Drug Delivery. ACS Omega 2018, 3, 15158–15167. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host-Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Simões, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular Cyclodextrin-Based Drug Nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-Based Host–Guest Supramolecular Hydrogel and Its Application in Biomedical Fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Ding, H.; Khan, S.T.; Liu, J.; Sun, L. Gelation Based on Host–Guest Interactions Induced by Multi-Functionalized Nanosheets. Gels 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.S.; Moon, K.; Park, S.H.; Chun, H.; Ko, Y.H.; Lee, J.Y.; Lee, E.S.; Samal, S.; Selvapalam, N.; Rekharsky, M.V.; et al. Complexation of Ferrocene Derivatives by the Cucurbit [7] Uril Host: A Comparative Study of the Cucurbituril and Cyclodextrin Host Families. J. Am. Chem. Soc. 2005, 127, 12984–12989. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Brzezinski, M.; Socka, M.; Basko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Dreiss, C.A.; Scherman, O.A. Sustained Release of Proteins From High Water Content Supramolecular Polymer Hydrogels. Biomaterials 2012, 33, 4646–4652. [Google Scholar] [CrossRef]
- Kehr, N.S. Periodic Mesoporous Organosilica-Based Nanocomposite Hydrogels for Enhanced Cell Adhesion in 3D Hydrogel Network. J. Appl. Biol. Sci. 2019, 13, 133–137. [Google Scholar]
- Feng, Q.; Xu, J.; Zhang, K.; Yao, H.; Zheng, N.; Zheng, L.; Wang, J.; Wei, K.; Xiao, X.; Qin, L.; et al. Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects. ACS Cent. Sci. 2019, 5, 440–450. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar Gum: Processing, Properties and Food Applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar Gum as a Promising Starting Material for Diverse Applications: A Review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-Cyclodextrin Cavity: Amount, Stability and Mechanism of Binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef]
- Tripathi, J.; Ambolikar, R.; Gupta, S.; Jain, D.; Bahadur, J.; Variyar, P.S. Methylation of Guar Gum for Improving Mechanical and Barrier Properties of Biodegradable Packaging. Film. Sci. Rep. 2019, 9, 14505. [Google Scholar] [CrossRef]
- Thurein, S.M.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical Properties of β-Cyclodextrin Solutions And Precipitates Prepared From Injectable Vehicles. AJPS 2018, 13, 438–449. [Google Scholar] [CrossRef]
- Lou, C.; Gu, J.; Di, M.; Ma, L.; Wang, Y.; Liu, X. Synthesis and Characterization of Trichlorophenol-Blocked Polyaryl Polyisocyanate. Iran. Polym. J. 2011, 20, 247–255. [Google Scholar]
- Gidwani, B.; Vyas, A. Synthesis, Characterization and Application of Epichlorohydrin-β-Cyclodextrin Polymer. Colloids Surf. B Biointerfaces 2014, 114, 130–137. [Google Scholar] [CrossRef]
- Osman, S.; Soliman, G.M.; Amin, M.; Zaky, A. Self-Assembling Hydrogels Based On β-Cyclodextrin Polymer And Poly (Ethylene Glycol) Bearing Hydrophobic Moieties For Protein Delivery. Int. J. Pharm. Pharm. Sci. 2014, 6, 591–597. [Google Scholar]
- Agarwal, R.; Mumtaz, H.; Ali, N. Role of Inositol Polyphosphates in Programmed Cell Death. Mol. Cell. Biochem. 2009, 328, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.S.; Rao, K.M.; Reddy, P.R.S.; Reddy, N.S.; Rao, K.S.V.K.; Subha, M.C.S. Synthesis and Characterisation of Guar Gum-g-Poly(Acrylamidoglycolic Acid) by Redox Initiator. Ind. J. Adv. Chem. Sci. 2012, 1, 28–32. [Google Scholar]
- Wong, C.S.; Badri, K.H. Chemical Analyses of Palm Kernel Oil-Based Polyurethane Prepolymer. Mater. Sci. Appl. 2012, 3, 78–86. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and Characterization of the Inclusion Complex of β-Cyclodextrin and Azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.; Agarwal, V.; Stack, K.; Richardson, D.E. Use of a New Novel Grafted Guar Gum-Copolymer as a Pitch Fixative. Appita 2011, 65–69. [Google Scholar]
- Zhao, R.; Tan, T.; Sandström, C. NMR Studies on Puerarin and Its Interaction with β-Cyclodextrin. J. Biol. Phys. 2011, 37, 387–400. [Google Scholar] [CrossRef]
- Kundu, S.; Abdullah, F.; Das, A.; Basu, A.; Halder, A.; Das, M.; Samanta, A.; Mukherjee, A. Antifungal Ouzo Nanoparticles from Guar Gum Propionate. RSC Adv. 2016, 6, 106563–106571. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Shruthi, S.B.; Bhat, C.; Bhaskar, S.P.; Preethi, G.; Sailaja, R.R.N. Microwave Assisted Synthesis of Guar Gum Grafted Acrylic Acid/Nanoclay Superabsorbent Composites and Its Use in Crystal Violet Dye Absorption. Green Sustain. Chem. 2016, 6, 11–25. [Google Scholar] [CrossRef]
- Pandey, P.P.; Ramrakhiani, M. X-Ray Diffraction Studies of Guar Gum, Polyacrylonitrile and Their Composites. Int. J. Educ. Appl. Res. 2015, 2, 7–14. [Google Scholar]
- Akduman, Ç.; Özgüney, I.; Kumbasar, E.P.A. Electrospun Thermoplastic Polyurethane Mats Containing Naproxen-Cyclodextrin Inclusion Complex. Autex Res. J. 2014, 14, 239–246. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-Thinning Hydrogels for Biomedical Applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Khedmat, S.; Momen-Heravi, F.; Pishvaei, M. A Comparison of Viscoelastic Properties of Three Root Canal Sealers. J. Dent. 2013, 10, 147–154. [Google Scholar]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A Protocol for Rheological Characterization of Hydrogels for Tissue Engineering Strategies. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul Med. 2014, 87, 215–219. [Google Scholar] [CrossRef]
- Francis, G.L. Albumin and Mammalian Cell Culture: Implications for Biotechnology Applications. Cytotechnology 2010, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Crow, B.B.; Nelson, K.D. Release of Bovine Serum Albumin from a Hydrogel-Cored Biodegradable Polymer Fiber. Biopolymers 2006, 81, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Naddaf, A.A.; Tsibranska, I.; Bart, H.J. Kinetics of BSA Release From Poly(N-Isopropylacrylamide) Hydrogels. Chem. Eng. Process. Process Intensif. 2010, 49, 581–588. [Google Scholar] [CrossRef]
- Hassan, F.; EI-Hiti, G.A.; Abd-Allateef, M.; Yousif, E. Cytotoxicity Anticancer Activities of Anastrozole against Breast, Liver Hepatocellular, and Prostate Cancer Cells. Saudi Med. J. 2017, 38, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Jha, G.; Potter, D.A. Anastrozole Use in Early Stage Breast Cancer of Post-Menopausal Women. Clin. Med. Ther. 2009, 1, 141–156. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Giangregorio, R.; Coonahan, E.; Kaplan, D.L. Silk Fibroin Rods for Sustained Delivery of Breast Cancer Therapeutics. Biomaterials 2014, 35, 8613–8620. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Singh, R. Ciprofloxacin Functionalized Biogenic Gold Nanoflowers as Nanoantibiotics Against Pathogenic Bacterial Strains. Int. J. Nanomed. 2019, 14, 9905–9916. [Google Scholar] [CrossRef]
- Sanopoulou, M.; Papadokostaki, K.G. Controlled Drug Release Systems: Mechanisms and Kinetics. Biomed. Membr. (Bio)Artif. Organs 2017, 2, 1–33. [Google Scholar]
- Cam, M.E.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.S.; Erdemir, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.S.; Kabasakal, L.; et al. Evaluation of Burst Release and Sustained Release of Pioglitazone-Loaded Fibrous Mats on Diabetic Wound Healing: An In Vitro and In Vivo Comparison Study. J. R. Soc. Interfaces 2020, 17, 20190712. [Google Scholar] [CrossRef]
- ASTM F2721-09; Standard Guide for Pre-Clinical In Vivo Evaluation in Critical Size Segmental Bone Defects. ASTM International: West Conshohocken, PA, USA, 2014.
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone Physiological Microenvironment and Healing Mechanism: Basis for Future Bone-Tissue Engineering Scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [PubMed]
- St John, T.A.; Vaccaro, A.R.; Sah, A.P.; Schaefer, M.; Berta, S.C.; Albert, T.; Hilibrand, A. Physical and Monetary Costs Associated with Autogenous Bone Graft Harvesting. Am. J. Orthop. 2003, 32, 18–23. [Google Scholar] [PubMed]
- Younger, E.M.; Chapman, M.W. Morbidity at Bone Graft Donor Sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of Synthetic Polymer Scaffolds for Bone Tissue Engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef]
- Lobb, D.C.; DeGeorge, B.R.; Chhabra, A.B. Bone Graft Substitutes: Current Concepts and Future Expectations. J. Hand Surg. Am. 2019, 44, 497–505. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, H.; RanguMagar, A.B.; Singh, P.; Oluremi, A.; Ali, N.; Watanabe, F.; Batta-Mpouma, J.; Kim, J.W.; Ghosh, A.; Ghosh, A. Guar-Based Injectable Hydrogel for Drug Delivery and In Vitro Bone Cell Growth. Bioengineering 2023, 10, 1088. https://doi.org/10.3390/bioengineering10091088
Poudel H, RanguMagar AB, Singh P, Oluremi A, Ali N, Watanabe F, Batta-Mpouma J, Kim JW, Ghosh A, Ghosh A. Guar-Based Injectable Hydrogel for Drug Delivery and In Vitro Bone Cell Growth. Bioengineering. 2023; 10(9):1088. https://doi.org/10.3390/bioengineering10091088
Chicago/Turabian StylePoudel, Humendra, Ambar B. RanguMagar, Pooja Singh, Adeolu Oluremi, Nawab Ali, Fumiya Watanabe, Joseph Batta-Mpouma, Jin Woo Kim, Ahona Ghosh, and Anindya Ghosh. 2023. "Guar-Based Injectable Hydrogel for Drug Delivery and In Vitro Bone Cell Growth" Bioengineering 10, no. 9: 1088. https://doi.org/10.3390/bioengineering10091088
APA StylePoudel, H., RanguMagar, A. B., Singh, P., Oluremi, A., Ali, N., Watanabe, F., Batta-Mpouma, J., Kim, J. W., Ghosh, A., & Ghosh, A. (2023). Guar-Based Injectable Hydrogel for Drug Delivery and In Vitro Bone Cell Growth. Bioengineering, 10(9), 1088. https://doi.org/10.3390/bioengineering10091088







