Revisional Endoscopic Foraminal Decompression via Modified Interlaminar Approach at L5-S1 after Failed Posterior Instrumented Lumbar Fusion in Elderly Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Inclusion & Exclusion Criteria
- (1)
- Clinical signs of unilateral lumbar monoradiculopathy after PILF;
- (2)
- Concordant imaging evidence of monosegmental FS at the same level within the fusion segment demonstrated on lumbar magnetic resonance imaging (MRI) and/or computer tomography (CT) scans;
- (3)
- Unsuccessful conservative treatment for at least 12 weeks;
- (4)
- Patients who agreed to sign informed consent to participate in this evaluation and are willing to return for follow-ups.
- (1)
- Bilateral symptoms or involving more than one dermatome;
- (2)
- Severe central stenosis on preoperative MRI or CT and/or Cauda equina syndrome;
- (3)
- Other diseases that may cause similar neurological symptoms, such as peripheral neuropathy;
- (4)
- Patients with systematic infection or bleeding diathesis difficult to improve;
- (5)
- Patients with unrealistic expectations and/or uncooperative patients.
2.2. Preoperative Work-Up
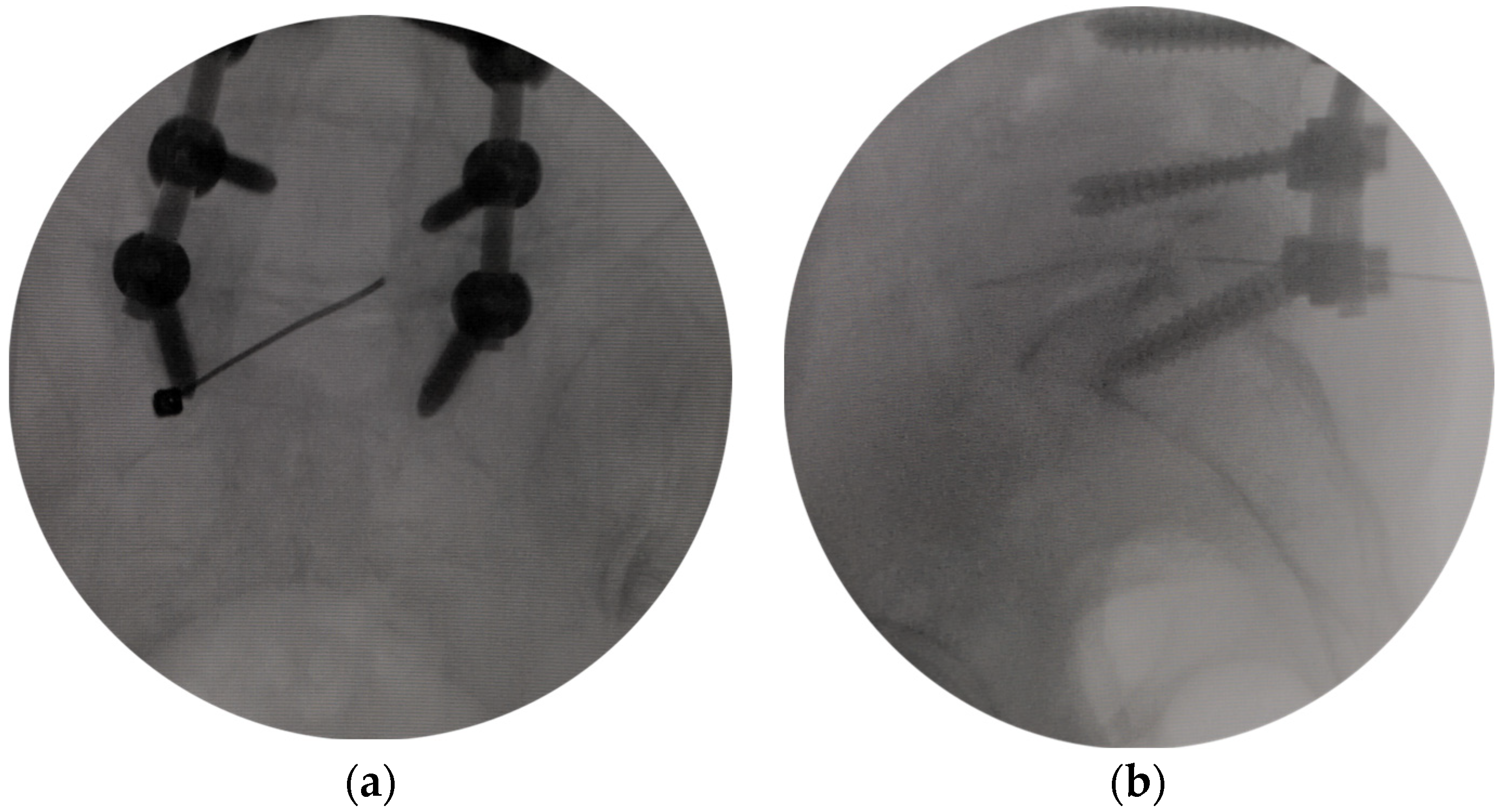
2.3. Surgical Procedures
2.4. Postoperative Care
3. Results
4. Discussion
- (1)
- Because of the preexisting internal fixation, there is no concern for iatrogenic instability, allowing more extensive decompression and enlargement of the intervertebral foramen to avoid irritating the DRG.
- (2)
- Under the endoscopic view, the structure of the foramen can be seen more clearly. Smaller operating instruments used in endoscopic operation can avoid irritating DRG.
- (3)
- The modified interlaminar approach we used can show an almost parallel trajectory to the L5 nerve root in the foramen and provide excellent visualization of the nerve root all along its course without the need for significant retraction. This technique was called “no touch decompression” [36], as FS can be treated without more retraction of DRG compared with that via transforaminal approach, especially in complicated cases and L5-S1 cases with anatomical limitations [37].
- (4)
- All operations were performed by a senior surgeon with extensive experience with the endoscopic operation. There is evidence that a lower incidence of complications has been observed when the contralateral interlaminar foraminotomy was performed by experienced surgeons [38].
- (1)
- Dissecting scar tissue from bone rather than nerve tissue is an effective method to reduce the risk of dural tear [42]. Bone tissue at the lateral and dorsal sides of the nerve structure was treated after the tip of the trephine, and anatomical landmarks were identified in X-ray view. The unscarred virgin tissue can be easily viewed and entered by endoscopic. Therefore, the operation can be safely performed without excessive retraction of the dura, nerve root, and DRG, which may be tethered at the foramen, and dissection of the scar tissues surrounding the nerve structure.
- (2)
- The entry direction of the instrument is not toward the nerve root and dural sac. The operation in the spinal canal is far from the dural sac and nerve root, and the operation in the intervertebral foramen is parallel to the nerve root. It reduces the risk of dural tear and neural injury.
- (3)
- Even if cerebrospinal fluid leakage occurs in endoscopic surgery, it is not easy to find it. There is usually no need for special treatment, and it does not affect postoperative recovery [39].
- (4)
- The number of cases is small. The incidence of neural injury may increase with higher caseloads, but it may be low.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, H.; Rahimi, S.Y.; Yeh, D.J.; Floyd, D. History of posterior thoracic instrumentation. Neurosurg. Focus 2004, 16, 1–4. [Google Scholar] [CrossRef]
- Arts, M.P.; Kols, N.I.; Onderwater, S.M.; Peul, W.C. Clinical outcome of instrumented fusion for the treatment of failed back surgery syndrome: A case series of 100 patients. Acta Neurochir. 2012, 154, 1213–1217. [Google Scholar] [CrossRef]
- Yeung, A.; Gore, S. Endoscopic foraminal decompression for failed back surgery syndrome under local anesthesia. Int. J. Spine Surg. 2014, 8, 22. [Google Scholar] [CrossRef]
- Irmola, T.M.; Hakkinen, A.; Jarvenpaa, S.; Marttinen, I.; Vihtonen, K.; Neva, M. Reoperation Rates Following Instrumented Lumbar Spine Fusion. Spine 2018, 43, 295–301. [Google Scholar] [CrossRef]
- Hazard, R.G. Failed back surgery syndrome: Surgical and nonsurgical approaches. Clin. Orthop. Relat. Res. 2006, 443, 228–232. [Google Scholar] [CrossRef]
- Lewandrowski, K.U. Endoscopic Transforaminal and Lateral Recess Decompression After Previous Spinal Surgery. Int. J. Spine Surg. 2018, 12, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Telfeian, A.E. Endoscopic foraminotomy for recurrent lumbar radiculopathy after TLIF: Technical report. Surg. Neurol. Int. 2015, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.B., Jr.; Madhavan, K.; Chieng, L.O.; Wang, M.Y.; Hofstetter, C.P. Early experience with endoscopic revision of lumbar spinal fusions. Neurosurg. Focus 2016, 40, E10. [Google Scholar] [CrossRef]
- Ahn, Y.; Keum, H.J.; Shin, S.H.; Choi, J.J. Laser-assisted endoscopic lumbar foraminotomy for failed back surgery syndrome in elderly patients. Lasers Med. Sci. 2020, 35, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Telfeian, A.E.; Moldovan, K.; Shaaya, E.; Syed, S.; Oyelese, A.; Fridley, J.; Gokaslan, Z.L. Awake, Endoscopic Revision Surgery for Lumbar Pseudarthrosis After Transforaminal Lumbar Interbody Fusion: Technical Notes. World Neurosurg. 2020, 136, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, S.; Komp, M.; Godolias, G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: Prospective 2-year results of 331 patients. Minim. Invasive Neurosurg. 2006, 49, 80–87. [Google Scholar] [CrossRef]
- Chen, K.T.; Jabri, H.; Lokanath, Y.K.; Song, M.S.; Kim, J.S. The evolution of interlaminar endoscopic spine surgery. J. Spine Surg. 2020, 6, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Kim, H.S.; Jang, I.T. How I do it? Uniportal full endoscopic contralateral approach for lumbar foraminal stenosis with double crush syndrome. Acta Neurochir. 2020, 162, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, H.S.; Jeon, J.B.; Lee, J.H.; Park, J.H.; Jang, I.T. The Novel Technique of Uniportal Endoscopic Interlaminar Contralateral Approach for Coexisting L5-S1 Lateral Recess, Foraminal, and Extraforaminal Stenosis and Its Clinical Outcomes. J. Clin. Med. 2021, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Berra, L.V.; Foti, D.; Ampollini, A.; Faraca, G.; Zullo, N.; Musso, C. Contralateral approach for far lateral lumbar disc herniations: A modified technique and outcome analysis of nine patients. Spine 2010, 35, 709–713. [Google Scholar] [CrossRef]
- Kim, H.S.; Patel, R.; Paudel, B.; Jang, J.S.; Jang, I.T.; Oh, S.H.; Park, J.E.; Lee, S. Early Outcomes of Endoscopic Contralateral Foraminal and Lateral Recess Decompression via an Interlaminar Approach in Patients with Unilateral Radiculopathy from Unilateral Foraminal Stenosis. World Neurosurg. 2017, 108, 763–773. [Google Scholar] [CrossRef]
- Zekaj, E.; Menghetti, C.; Saleh, C.; Isidori, A.; Bona, A.R.; Aimar, E.; Servello, D. Contralateral interlaminar approach for intraforaminal lumbar degenerative disease with special emphasis on L5-S1 level: A technical note. Surg. Neurol. Int. 2016, 7, 88. [Google Scholar] [CrossRef]
- Chen, K.T.; Song, M.S.; Kim, J.S. How I do it? Interlaminar contralateral endoscopic lumbar foraminotomy assisted with the O-arm navigation. Acta Neurochir. 2020, 162, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Hou, S.X.; Shang, W.L.; Song, K.R.; Zhao, H.L. Modified Percutaneous Lumbar Foraminoplasty and Percutaneous Endoscopic Lumbar Discectomy: Instrument Design, Technique Notes, and 5 Years Follow-up. Pain Physician 2017, 20, E85–E98. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Aota, Y.; Higashi, T.; Ishida, K.; Niimura, T.; Konno, T.; Saito, T. Roentgenographic and computed tomographic findings in symptomatic lumbar foraminal stenosis. Eur. Spine J. 2015, 24, 333–338. [Google Scholar] [CrossRef]
- Kim, C.H.; Chung, C.K.; Choi, Y.; Kim, M.J.; Yim, D.; Yang, S.H.; Lee, C.H.; Jung, J.M.; Hwang, S.H.; Kim, D.H.; et al. The Long-term Reoperation Rate Following Surgery for Lumbar Herniated Intervertebral Disc Disease: A Nationwide Sample Cohort Study With a 10-year Follow-up. Spine 2019, 44, 1382–1389. [Google Scholar] [CrossRef]
- Maruenda, J.I.; Barrios, C.; Garibo, F.; Maruenda, B. Adjacent segment degeneration and revision surgery after circumferential lumbar fusion: Outcomes throughout 15 years of follow-up. Eur. Spine J. 2016, 25, 1550–1557. [Google Scholar] [CrossRef]
- Ahn, Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev. Med. Devices 2014, 11, 605–616. [Google Scholar] [CrossRef]
- Knight, M.T.; Vajda, A.; Jakab, G.V.; Awan, S. Endoscopic laser foraminoplasty on the lumbar spine-early experience. Minim. Invasive Neurosurg. 1998, 41, 5–9. [Google Scholar] [CrossRef]
- Knight, M.T.; Goswami, A.; Patko, J.T.; Buxton, N. Endoscopic foraminoplasty: A prospective study on 250 consecutive patients with independent evaluation. J. Clin. Laser Med. Surg. 2001, 19, 73–81. [Google Scholar] [CrossRef]
- Knight, M.; Goswami, A. Management of isthmic spondylolisthesis with posterolateral endoscopic foraminal decompression. Spine 2003, 28, 573–581. [Google Scholar] [CrossRef]
- Wu, J.J.; Chen, H.Z.; Zheng, C. Transforaminal Percutaneous Endoscopic Discectomy and Foraminoplasty after Lumbar Spinal Fusion Surgery. Pain Physician 2017, 20, E647–E651. [Google Scholar]
- Lee, C.K.; Rauschning, W.; Glenn, W. Lateral lumbar spinal canal stenosis: Classification, pathologic anatomy and surgical decompression. Spine 1988, 13, 313–320. [Google Scholar] [CrossRef]
- Stephens, M.M.; Evans, J.H.; O’Brien, J.P. Lumbar intervertebral foramens. An in vitro study of their shape in relation to intervertebral disc pathology. Spine 1991, 16, 525–529. [Google Scholar] [CrossRef]
- Guyer, R.D.; McAfee, P.C.; Banco, R.J.; Bitan, F.D.; Cappuccino, A.; Geisler, F.H.; Hochschuler, S.H.; Holt, R.T.; Jenis, L.G.; Majd, M.E.; et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Five-year follow-up. Spine J. 2009, 9, 374–386. [Google Scholar] [CrossRef]
- Orita, S.; Inage, K.; Eguchi, Y.; Kubota, G.; Aoki, Y.; Nakamura, J.; Matsuura, Y.; Furuya, T.; Koda, M.; Ohtori, S. Lumbar foraminal stenosis, the hidden stenosis including at L5/S1. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Aota, Y.; Higashi, T.; Ishida, K.; Nimura, T.; Konno, T.; Saito, T. Lumbar foraminal stenosis causes leg pain at rest. Eur. Spine J. 2014, 23, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Van Isseldyk, F.; Kotheeranurak, V.; Quillo-Olvera, J.; Bae, J.; Choi, K.C.; Kim, J.S. Transforaminal Endoscopic Decompression for Foraminal Stenosis: Single-Arm Meta-Analysis and Systematic Review. World Neurosurg. 2022, 168, 381–391. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mikawa, Y.; Watanabe, R.; An, H.S. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine 1996, 21, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, H.; Wu, J.; Liu, H.; Zhang, Z.; Tang, Y.; Zhou, Y. Comparison of Three Minimally Invasive Spine Surgery Methods for Revision Surgery for Recurrent Herniation After Percutaneous Endoscopic Lumbar Discectomy. World Neurosurg. 2017, 100, 641–647.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Wu, P.H.; Jang, I.T. Current and Future of Endoscopic Spine Surgery: What are the Common Procedures we Have Now and What Lies Ahead? World Neurosurg. 2020, 140, 642–653. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Wu, P.H.; Jang, I.T. Effect of Dorsal Root Ganglion Retraction in Endoscopic Lumbar Decompressive Surgery for Foraminal Pathology: A Retrospective Cohort Study of Interlaminar Contralateral Endoscopic Lumbar Foraminotomy and Discectomy versus Transforaminal Endoscopic Lumbar Foraminotomy and Discectomy. World Neurosurg. 2021, 148, e101–e114. [Google Scholar] [CrossRef]
- Kim, H.S.; Wu, P.H.; Jie Chin, B.Z.; Jang, I.T. Systematic Review of Current Literature on Clinical Outcomes of Uniportal Interlaminar Contralateral Endoscopic Lumbar Foraminotomy for Foraminal Stenosis. World Neurosurg. 2022, 168, 392–397. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Hellinger, S.; De Carvalho, P.S.T.; Freitas Ramos, M.R.; Soriano-Sanchez, J.A.; Xifeng, Z.; Calderaro, A.L.; Dos Santos, T.S.; Ramirez Leon, J.F.; de Lima, E.S.M.S.; et al. Dural Tears During Lumbar Spinal Endoscopy: Surgeon Skill, Training, Incidence, Risk Factors, and Management. Int. J. Spine Surg. 2021, 15, 280–294. [Google Scholar] [CrossRef]
- Albayrak, S.; Ozturk, S.; Ayden, O.; Ucler, N. Dural Tear: A Feared Complication of Lumbar Discectomy. Turk. Neurosurg. 2016, 26, 918–921. [Google Scholar] [CrossRef]
- El Shazly, A.A.; El Wardany, M.A.; Morsi, A.M. Recurrent lumbar disc herniation: A prospective comparative study of three surgical management procedures. Asian J. Neurosurg. 2013, 8, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chung, C.K.; Jahng, T.A.; Yang, H.J.; Son, Y.J. Surgical outcome of percutaneous endoscopic interlaminar lumbar diskectomy for recurrent disk herniation after open diskectomy. J. Spinal Disord. Tech. 2012, 25, E125–E133. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Li, Z.; Zhao, H.; Wang, J.; Hou, S. Revisional Endoscopic Foraminal Decompression via Modified Interlaminar Approach at L5-S1 after Failed Posterior Instrumented Lumbar Fusion in Elderly Patients. Bioengineering 2023, 10, 1097. https://doi.org/10.3390/bioengineering10091097
Cao Z, Li Z, Zhao H, Wang J, Hou S. Revisional Endoscopic Foraminal Decompression via Modified Interlaminar Approach at L5-S1 after Failed Posterior Instrumented Lumbar Fusion in Elderly Patients. Bioengineering. 2023; 10(9):1097. https://doi.org/10.3390/bioengineering10091097
Chicago/Turabian StyleCao, Zheng, Zhenzhou Li, Hongliang Zhao, Jinchang Wang, and Shuxun Hou. 2023. "Revisional Endoscopic Foraminal Decompression via Modified Interlaminar Approach at L5-S1 after Failed Posterior Instrumented Lumbar Fusion in Elderly Patients" Bioengineering 10, no. 9: 1097. https://doi.org/10.3390/bioengineering10091097
APA StyleCao, Z., Li, Z., Zhao, H., Wang, J., & Hou, S. (2023). Revisional Endoscopic Foraminal Decompression via Modified Interlaminar Approach at L5-S1 after Failed Posterior Instrumented Lumbar Fusion in Elderly Patients. Bioengineering, 10(9), 1097. https://doi.org/10.3390/bioengineering10091097